Artigo
| Asymmetric sulfoxidation of albendazole to ricobendazole by fungi: effect of pH |
|
Thiago BarthI,*; Viviane C. HilárioII; Bruno A. RochaII; Niege A. J. C. FurtadoIII; Mônica T. PupoIII; Anderson R. M. de OliveiraII
IUniversidade Federal do Rio de Janeiro, Campus Macaé, 27930-560 Macaé - RJ, Brasil Recebido em 24/02/2015 *e-mail: barththiago@yahoo.com.br Albendazole (ABZ) is an anthelmintic drug used for the treatment of infectious diseases in veterinary and human medicine. This drug is a prochiral drug that after administration, is rapidly oxidized in the pharmacologically active sulfoxide metabolite, which is also known as ricobendazole (ABZSOX). ABZSOX has a stereogenic center and possibly two enantiomers, (+)-ABZSOX and (-)-ABZSOX. In the present work, we investigate the pH effect on the asymmetric stereoselective sulfoxidation of ABZ into ABZSOX by employing the fungi Nigrospora sphaerica, Papulaspora immera Hotson, and Mucor rouxii. The results show a possibility of obtaining the pure enantiomers of the ricobendazole drug using fungi as biocatalytic agents. The three fungi showed a high degree of enantioselectivity expressed by enantiomeric excess. In addition, M. rouxii can be used as an alternative to obtain the (+)-ABZSOX enantiomer (ee 89.8%). INTRODUCTION The biotransformation of organic compounds by fungi is considered an economically and ecologically viable technology. Moreover, it is a useful tool for the structural modification of bioactive compounds promoting the addition of groups of interest in the molecule in certain positions where it would be difficult by conventional synthesis methods.1-3 In addition, some microorganisms can simulate the mammalian cytochrome P450 metabolism of many molecules of pharmacological importance such as drugs and natural products.4-6 The use of microbial system as models to mimic the metabolism of drugs in humans has received considerable attention.1-4 This tool can be also applied to the production of large quantities of the major and minor metabolites, which is more desirable than isolation of these compounds from treated dosed animals, and with lower cost, greater efficiency and less time than animal tests. So, it avoids the concerns often associated with chemical synthesis, such as the use of toxic reagents and drastic reaction conditions.2-3 The synthesis of optically active compounds by using fungal models offers a few advantages compared to chemical synthesis, because it can be highly enantiomeric and regio-selective under mild conditions. Pharmaceutical compounds have often several chiral centers, consequently it is not easy to prepare such compounds through the conventional chemical synthesis.1-3,7-10 Albendazole (ABZ) is a widely drug used in veterinary and human medicine for the treatment of helmintic infection diseases. This drug is a prochiral drug that after parenteral and oral administration, it is rapidly oxidized in the human liver to the pharmacologically active sulfoxide metabolite which is also known as ricobendazole (ABZSOX) (Figure 1).11 In sequential oxidation, this metabolite is converted to the inactive metabolite albendazole sulfone (ABZSO2) and then in albendazole aminesulfone. The anthelmintic activity of albendazole is attributed to its bioactive metabolite ricobendazole.12 The Cytochrome isoform (CYP3A) and the flavin-containing monooxygenases appear to mediate the metabolism of ABZ to ABZSOX, whereas the biotransformation of ABZSOX to ABZSO2 is only influenced by CYP.13 Despite the interest in the activities of the ABZSOX enantiomers, Bolás-Fernández et al. showed that at low concentrations of rac-ABZSOX, (+)-ABZSOX and (-)-ABZSOX, only the (+)-ABZSOX has a significant reduction of the Trichinella spiralis viability in a murine model, that is a well-established procedure to evaluate anthelmintic activity.14
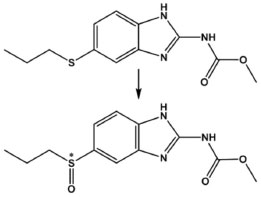 Figure 1. Chemical structures of albendazole and its main metabolite, albendazole sulfoxide *Denote the chiral center
Our earlier work reported the development of a high-performance liquid chromatographic method using polar organic mode to analyze ABZ, ABZSO2 and the chiral and active metabolite ABZSOX, ricobendazole, that was applied in stereoselective fungal biotransformation studies. This study was the initial step to use fungi to obtain the active metabolite, ricobendazole. Nigrospora sphaerica (Sacc.) E. W. Mason SS67, Pestalotiopsis foedans VR8, Papulaspora immersa Hotson SS13 and Mucor rouxii NRRL 1894 were able to convert ABZ stereoselectively into its chiral metabolite (+)-ABZSOX. In order to improve the enantiomeric excess (e.e.) on the (+)-ABZSOX formation, the pH influence was investigated.15 So, the present work shows the influence of pH on the biotransformation of albendazole by using the same microorganisms of earlier work to improve the e.e. of (+)-ABZSOX.
