Artigo
| Variation of essential oil composition of Tapirira guianensis Aubl. (Anacardiaceae) from two sandbank forests, North of Brazil |
|
Maria das Graças Bichara Zoghbi*; Raimunda Alves Pereira; Giselle do Socorro Luz de Lima; Maria de Nazaré do Carmo Bastos
Coordenação de Botânica, Museu Paraense Emílio Goeldi, Av. Perimetral 1901, 66077-901 Belém - PA, Brasil Recebido em 28/01/2014 * e-mail: zoghbi@museu-goeldi.br Tapirira guianensis (Anacardiaceae) is used in traditional medicine and is important for the recovery of degraded areas and riparian forests because the T. guianensis fruits are highly consumed by wildlife. Volatile components from dried leaves and branches of five individual plants of T. guianensis were collected in two sandbank forests of the State of Pará (Extractive Reserve Maracana and Area of Environmental Protection Algodoal/Maiandeua), extracted by hydrodistillation using a Clevenger-type apparatus, and analyzed by GC/MS. The ten oils obtained are comprised mostly of sesquiterpene hydrocarbons (58.49 to 100%), with (E)-caryophyllene, β-selinene, α-selinene, β-sesquiphellandrene, and α-zingiberene being the most prominent. The results of the oil compositions were processed by Hierarchical Component Analysis (HCA) allowing the establishment of three groups of essential oils for T. guianensis differentiated by the content of β-selinene/α-selinene (Type I), (E)-caryophyllene (Type II), and β-sesquiphellandrene/α-zingiberene (Type III). INTRODUCTION The Anacardiaceae family consists of 81 genera and 800 species that are mostly tropical. Diversity in Brazil is represented by 14 genera and 54 species that are concentrated in the northern and southern regions.1 Tapirira guianensis Aubl. is widely distributed throughout Brazil, mainly in humid soils, from the Amazon to the Atlantic Forest, crossing Central Brazil by gallery forests.2 In the North of Brazil, T. guianensis is popularly known as tapirira; other common names of this species in Brazil are pau-pombo, cupiúva, tatapiririca, and jobo.3-5 This species is used in traditional medicine and is important for the recovery of degraded areas and riparian forests due to the production of fruits that are highly consumed by wildlife.6 On the sandbank forest of Marieta beach, which is under the domain of Extractive Reserve of Maracanã (Resex Maracanã), T. guianensis was considered the most important aromatic species. Density and dominance of T. guianensis showed the highest values in the ecotone site.7 In the State of Pará, the wood and the inner bark of the trunk have been used, respectively, to treat oral thrush in children and sore throat and mouth.8 The ethanol extract of the leaves and stem bark of T. guianensis showed important cytotoxic activity.9 Phytochemical studies on the methanol extract of the seeds of T. guianensis resulted in the isolation of β-sitosterol and alkylphenols10 while the hexane extract of the bark furnished triterpenoids and ferulates.11 Four anti-protozoal and anti-bacterial compounds were isolated from dichloromethane extract of the bark of the plant collected in French Guiana.12 The concentration and distribution of nutrients in distinct organs of the plant was evaluated.4 Ultra-structural aspects of secretory canals in vegetative and reproductive organs of T. guianensis were study by Lacchia and Guerreiro.13 A survey of the literature revealed that no prior studies of essential oil from T. guianensis have been reported. The aim of this paper was to characterize the chemical composition of leaf and branch essential oils of T. guianensis growing wild in two sandbank forests of the State of Pará, Brazil.
