Educação
| Nanoporous materials: pillared clays and regular silicas as an example of synthesis and their porosity characterization by X-ray diffraction |
|
Joao Pires
Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa, Ed. C8, Campo Grande, 1749-016 Lisboa, Portugal Recebido em 04/03/2013 *e-mail: jpsilva@fc.ul.pt Because of their practical applications, porous materials attract the attention of undergraduate students in a way that can be used to teach techniques and concepts in various chemistry disciplines. Porous materials are studied in various chemistry disciplines, including inorganic, organic, and physical chemistry. In this work, the syntheses of a microporous material and a mesoporous material are presented. The porosity of the synthesized materials is characterized by X-ray diffraction analysis. We show that this technique can be used to determine the pore dimensions of the synthesized materials. INTRODUCTION Nanoporous materials, i.e., materials with pore widths between 1 and 100 nm, have a wide range of effective and potential applications in fields such as catalysis, separation/purification, electronics, and drug delivery.1-3 The term "nanoporous" is relatively recent; in fact, the International Union of Pure and Applied Chemistry (IUPAC) classifies porous materials, according to their pore widths, as microporous (pore widths less than 2 nm), mesoporous (pore widths between 2 and 50 nm), and macroporous (pore widths greater than 50 nm).2,4 A large number of applications of nanoporous materials can be readily exemplified and understood, which usually attracts students' attention. Therefore, these types of solids can be used as a route to introduce undergraduate students to or to deeply explore various aspects of chemistry, particularly in the fields of inorganic and physical chemistry. In the experiments described in this text, X-ray diffraction (XRD) is used to characterize the porous structures of two types of materials obtained using two different synthetic approaches: the intercalation of a natural clay to produce a microporous material and a micelle-templated synthesis to obtain a mesoporous material. Pillared interlayered clays (PILCs) are a relatively recent family of microporous materials.5 Students find the study of PILCs interesting because these solids are produced from natural clays,6,7 and their preparation is therefore an example of adding value to a natural product. Natural clays are layered solids but do not have definite porosity because they reversibly swell in the presence of water and a number of other molecules.6 Clays exhibit ion-exchange capabilities; therefore, PILCs are prepared by exchanging the charge-compensating cations present in the interlamellar space of clays with large hydroxy-metal polycations. After heating the PILCs, the inserted polycations yield rigid, thermally stable oxide species, which prop the clay layers apart and prevent their collapse.5 Figure 1 presents the main steps involved in the preparation of PILCs. A wide range of polyoxocations can be used as pillaring agents, but the most extensively studied polyoxocations are those of aluminum and zirconium.5,8
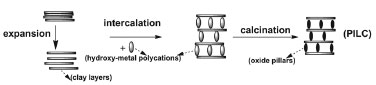 Figure 1. Main steps of the pillaring process
The so-called ordered mesoporous materials include micelle-templated materials, which are an interesting group of nanoporous solids; these materials usually consist of silica pore walls formed around organic micelles, which are often long-chain quaternary ammonium ions that act as a mold for the structure. The first materials of this type were reported in 1969,9 but it was only in 1992 after a similar material was obtained by scientists in Mobil Oil Corporation10 that the properties of such materials gained recognition.11 The MCM-41 solid, in particular, shows a highly ordered hexagonal array of one-dimensional pores with walls made of amorphous silica.12 From a more specific view, the work described here exemplifies how a porous material can be obtained from entirely synthetic products or from a natural product, such a clay, and explores the technique of X-ray diffraction for the characterization of pore sizes. In a more general sense, the described experiments, while involving relatively simple methodologies, are expected to contribute to the students' general skills with respect to materials preparation and to stress to students, using the example of porous materials, the interdisciplinary nature of some basic concepts of various disciplines of chemistry. This approach therefore counters the belief of a large number of students that chemistry disciplines are well segmented.
EXPERIMENTAL OVERVIEW Pillared clay Many natural clays can be used as raw materials for this synthesis, provided that they contain smectite (expandable) clays, particularly montmorillonite, as a major fraction. Bentonites, such as Volclay SPV-200 from Ward's National Science Est., satisfy this requirement. Particularly interesting and of high pedagogical value is the case in which your university has a geology department that can provide a few grams of a local smectite clay. In this activity, a Zr-PILC is prepared because the preparation Zr-polyoxocations is experimentally simple as they can be readily obtained from an aqueous solution of zirconium oxychloride (ZrOCl2·8H2O)13 (Figure 2). Experimental and time-management details are given in the Supplementary Material.
