Artigo
| Evaluation of a buffered solid phase dispersion procedure adapted for pesticide analyses in the soil matrix |
|
Ana María DomínguezI,*; Mario FunesII; Ximena FadicII; Fabian PlacenciaII; Francisco CerecedaII; Juan Pablo MuñozII
IDepartamento de Química, Universidad Técnica Federico Santa María, Av. España 1680, Valparaíso, Chile Recebido em 09/12/2014 *e-mail: anamaria.dominguez@usm.cl An evaluation of the pesticides extracted from the soil matrix was conducted using a citrate-buffered solid phase dispersion sample preparation method (QuEChERS). The identification and quantitation of pesticide compounds was performed using gas chromatography-mass spectrometry. Because of the occurrence of the matrix effect in 87% of the analyzed pesticides, the quantification was performed using matrix-matched calibration. The method's quantification limits were between 0.01 and 0.5 mg kg-1. Repeatability and intermediate precision, expressed as a relative standard deviation percentage, were less than 20%. The recoveries in general ranged between 62% and 99%, with a relative standard deviation < 20%. All the responses were linear, with a correlation coefficient (r) >0.99. INTRODUCTION The world's population has grown twice over the last 50 years,1 requiring an increase in global food production. To solve these problem two practices were implemented: greenhouse cultivation to satisfy the year round demand for some vegetables; and the extensive use of pesticides to fight against pests and reach production goals. Over the course of many years, the intensive use of pesticides led to them becoming environmental contaminants.2 Insecticides are toxic to harmful insects but also to beneficial insects like pollinators; 3,4 birds, fish, amphibian, and other organisms including humans.5-7 Pesticides can cause ecological unbalance, contaminating soils and waters (groundwater, rivers and lakes),8,9 by drainage, from agricultural soils and run-off; resulting in the accumulation of contaminants in different parts of the hydrosphere.10 Soil is an important resource and in agriculture is the direct receptor of pesticides which are applied directly to it or by foliar application. Soil works as the main reservoir for pesticides and other environmental contaminants.11 The adsorption of pesticides in the soil is affected by the soil and the pesticide's physico-chemical properties. These compounds can be retained in the soil clay and organic matter, and could affect the uptake of nutrients by plants and the soil fertility. There are reports about the detrimental effects on the earthworm population from pesticides like dimethoate (even at doses lower than those recommended for application)12 and other organophosphates.13 In addition, some authors report the negative effect on nitrogen-fixing and phosphorus solubilizing microorganisms by pesticides in contaminated soil.14 Chowdhury et al, report a reduction of soil microbial biomass due to the application of pesticides like captan, carbosulfan, 2,4-D, thiram, among other.15 Lo reports the harmful effects of butachlor on the Azospirillum population and aerobic nitrogen fixers in non-flooded soil or fenamiphos on nitrification bacterias.16 On the other hand, the degradation of highly lipophilic and persistent organochloride pesticides, takes many years generating breakdown products, sometimes more toxic than their precursors17,18 and producing bioaccumulation in some soil microorganisms19 and thus, in the trophic chain.20 Many techniques have been used for the extraction of pesticides from soils, such as: Soxhlet extraction,21 microwave assisted extraction (MAE),22,23 solvent assisted extraction (ASE),24 ultrasound assisted extraction,25 supercritical fluid extraction,22,26 solid phase extraction (SPE),27 among others. The "Quick, Easy, Cheap, Effective, Robust and Safe" (QuEChERS) procedure was implemented some years ago, for the extraction of pesticides in fruits and vegetables. QuEChERS extraction requires simple and low cost equipments; it's a fast method with good results.28 But, with time this solid phase dispersion methodology has been widely applied to other matrices like soil and biological samples;20,29-31 and also to environmental contaminants such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), benzene, toluene, ethylbenzene, and xylenes (BTEX), among others.31,32 This procedure has been applied either alone or in combination with other extractive techniques like supercritical fluid extraction.26 In our scope was evaluated the simultaneous extraction of eight pesticides including: two herbicides: 2,6-dinitro-N,N-dipropyl-4-(trifluoromethyl)aniline (trifluralin) and 6-chloro-N,N'-diethyl-1,3,5-triazine-2,4-diamine (simazine); one fungicide: 3-(3,5-dichlorophenyl)-N-isopropyl-2,4-dioxoimidazolidine-1-carboxamide (iprodione); three insecticides: O,O-dimethyl S-[2-(methylamino)-2-oxoethyl] dithiophosphate (dimethoate), 3-phenoxybenzyl (1RS)-cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate (permethrin), O,O-dimethyl S-[(4-oxo-1,2,3-benzotriazin-3(4H)-yl)methyl]dithiophosphate (azinphos methyl) and two persistent organic compounds 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane (4,4'-DDT) and its main breakdown product 1,1-bis-(4-chlorophenyl)-2,2-dichloroethene (4,4'-DDE). DDT was widely used in the 80's but it could stay in soils for many years as DDE, due to its persistence. A citrate buffered solid phase dispersive procedure (AOAC EN 15662), originally employed for vegetable matrices33 was used to extract pesticides from soil, with minor modifications. GC/MS-SIR technique was employed for detection and quantification. The procedure validation was carried out using spiked organic farming soil samples.