EXPERIMENTAL Botanical material Sample A (September, 2007) was taken from the sandbank forest of Marieta beach, which is located near to Vila da Penha Community (00º35'50" S/47º26'35.5" W), which is located in the municipality of Maracanã and is under the domain of the Extractive Reserve of Maracanã (Resex Maracanã). Samples B, C, and D (August, 2009) were taken in the sandbank forest of Algodoal Island (Maracanã), in the municipality of Maracanã, which is under the domain of the Environmental Protection Area Algodoal/Maiandeua (00º35'19.8" S/47º34'27.7" W). Sample E (October, 2009) was taken in the area closer to the shore of Vila da Penha Community. Voucher specimens (Sample A: MG 189,996; Sample B: 196,815; Sample C: 196,819; Sample D: 196,826; Sample E was identified by comparison with the other cited voucher of T. guianensis. Vouchers were deposited in the Herbarium MG of the Museu Paraense Emílio Goeldi (MPEG). The samples were dried for 7 days in an air-conditioned room (at low humidity) and then ground. Extraction of volatile compounds The dry plant material was hydrodistilled for 3 h, using a Clevenger-type apparatus with maintenance of the refrigeration water in 15 ºC. The oils obtained were centrifuged for 5 min (3,000 rpm), dried over Na2SO4, and centrifuged again in the same conditions. The hexane solution (1 mL) containing 2 µL of the oil was submitted for GC-MS analysis. The total oil yield was expressed in percentage (volume∙mass-1) on the basis of dried material. The amount of water was measured using infrared light on a Marte ID-50 device. Analysis of volatile compounds The oils were analyzed using a Shimadzu GC/MS Model QP 2010 Plus, equipped with a Rtx-5MS (30 m × 0.25 mm; 0.25 µm film thickness) fused silica capillary column. Chromatographic conditions included helium as carrier gas at 1.2 mL∙min-1; splitless injection of 1 µL, of the hexane solution; injector and interface temperature at 250 ºC; oven temperature program 60-240 ºC at 3 ºC∙min-1. EIMS: electron energy, 70 eV; ion source temperature at 200 ºC. Identification of the compounds were made by comparison of their GC mass and retention data with those in NIST-05 library, and cited in the literature.14-18 Retention indices were calculated using n-alkane standard solutions (C8-C26) available from Fluka S. A., in the same chromatographic conditions.
RESULTS AND DISCUSSION The percentage of the compounds identified in the leaf and branch oils are listed in Table 1 in sequence of their retention indices. Higher yield in oils were obtained from the leaves (Samples A to E: 0.24%, 0.23%, 0.23%, 0.27%, and 0.13%, respectively). The yields of the oils isolated from the branches of Samples A to E were 0.14%, 0.05%, 0.05%, 0.09%, and 0.04%, respectively. In total, 119 compounds were identified, accounting for 87.06%-100% of the total volatiles. The oils from the ten samples analyzed were terpenoid in nature, predominated by sesquiterpenes (sesquiterpene hydrocarbons change from 58.49% to 100.00%; oxygenated sesquiterpenes change from zero to 28.86%). In total, monoterpenes accounted for 3.27% (monoterpene hydrocarbons changes from zero to 1.15%; oxygenated monoterpenes changes from zero to 0.39%), mostly present in Sample E. Monoterpenes were present in small amounts in Samples B to E and not detected in Sample A, and were represented by α-pinene, β-pinene, α-phellandrene, α-terpinene, sylvestrene, (E)-β-ocimene, γ-terpinene, terpinolene, linalool, terpinen-4-ol, and geraniol. Among the sesquiterpenes only β-elemene and (E)-caryophyllene were detected in all oils analyzed.