MATERIALS AND METHODS Chemicals and reagents ABZ and ABZSOX (analytical standard, purity > 98%) were purchased from Sigma-Aldrich (Steinheim, Germany). The solvents used in hollow fiber liquid-phase microextraction (HF-LPME) procedure and in the biotransformation procedure were: 1-octanol (analytical grade, purity > 99%) purchased from Sigma-Aldrich, Steinheim, Germany and N,N-dimethylformamide (analytical grade, purity > 99%) purchased from Mundial Química (Ribeirão Preto, São Paulo, Brazil). The reagents (analytical grade and purity > 98%) potassium chloride monosodium phosphate 1-hydrated, disodium phosphate 2-hydrated, magnesium sulfate 7-hydrated and iron sulfate 7-hydrated were obtained from Merck (Darmstadt, Germany). Sodium hydroxide (purity > 98%) was purchased from Nuclear (Diadema, São Paulo, Brazil), hydrochloric acid and acetic acid (purity > 99%) were obtained from Zilquímica (Ribeirão Preto, São Paulo, Brazil). Potato dextrose agar, sucrose, malt extract, dextrose, tryptone and yeast extract were obtained from Acumedia with grade for microbiology assays (Lansing, MI, USA). Water used to prepare the solutions was purified using a Milli-Q plus system (Millipore, Bedford, MA, USA). HPLC grade ethanol, methanol, acetonitrile, and acetic acid were purchased from Merck (Darmstadt, Germany) and from J. T. Baker (Philipsburg, NJ, USA), all HPLC grade solvents with purities > 99.9%, while diethylamine (purity > 99.5%) was obtained from Fluka (Buchs, Switzerland). Fungus isolation and maintenance The selected strains of endophytic fungi were Papulaspora immersa Hotson SS13 and Nigrospora sphaerica (Sacc.) E. W. Mason SS67 isolated from Smallanthus sonchifolius. The studied fungi were identified as previously described by Gallo et al..16 The fungi have been maintained as agar plugs in sterile water, or in glycerol: water (8: 2, v/v) at 10 ºC. The strains have been deposited in the Laboratory of Microbial Chemistry, Faculty of Pharmaceutical Sciences of Ribeirão Preto (University de São Paulo, Ribeirão Preto, Brazil). The fungus Mucor rouxii strain NRRL 1894 was obtained as a courtesy of Dr. C.W. Hesseltine (Northern Utilization Research and Development Division, ARS, USDA, Peoria, IL, USA) and belongs to a collection of fungal cultures of the Biology Department, Faculty of Philosophy, Sciences and Letters of Ribeirão Preto, University of São Paulo. The microorganism is stored as a conidial suspension on silica gel (6-12 mesh, grade 40, desiccant activated) at 4 ºC and on slants of solid oatmeal baby food consisting of 0.4% (w/v) oatmeal and 1.8% (w/v) agar. Biotransformation study Three discs of 0.5 cm of diameter (potato dextrose agar plugs) containing the fungal mycelia were aseptically transferred to 9.0 cm diameter Petri dishes containing PDA medium (potato, dextrose, agar) and allowed to grow for 6 days at 25 ºC. Biotransformation was performed using a two-stage fermentation protocol.17 In the first stage (pre-culture), three 0.5 cm uniform discs were cut with a transfer tube (Fischer Scientific, Pittsburgh, PA, USA) and then inoculated in 50 mL Falcon tubes containing 20 mL of pre-fermentative liquid broth (glucose 10 g, tryptone soy broth 5 g, yeast extract 3 g, and malt extract 10 g, per litre and pH adjusted to 6.2 with 0.5 mol L-1 HCl solution). The Falcon tubes were incubated for 4 days (96 h) at 30 ºC on a rotatory shaker (CIENTEC, model CT-712RN, Piracicaba, São Paulo, Brazil) operating at 120 rpm. In the second stage (biotransformation), the resulting mycelium was transferred into 250 mL Erlenmeyer flasks containing 75 mL of modified Czapek medium (25 g sucrose, 2 g NaNO3, 1 g KH2PO4, 0.5 g MgSO4.7H2O, 0.5 g KCl, 0.01 g FeSO4.7H2O, and deionized water to 1.0 L. The pH was adjusted to 3.0, 4.0, 5.0, 6.0, and 7.0 with a solution of 1 mol L-1 HCl or NaOH). Subsequently, 2 mg of ABZ dissolved in 200 µL of N,N-dimethylformamide was added to the flask. Control