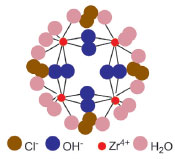 Figure 2. Model of the tetramer of zirconium cations (adapted from ref. 16)
X-ray diffractograms are obtained to investigate the physical changes that occur in the space between the clay layers during the pillaring process, i.e., the changes in the d001 basal spacing (Figure 3). Sample diffractograms obtained by students are shown in Figure 4.
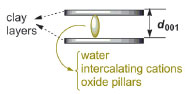 Figure 3. Schematic representation of the d001 basal spacing in smectites
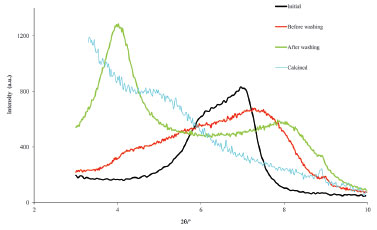 Figure 4. X-ray diffractograms in the 2θ region ascribed to d001 for an initial clay and the samples in various phases of the pillaring process
MCM-41 Tetraethyl orthosilicate (TEOS) is the most common silica source used in the synthesis of MCM-41-type materials, although various templating micelles can be used.11,14 These micelles are obtained from cationic organic surfactants such as cetyltrimethylammonium bromide, H3C(CH2)15N(CH3)3Br (CTAB). In the present activity, in addition to CTAB, tributylheptylammonium bromide (H3C(CH2)6N(CH2CH2CH2CH3)3Br) was also used. In this way, the students can observe the effect of the template on the pore size. These two surfactants are structurally different because, in the latter case, the main chain is shorter (C7 vs. C16 in CTAB) and the remaining groups linked to the nitrogen atom are bulkier. Examples of the X-ray diffractograms obtained by students are shown in Figure 5 (and Figure 1S in the Supplementary Material). Experimental and time-management details for the preparation of both MCM-41 samples are given in the Supplementary Material.
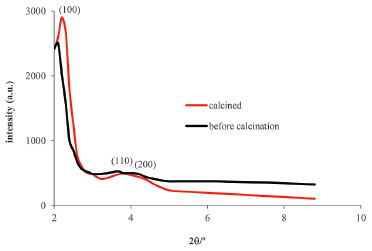 Figure 5. X-ray diffractogram for the MCM-41 sample, which was prepared using a mixture of CTAB and tributylheptylammonium bromide, before and after calcination
DISCUSSION The described experiments are appropriate for senior undergraduate students, particularly, but not exclusively, for those working on a mini-project basis in the areas of inorganic chemistry and materials chemistry or those enrolled in laboratory classes where the interdisciplinary nature of these disciplines is valued. The reagents are affordable, and the methodologies used allow the students to work with a considerable degree of autonomy, which may lead them to propose extensions of the work. The first level of characterization of the prepared materials is conducted using XRD. An example of the discussion of the results for Zr-PILC in Figure 4 is presented in the Supplementary Material. In summary, starting from a natural clay, which has no fixed pore dimension because the d001 value depends, in this case, even on the humidity, a microporous material with a fixed porosity value was obtained. Notably, although the synthesis of other types of microporous materials have been proposed for laboratory activities, such as the synthesis of zeolites,15 information on pore dimensions obtained from XRD results cannot be obtained and analyzed by undergraduate students as readily as in the case of pillared clays. Diffractograms of the MCM-41-type materials are given in Figure 5. Students synthesized the materials and obtained the diffractograms. Students should note that because of the large d spacing and sometimes low degree of regularity in mesoporous materials compared to solids that can be obtained as single-crystals, only a few peaks are often observed in the XRD patterns of MCM-type solids. A more detailed discussion is presented in the Supplementary Material. The values listed in Table 1 are within the range of those presented in the literature for MCM-41-type materials.12 Although the silicates that form the walls of MCM-41 partially condensate at room temperature, the calcination step is mandatory to complete this condensation and avoid structural collapse. The values of the cell parameter a0 listed in Table 1 show the calcination effect. The a0 values decrease after calcination, i.e., upon the completion of the silicate condensation, and this effect is more evident in the case where only CTAB was used in the synthesis. When tributylheptylammonium bromide was used in addition to CTAB, an increase in the unit cell parameter, i.e., an increase in the pore size, was observed.