MATERIALS AND METHODS Reagents Acetone and acetonitrile (pesticide grade), were purchased by Fisher Chemical, USA. Pesticide neat standards (> 98% of purity): dimethoate, trifluralin, simazine, 4,4'-DDT, 4,4'-DDE, iprodione, azinphos-methyl, permethrin, and the internal standard triphenylphosphate (TPP) were obtained from Accustandard, USA. Sodium citrate dibasic sesquihydrate (>99% of purity for analysis), were acquired from Sigma-Aldrich-Fluka, Spain and; the Emsure sodium chloride (> 99% of purity), tri-sodium citrate dehydrate (> 98% of purity), and anhydrous sodium sulphate (>99% of purity) from Merck, Germany. Selectra PSA (primary and secondary amine) was purchased by UCT, USA. Individual and multi-residue pesticides stock solutions Individual pesticides stock solutions between 1000 to 3000 mg kg-1 were prepared in acetone, using the proper amount of each neat pesticide solid standard. The multi-residue working solution (20 mg kg-1) was prepared in acetone, from the individuals stock solutions. The stocks and working solutions were stored in an amber bottle at 4 ºC. Working solutions were prepared every week. Sample collection and treatment Two soils were collected at the same location (Fundo San Jorge, Valparaiso, Chile): one uncultivated soil and one soil using for organic farming (chemical free agriculture soil). A composite sample of two kilograms for each soil was collected, from 0 to 15 cm of depth. Sampling was performed using the zig-zag method.34 Samples were dried at room temperature, sieved with a 2 mm mesh and stored at -20 ºC in a glass container, until analysis. Soil characterization pH, conductivity and humidity percentage were determined for each soil sample. pH and conductivity were measured in a Sper Scientific equipment, Bench-Top Meter, USA. pH 50 mL of calcium chloride solution 0.01 mol L-1 was added to 20 g of soil. The mixture was shaken for 2 hours and allowed to stand for 1 hour. pH was registered in the supernatant solution. Conductivity 150 mL of deionized water was mixed with 30 g of soil. The samples were shaken for 2 hours and allowed to stand by 1 hour. The conductivity measurements were taken in the supernatant solution. Humidity percentage 5 g soil, in triplicate, was heated at 105 ºC for 48 hours; then the sample let cold down at ambient temperature and weighted. The procedure was repeated until constant weight. Spike soil procedure The spiked soil samples were prepared adding to 5 g of soil, the appropriated volume of multi-residues working solution to reach concentrations of 0.2 and 0.8 mg kg-1. Then 5 mL of acetone was added to the sample and shaked for 2 hours at 200 rpm in order to achieve a better analyte homogenization into de soil matrix. Later, the sample was dried and aged for 48 hours at room temperature before the extraction procedure. Solid phase dispersion procedure The extraction procedure was based on a buffered QuEChERS methodology (EN 15662) for food and plant origin,35 with some modifications. The procedure had been employed by Rouvière with dichloromethane