flasks consisted of culture broth (Czapek) without ABZ and fungi, sterile broth with fungal mycelium, sterile broth with ABZ, and sterile broth added of N,N-dimethylformamide. Biotransformation experiments were carried out at 30 ºC, with shaking at 120 rpm for 120 h. Aliquots of 1 mL were collected from the culture flasks, submitted to extraction procedure and analyzed by HPLC. The kinetic studies were presented as chemical yield (%) versus collecting interval (hours) profiles. In addition, the results obtained in the biotransformation processes were also expressed as enantiomeric excess (ee), determined by the equation: ee = (A-B/A+B) x 100; where: A is the enantiomer with higher concentration and B is the enantiomer with lower concentration. The efficiency of each biotransformation process was calculated as the chemical yield (in percentage). The chemical yield (%) of ABZSOX enantiomers formed was calculated considering the addition of 2 mg of ABZ into 75 mL of modified Czapek medium (26,666 ng mL-1). Thus, the concentration of 26,666 ng mL-1 of ABZSOX was considered 100% of chemical yield. Chromatographic conditions The liquid chromatographic analyses were conducted according to the validated method described by our group using a Shimadzu chromatograph (Kyoto, Japan), equipped with an LC-ATVP solvent pump unit and a SPD-10AVP UV-Vis detector operating at 290 nm. The system control was carried out by SCL-10A.15 Injections were performed manually through a 20 µL loop with a Rheodyne model 7125 injector (Cotati, CA, USA). The software Shimadzu LC solution, version 1.22 SP1, was used for system control and data acquisition. The resolution of the ABZ and ABZSOX enantiomers was carried out at room temperature (23ºC ± 2) on a Chiralpak AS column (250 x 4.6 mm i.d, 10 µm particle size, Chiral Technologies, Exton, PA, USA) using acetonitrile/ethanol (97:3, v/v) plus 0.2% triethylamine and 0.2% acetic acid as the mobile phase at the flow rate of 0.5 mL min-1. A C18 guard column (4 mm × 12.5 mm, 5 µm particle size, Merck, Darmstadt, Germany) was used to protect the analytical column. Employing this condition, the retention time for ABZ, (-)-ABZSOX and (+)-ABZSOX was 9.7, 14.5, and 16.2 min, respectively. Standard stock solutions of ABZSOX were prepared at the concentration of 1000 mg mL-1 in methanol. Calibration curve solutions of rac-ABZSOX were prepared at concentrations of 2, 8, 20, 40, 200 and 400 mg mL-1. All these solutions were stored at -20 ºC. Hollow fiber liquid-phase micro-extraction (HF-LPME) procedure Pedersen-Bjegaard and Rasmussen 199918 devised a new extraction methodology combining the concepts of extraction with membranes. In the HF-LPME, considered an evolution of micro-extraction methods with solvents, a capillary, porous, and hydrophobic membrane (polypropylene) is impregnated with an organic solvent, while the lumen of the membrane is filled with an acceptor phase. The hollow fiber used was an Accurel PP Q3/2 porous polypropylene hollow fiber purchased from Membrana (Wuppertal, Germany). The inner diameter of the fiber was 600 µm, the thickness of the wall was 200 µm, the pore size was 0.2 µm, and with 15 cm of length. To perform the extraction of ABZ and ABZSOX from the liquid culture medium, the procedure developed by Hilário et al. was employed.15 Briefly, the hollow fibers were immersed in n-octanol and the excess of solvent was removed by ultra-sonication in purified water. After impregnation, 50 µL of 0.1 mol L-1 HCl was injected into the lumen of the hollow fiber and, further, the fiber was immersed in the donor phase (liquid culture medium, pH 7.0). The extraction was carried out for 50 min at 1,500 rpm. After extraction, the acceptor solution was withdrawn from the fiber, transferred to a conical tube and evaporated to dryness. The residues were then dissolved in 50 µL of the LC mobile phase and 20 µL were introduced into the LC system.