CONCLUSIONS The work presented here uses a relatively simple experimental approach for the preparation and characterization of nanoporous materials. This approach allows students of chemistry or other courses that involve materials chemistry to engage in material synthesis and to characterize by X-ray diffraction the samples they have synthesized. This experiment provides students the opportunity to apply learned competencies that are interdisciplinary. In addition, because of the perception of students that nanoporous materials have applications in a wide range of fields, students view the work favorably, which may contribute to the decision of some students to proceed with the theme of nanoporous materials in the final project of their bachelor or master course.
SUPPLEMENTARY MATERIAL Supplementary material is freely available at http://quimicanova.sbq.org.br in the form of a PDF file. This material includes detailed experimental instructions, guidelines for synthesis, characterization, and time management, and complementary activities.
ACKNOWLEDGMENT J. Pires is grateful to the CQB (strategic projects PEst-OE/QUI/UI0612/2013) and the Department of Chemistry and Biochemistry, University of Lisbon, for resources.
REFERENCES 1. Lu, G. Q.; Zhao, X. S.; in Nanoporous Materials: Science and Engineering; Lu, G. Q.; Zhao, X. S., eds.; Imperial College Press: London, 2004, cap. 1. 2. Polarz, S.; Smarsly, B.; J. Nanosci. Nanotechnol. 2002, 2, 581. 3. Wang, S.; Microporous Mesoporous Mater. 2009, 117, 1. 4. Sing, K. S. W.; Everett, D. H.; Haul, R. A. W.; Moscou, L.; Pierotti, R. A.; Rouquerol, J.; Siemieniewska, T.; Pure Appl. Chem. 1985, 57, 603. 5. Gil, A.; Korili, A.; Trujillano, R.; Vicente, M. A.; Pillared Clays and Related Catalysts, Springer: New York, 2010; Gil, A.; Korili, A.; Vicente, M. A.; Catal. Rev. Sci. Eng. 2008, 50, 153. 6. Velde, B.; Introduction to Clay Minerals - Chemistry, Origins, Uses and Environmental Significance, Chapman & Hall: London, 1992. 7. Grim, R. E.; Clay Mineralogy, 2nd ed.; McGraw-Hill: New York, 1968. 8. Suzuki, K.; Horio, M.; Masuda, H.; Mori, T.; Bull. Chem. Soc. Jpn. 1991, 64, 732. 9. Di Renzo, F.; Cambon, H.; Dutartre, R.; Microporous Mater. 1997, 10, 283. 10. Beck, J. S.; Vartuli, J. C.; Roth, W. J.; Leonowicz, M. E.; Kresge, C. T.; Schmitt, K. D.; Chu, C. T. W.; Olson, D. H.; Sheppard, E. W.; McMullen, S. B.; Shepard E. W.; J. Am. Chem. Soc. 1992, 114, 10834. 11. Taguchi, A.; Schüth, F.; Microporous Mesoporous Mater. 2005, 77, 1. 12. McCullen, S. B.; Vartuli, J. C.; Kresge, C. T.; Roth, W. J.; Beck, J. S.; Schmitt, K. D.; Leonowicz, M. E.; Schlenker, J. L.; Shih, S. S.; Lutner, J. D. In Access in Nanoporous Materials; Pinnavaia, T. J.; Thorpe, M. F., eds.; Plenum Press: New York, 1995. 13. Pereira, P. R.; Pires, J.; Carvalho M. B.; Langmuir 1998, 14, 4584; Figueras, F.; Mattrod-Bashi, A.; Fetter, G.; Thrierr, A.; Zanchetta, J. V.; J. Catal. 1989, 119, 91. 14. Hu, J.; Yin, J.; Lin, T.; Li, G.; J. Chem. Educ. 2012, 89, 284. 15. Copperthwaite, R. G.; Hutchlngs, G. J.; van der Rlet, M.; J. Chem. Educ. 1986, 63, 632; Blalier, F.; Schumacher, E.; J. Chem. Educ. 1990, 67, 519; Balkus, K. J.; Ly, K. T.; J. Chem. Educ. 1991, 68, 875; Belver, C.; Vicente, M. A.; J. Chem. Educ. 2006, 83, 1541. 16. Ohtsuka, K.; Hayashi, Y.; Suda, M.; Chem. Mater. 1993, 5, 1823; Muha, G.; Vaughan, P. A.; J. Chem. Phys. 1960, 33, 194. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access