partition;36 but, in this study, acetonitrile was used as partition solvent for pesticide extraction from soils samples and the purification step was slightly modified. A sample of 5 g of soil spiked with 100 µL of TPP was mixed with 10 mL of acetonitrile and vortex mix for 1 minute, in a 50 mL centrifuged tube. After that, 0.5 g of sodium chloride, 0.5 g of tri-sodium citrate dehydrate, 250 mg of sodium citrate dibasic sesquihydrate and 3 g of anhydrous sodium sulphate (replacing the magnesium sulphate) were added and mix again for 1 minute. The organic extract containing the pesticides was isolated from the soil after centrifuging the mixture for 15 minutes at 5000 rpm at 15 ºC. Finally, 2 mL of the organic extract was filtered through a glass Pasteur pipette packed with silanized glass wool and anhydrous sodium sulphate, and 1 µL of the filtrated organic extract was introduced directly in the injector port for the chromatographic analysis. Extract clean-up using PSA The cleanup step was carried out in a 15 mL falcon centrifuged tube adding 25 mg PSA and 150 mg anhydrous sodium sulphate, per organic extract milliliter. Then, the extract was agitated for 30 s and centrifuged for 5 min at 5000 rpm28 before the analysis. Matrix match calibration solutions Six calibration points, ranging from 0 to 1 mg kg-1, were prepared by spiking the soil blank extract with the appropriated amount of the multi-residue working solution, and 100 µL of the internal standard (TPP). The soil blank extract was obtained by extracting uncultivated soil with the proposed QuEChERS procedure. Matrix effect evaluation In order to study the matrix effect a calibration curve prepared with the pesticides working solution, from 0 to 1 mg kg-1, on a soil blank extract was injected in the chromatographic system. Moreover a calibration curve prepared with a pesticides working solution on pure acetonitrile was also injected. The matrix effect (%) was determined by statistical comparisons between the slopes of these curves. Chromatographic analysis The chromatographic analysis was performed using Perkin Elmer-USA, equipment Clarus 680 GC, hyphenated to SQ8C single quadrupole mass spectrometry detector. One microliter of the sample was injected in splitless mode; setting the injector port temperature at 200 ºC and using a pulse of 2 mL min-1 for 1 min. Analytes were isolated employing the Elite-5ms (Perkin Elmer, United States) capillary column of 30 m x 0.25 mm x 0.25 µm. The column oven was set at 80 ºC for 2 min. The temperature was increased to 180 ºC at 20 ºC min-1, hold constant for 12 minutes, and then raised again to 250 ºC using a heating ramp of 4.5 ºC min-1. The helium working flow was set at 1 mL min-1. The mass spectrometer transfer line and the ionization source temperature were set at 200 ºC and 170 ºC, respectively; and the Register Ion Monitoring (SIR = SIM) mode method was used for the analysis, selecting three fragments of each pesticide (see Table II), two for verification purpose and one for quantification (underlined).