RESULTS AND DISCUSSION Stereoselective fungal biotransformation study The ABZSOX metabolite is also known as ricobendazole and it is available in the market as a more potent antihelminthic drug than ABZ. Previous studies performed by Prasad et al. showed that the filamentous fungus Cunninghamella blakesleeana transformed albendazole in three metabolites at significant amounts.12,19 Two metabolites were predicted to be albendazole sulfoxide and albendazole sulfone, the major mammalian metabolites reported previously. A new N-methylated metabolite of albendazole sulfoxide was also produced, where the methylation took place on the N-atom of the imidazole ring system. Besides, the authors also demonstrated that this biotransformation is temperature, pH, substrate concentration and carbon source dependent. This study did not evaluate the stereoselectivity in the process of ABZSOX enantiomers formation. 12,19 In a previously work published by our group it was performed a screening using five endophytic fungi strains Glomerella cingulata VA1, Pestalotiopsis foedans VR8, Papulaspora immersa Hotson SS13, Fusarium oxysporum SS50 and Nigrospora sphaerica (Sacc.) E.W. Mason SS67 and a soil fungus Mucor rouxii strain NRRL 1894. Among these six studied strains, three presented more than 15% of enantiomeric excess, including the fungi P. immersa SS13, N. sphaerica SS67, and M. rouxii. Under the previous study, all evaluated fungi were able to biotransform ABZ to ABZSOX. In order to improve the stereoselectivity on the ricobendazole formation we decided to investigate the pH influence on the biotransformation process.15 Biotransformations are enzyme catalyzed reactions and enzymes are polyelectrolytes containing both positive and negative ionizable amino acid at the surface, which may be protonated or non-protonated depending on the pH of the surrounding medium, leading to different enantioselectivities.20 It is well know that pH plays a crucial role in enzymatic reactions. The pH variation alters the ionic state of the substrate and enzymes involved in this reaction, thus leading to changes in enzymatic activity and enantioselectivity, resulting in changes in yields and e.e. values.21-23 The monitored biotransformation reaction of ABZ to ABZSOX involves a sulfoxidation reaction. The enantioselective oxidations of a prochiral sulfides, like ABZ, is undoubtly the most direct and economical method for the synthesis of enantiomerically pure sulfoxides. For this purpose, the use of biological sulfoxidation approaches has been featured. Both isolated enzymes and whole cells have been applied in the enantioselective oxidation of prochiral sulfides. But, the use of biotransformations, with whole cells, presents great advantages like enzyme isolation and cofactors provision absence.24 The biotransformation reactions of ABZ using P. immersa SS13, N. sphaerica SS67 and M. rouxii fungi were followed for five days (120 h). The aliquots were collected after 48, 96 and 120 hours and analyzed by HPLC. The amount of (-)-ABZSOX and (+)-ABZSOX was quantified using a calibration curve prepared daily. After extraction and analysis, the ABZSOX enantiomers concentrations were obtained and graphs of chemical yield (Figure 2) and e.e. (Figure 3) vs incubation time and pH were plotted.