(E)-Caryophyllene is a common sesquiterpene encountered in essential oils; other Anacardiaceae species rich in (E)-caryophyllene in the essential oils of the leaves were Anacardium humile A. St.-Hil. (31.00%) and A. occidentale L. (15.40%).19 Two samples, taken at APA Algodoal/Maiandeua (Samples B and D) and one collected at Resex Maracanã (Sample E), exhibited (E)-caryophyllene as the most abundant component in the oils of leaves and branches (Sample B - leaf: 29.47%, branch: 23.39%; Sample D - leaf: 66.87%, branch: 59.17%; Sample E - leaf: 28.15%, branch: 19.25%). The second sample collected at Resex Maracanã (Sample A) showed α-selinene (leaf: 24.37%, branch: 31.07%) and β-selinene (leaf: 42.58%, branch: 57.56%) as major constituents. The third sample collected at APA Algodoal/Maiandeua (Sample C), presented two major constituents: α-zingiberene (leaf: 24.49%, branch: 18.61%) and β-sesquiphellandrene (leaf: 20.00%, branch: 17.00%). In this study, we found that leaves and branches of all analyzed samples of T. guianensis accumulated more sesquiterpene hydrocarbons (leaves: 90.50%, 80.09%, 91.92%, 91.68% and 75.24%; branches: 100.00%, 65.7%, 75.51%, 78.04%, and 58.49%) than oxygenated sesquiterpenes (leaves: 2.77%, 12.79%, 1.97%, 6.42%, and 14.17%; branches: zero, 20.77%, 7.86%, 14.08% and 28.86%). On the other hand, some compounds also appear to be restricted to one tissue: several compounds were encountered only in the branches. As a good example, β-pinene, α-phellandrene, terpinolene, nonanal, (2E)-nonen-1-al, decanal, geraniol, (2E)-decen-1-al, safrole, (2E,4Z)-decadienal, and (2E,4E)-decadienal were detected in branches of the Sample E, but absent in the leaves. A similar result was obtained by Courtois et al.20 for bark and leaves of 55 tropical tree species. The intraspecific variability of all 119 compounds from the ten samples was included in the multivariate analysis using Minitab 14 software for Hierarchical Component Analysis (HCA). HCA distinguishes the Samples B, D, and E for the content of (E)-caryophyllene, and the Samples A and C by the content of β-selinene/α-selinene and β-sesquiphellandrene/α-zingiberene, respectively. HCA also revealed that the chemical composition of leaves and branches of T. guianensis do not exhibit expressive differences. Therefore, three main types of essential oils of individuals of the T. guianensis were found: Cluster I (Sample A) was characterized by the presence of β-selinene/α-selinene. Cluster II (Samples B, D and E) had (E)-caryophyllene as major compound. Cluster III (Sample C) showed substantial percentages of b-sesquiphellandrene/α-zingiberene. In the oils of T. guianensis large variation was observed in the content of the major and minor constituents, besides high qualitative variation. This variation is especially important because the samples were collected in the same environment (sandbank), municipality, and Amazonian climatic period (dry season). Additionally, all of the plants were in the flowering stage and were free from injuries by microorganisms. Our results suggest that chemical types may exist for T. guianensis.
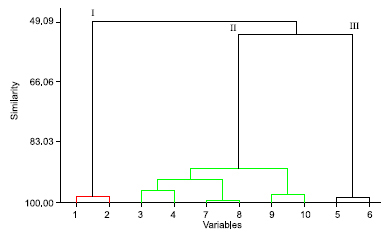 Figure 1. Hierarchical Component Analysis (HCA) of Tapirira guianensis plants collected from five populations of the North of Brazil
CONCLUSION The essential oils of T. guianensis, growing wild in two sandbanks of the State of Pará were characterized by the predominance of hydrocarbon sesquiterpenes. Three types of the oils were encountered for this species: 1) β-selinene/α-selinene, 2) (E)-caryophyllene, 3) α-zingiberene/β-sesquiphellandrene. The chemical variability of T. guianensis is important in light of their use in traditional medicine.
ACKNOWLEDGMENTS The authors are grateful to Fundação de Amparo à Pesquisa do Estado do Pará (FAPESPA) for financial support, and Osvaldo Nascimento (MPEG) for plant collection. R. A. Pereira and G. S. L. de Lima are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the fellowship.