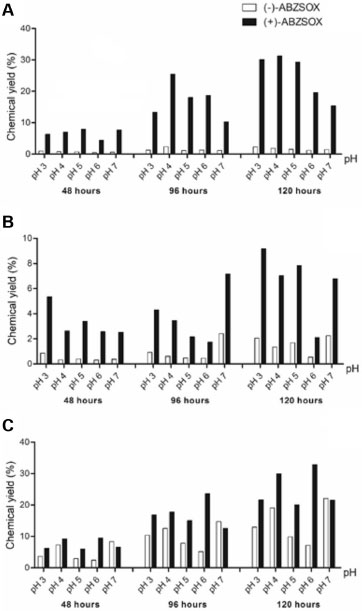 Figure 2. The influence of reaction time on the (-)-ABZSOX and (+)-ABZSOX concentration in the medium incubated with Mucor rouxii (A), Papulaspora immersa Hotson (B) and Nigrospora sphaerica (C). Reaction conditions: 75 mL of Czapek culture medium, 30 ºC, 120 rpm, and 2 mg of albendazole
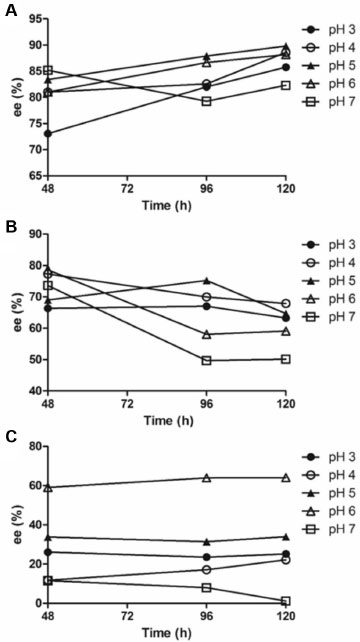 Figure 3. Variation of (+)-ABZSOX enantiomeric excess ee (%) as a function of time in culture medium incubated with Mucor rouxii (A), Papulaspora immersa Hotson (B) and Nigrospora sphaerica (C). Reaction conditions: 75 mL of Czapek culture medium, 30 ºC, 120 rpm, and 2 mg of albendazole
Figures 2C and 3C shows the enantiomeric excess profile for the different studied pHs, during 120 hours, for the fungus N. sphaerica SS67. Figures 2C and 3C show that concentrations of the ABZSOX increases with the incubation time, moreover the highest enantiomeric excess was achieved at pH 6, for the metabolite (+)-ABZSOX (ee 64.1%). On the other hand, at pH 7, it was observed a greater formation of the metabolite (-)-ABZSOX with a decrease in enantiomeric excess for the (+)-ABZSOX metabolite. The results suggest that this fungus can be used to obtain the two ABZSOX enantiomers, and the preferential optical configuration can be achieved by controlling the culture medium pH. Figure 2B and 3B shows the results of ABZ biotransformation for the endophytic fungus P. immersa SS13. In this case the ABZSOX enantiomer concentration is also time-dependent. The highest concentrations of ABZSOX enantiomers, employing this microorganism, were observed at 120 hours. Also, it can be seen in Figure 2B and 3B that the highest enantiomeric excess was achieved in 48 hours at pH 6 (ee 78.5%). For all studied pH values the preferably formed enantiomer was (+)-ABZSOX and the highest amount were obtained at pH 3. In turn, the microbial transformation of ABZ into ABZSOX by M. rouxii can be considered the most promising tool for the ABZSOX formation. Among all evaluated fungi, the biontransformation by the fungus M. rouxii presented high formation, comparable with the fungus N. sphaerica SS67, but with the highest enantiomeric excess for (+)-ABZSOX metabolite, as showed in Figure 2A and 3A. Figures 2A and 3A showed that the maximal ee was observed after 96 hours of incubation at pH 5, 89.8% of ee% of (+)-ABZSOX in comparison to (-)-ABZSOX. Moreover, the highest amount was produced after 120 hours of incubation.
CONCLUSIONS In conclusion, our results have shown the fungi ability to mimic the action of enzymes in drugs metabolism. The ability of these models to mimic mammalian metabolism, to perform biotransformation reactions, and to produce significant amounts of drug metabolites suggest that this microbial system represents a suitable alternative for drug biotransformation studies. This paper describes for the first time the pH influence in stereoselectivity microbial biotransformation of ABZ into ABZSOX. The three fungi tested showed a high degree of enantioselectivity expressed by the enantiomeric excess. Besides, the fungus M. rouxii can be an excellent alternative to obtain the (+)-ABZSOX enantiomer with higher efficiency after microbial transformation bioprocess.
ACKNOWLEDGMENTS The authors are grateful to the São Paulo Research Foundation (FAPESP, grant number 2013/17658-9) and to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for financial support and for granting a research fellowship.
REFERENCES 1. Asha, S.; Vidyavathi, M.; Biotechnol. Adv. 2009, 27, 16. DOI: http://dx.doi.org/10.1016/j.biotechadv.2008.07.005 PMID: 18775773 2. Faber, K.; Biotransfomations in Organic Chemistry, Springer-Verlag: Berlin, 2011. 3. Pupo, M. T.; Borges, K. B.; Borges, W. S.; Bonato, P. S. In Microbial Biotechnology; Saikai, R.; Bezbaruah, R. L.; Bora, T. C., org.; New India Publishing Agency: New Delhi, 2008, pp. 47-66. 4. Borges, W. S.; Borges, K. B.; Bonato, P. S.; Said, S.; Pupo, M. T.; Curr. Org. Chem. 2009, 13, 1137. DOI: http://dx.doi.org/10.2174/138527209788921783 5. Liu, J. H.; Yu, B. Y.; Curr. Org. Chem. 2010, 14, 1400. DOI: http://dx.doi.org/10.2174/138527210791616786 6. Parshikov, I. A.; Netrusov, A. I.; Sutherland, J. B.; Biotechnol. Adv. 2012, 30, 1516. DOI: http://dx.doi.org/10.1016/j.biotechadv.2012.03.010 PMID: 22484051 7. Borges, K. B.; Borges, W. S.; Duran-Patron, R.; Pupo, M. T.; Bonato, P. S.; Collado, I. G.; Tetrahedron: Asymmetry 2009, 20, 385. DOI: http://dx.doi.org/10.1016/j.tetasy.2009.02.009 8. Patel, R. N.; ACS Catal. 2011, 1, 1056. DOI: http://dx.doi.org/10.1021/cs200219b 9. Solano, M.; Hoyos, P.; Hernaiz, M. J.; Alcantara, A. R.; Sanchez-Montero, J. M.; Bioresource Technol, 2012, 115, 196. DOI: http://dx.doi.org/10.1016/j.biortech.2011.11.131 10. Sikora, A.; Siódmiak, T.; Marszall, M. P.; Chirality 2014, 26, 663. DOI: http://dx.doi.org/10.1002/chir.22362 PMID: 25080075 11. Delatour, P.; Benoit, E.; Caude, M.; Tambute, A.; Chirality 1990, 2, 156. DOI: http://dx.doi.org/10.1002/chir.530020306 PMID: 2147560 12. Prasad, G. S.; Girisham, S.; Reddy, S. M.; Srisailam, K.; World J. Microbiol. Biotechnol. 2008, 24, 1565. DOI: http://dx.doi.org/10.1007/s11274-007-9645-7 13. Moroni, P.; Buronfosse, T.; Longin-Sauvageon, C.; Delatour, P.; Benoit, E.; Drug Metab. Dispos. 1995, 23, 160. PMID: 7736906 14. Bolás-Fernández, F.; Rama-Iñiguez, S.; Torrado, J. J.; J. Parasitol. 2004, 90, 407. DOI: http://dx.doi.org/10.1645/GE-3212RN PMID: 15165068 15. Hilário, V. C.; Carrao, D. B.; Barth, T.; Borges, K. B.; Furtado, N. A.; Pupo, M. T.; de Oliveira, A. R.; J. Pharm. Biomed. Anal. 2012, 61, 100. DOI: http://dx.doi.org/10.1016/j.jpba.2011.12.012 PMID: 22230802 16. Gallo, M. B. C.; Chagas, F. O.; Almeida, M. O.; Macedo, C. C.; Cavalcanti, B. C.; Barros, F. W.; de Moraes, M. O.; Costa-Lotufo, L. V.; Pessoa, C.; Bastos, J. K.; Pupo, M. T.; J. Basic Microbiol. 2009, 49, 142. DOI: http://dx.doi.org/10.1002/jobm.200800093 PMID: 18798172 17. Borges, K. B.; Borges, W. S.; Pupo, M. T.; Bonato, P. S.; Appl. Microbiol. Biotechnol. 2007, 77, 669. DOI: http://dx.doi.org/10.1007/s00253-007-1171-x PMID: 17876580 18. Pedersen-Bjergaard, S.; Rasmussen, K. E.; Anal. Chem. 1999, 71, 2650. DOI: http://dx.doi.org/10.1021/ac990055n PMID: 10424162 19. Prasad, G. S.; Girisham, S.; Reddy, S. M.; Srisailam, K.; World J. Microbiol. Biotechnol. 2008, 24, 2055. DOI: http://dx.doi.org/10.1007/s11274-008-9709-3 20. Long, W. S.; Kow, P. C.; Kamaruddin, A. H.; Bhatia, S.; Process Biochem. 2005, 40, 2417. DOI: http://dx.doi.org/10.1016/j.procbio.2004.09.014 21. Lou, W. Y.; Zong, M. H.; Zhang, Y. Y.; Wu, H.; Enzyme Microb. Technol. 2004, 35, 190. DOI: http://dx.doi.org/10.1016/j.enzmictec.2004.04.009 22. Li, Y. N.; Shi, X. A.; Zong, M. H.; Meng, C.; Dong, Y. Q.; Guo, Y. H.; Enzyme Microb. Technol. 2007, 40, 1305. DOI: http://dx.doi.org/10.1016/j.enzmictec.2006.10.016 23. Silva, V. D.; Stambuk, B. U.; Nascimento, M. G.; J. Mol. Catal. B: Enzym. 2012, 77, 98. DOI: http://dx.doi.org/10.1016/j.molcatb.2011.12.006 24. Fernández, I.; Khiar, N.; Chem. Rev. 2003, 103, 3651. DOI: http://dx.doi.org/10.1021/cr990372u PMID: 12964880 |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access