REFERENCES 1. Luz, C. L. S.; Pirani, J. R.; Valente, A. S. M.; Fernandez, E. P.; Penedo, T. S. A.; Borges, R. A. X.; Anacardiaceae; In: Livro vermelho da flora do Brasil; Martinelli, G.; Moraes, M. A. (Orgs.). Instituto de Pesquisas Jardim Botânico do Rio de Janeiro: Rio de Janeiro, 2013, p. 140. 2. Ribeiro-Filho, A. A.; Funch, L. S.; Rodal, M. J. N.; Rodriguésia 2009, 60, 265. 3. Santana, W. M. S.; Silva-Mann, R.; Ferreira, R. A.; Arrigoni-Blank, M. F.; Blank, A. F.; Poderoso, J. C. M.; Scientia Forestalis 2009, 37, 47. 4. Nascimento, S. M.; Costa, J. C. A.; Barreto, L. P.; Bezerra Neto, E.; Passos, M. A. A.; Ribeiro, J. S.; Revista Brasileira de Ciências Agrárias 2007, 2, 128. 5. Cesarino, F.; Leão, J. A.; Pantoja, T. F.; Silva, B. M. S.; Revista Brasileira de Biociências 2007, 5, 348, supl. 2. 6. Lorenzi, H.; Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil, v. 1, Editora Plantarum: Nova Odessa, 1998. 7. Pereira, R. A.; Dissertação de Mestrado, Universidade Federal Rural da Amazônia e Museu Paraense Emílio Goeldi, Brasil. 8. Coelho-Ferreira, M.; J. Ethnopharmacol. 2009, 126, 159. 9. Mahmoud, T. S.; Marques, M. R.; Ó Pessoa, C.; Lotufo, L. V. C.; Magalhães, H. I. F.; Moraes, M. O.; Lima, D. P.; Tininis, A. G; Oliveira, J. E.; Rev. Bras. Farmacogn. 2011, 21, 456. 10. David, J. M.; Chávez, J. P.; Chai, H-B.; Pezzuto, J. M.; Cordell, G. A.; J. Nat. Prod. 1998, 61, 287. 11. Correia, S. J.; David, J. P.; David, J. M.; Quim. Nova 2003, 26, 36. 12. Roumy, V.; Fabre, N.; Portet, B.; Bourdy, G.; Acebey, L.; Vigor, C.; Valentin, A.; Moulis, C.; Phytochemistry 2009, 70, 305. 13. Lachia, A. P. S.; Guerreiro, S. M. C.; Acta Bot. Bras. 2009, 23, 376. 14. Adams, R. P.; Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectrometry. Allured Publishing Corporation: Carol Stream IL, 2007, 804p. 15. Indrayan, A. K.; Bhojak, N. K.; Kumar, N.; Shatru, A.; Gaur, A.; Indian J. Chem., Sect B 2011, 50B, 1136. 16. Bi Kouame, F. P.; Bedi, G.; Koffi, A. M.; Chalchat, J. C.; N'Guessan, T. Y.; The Open Natural Products Journal 2010, 3, 6. 17. Yayli, N.; Yasar, A.; Güleç, C.; Usta, A.; Kolayh, S.; Coskunçelebi, K.; Karaoğlu, Ş.; Phytochemistry 2005, 66, 1741. 18. Yayli, N.; Yaşar, A.; Yayli, N.; Albay, C.; Aşamaz, Y.; Coşkunçelebi, K.; Karaoğlu, Ş.; Pharm. Biol. 2009, 47, 7. 19. Montanari, R. M.; Barbosa, L. C. A.; Demuner, A. J.; Silva, C. J.; Andrade, N. J.; Ismail, F. M. D.; Barbosa, M. C. A.; Molecules 2012, 17, 9728. 20. Courtois, E. A.; Baraloto, C.; Paine, C. E. T.; Petronelli, P.; Blandinieres, P.-A.; Stien, D.; Höuel, E.; Bessière, J.-M.; Chave, J.; Phytochemistry 2012, 82, 81. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access