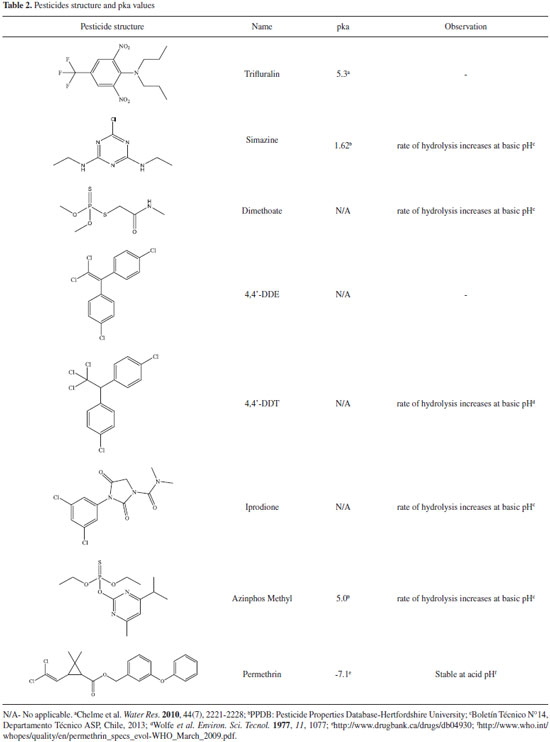
RESULTS AND DISCUSSION Soil characterization Humidity, pH and conductivity of uncultivated soil and organic farming soil were measured. As can be seen in Table I, both soils had similar percentage of humidity. The organic farming soil that was used for organic tomato cultivation showed slightly acidic pH, as expected. This pH value was higher than the one founded on uncultivated soil. The electric conductivity (EC) indicates that both soils had low indirect salinity values, meaning a low amount of soluble salts.37 Although, as expected, the organic farming soil presented slightly higher soluble salt content than the uncultivated soil. Pesticides The pesticides structure and acid dissociation constant (pka) values are shown in Table II. The pka values indicate that we have acidic pesticides like simazine but also strong basics such as permethrin. In order to make a simultaneous determination of these pesticides it was necessary to make a compromise, also taking into account some reports about the rate of hydrolysis of some pesticides with pH. So we determined to work under buffered conditions with pH values of about 5 to 5.5 to protect the base and acid sensitive species, also allowing the extraction of the acids. Selectivity The presence of interferent compounds in the soil matrix, from the studied pesticides retention time segments, was determined through GC/MS-SIR analysis for both soil (uncultivated and organic), after its extraction by QuEChERS. Both soils chromatograms: uncultivated (bottom chromatogram) and organic (medium chromatogram) were compared in Figure 1, with the 0.8 mg kg-1 pesticide spiked uncultivated soil extract (upper chromatogram).
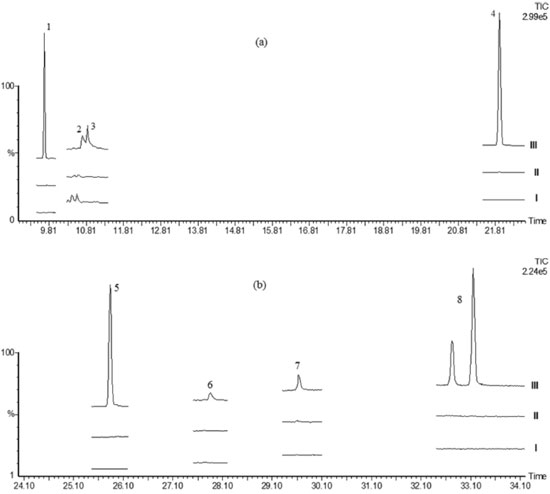 Figure 1. Chromatograms of uncultivated soil (I), organic farming soil (II) and organic farming soil spiked (III) with 0.8 mg kg-1 of a multi-standard solution of pesticide. Figure (a): trifluralin (1), dimethoate (2), simazine (3) and 4,4'-DDE (4); Figure (b): 4,4'-DDT (5), iprodione (6), azinphos-methyl (7) and permethrin mixture cis-trans (8)
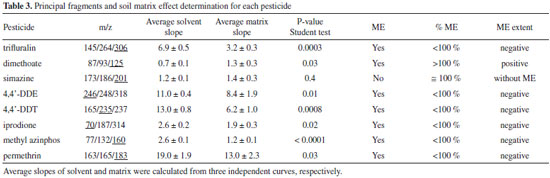
The uncultivated soil chromatogram shows two signals in the GC/MS-SIR segment of dimethoate and simazine, close to the retention time of these pesticides. But as can be seen in the figure the signals are well resolved and those matrix compounds will not interfere with our analytes determination in the SIR mode, although they are close to them. The purity analysis of the signals reveals values less than 48%; probably corresponding to more than one compound with low resolution. The responsible for those signals could not be identified, neither using full scan, nor was there a match in the Nist library v. 2.0. Their mass spectrum shows an intense mass fragment of m/z 91 and less intense m/z 77, associated commonly with tropylium and benzene, which can be formed by alkyl, substituted aromatic compounds fragmentation. The organic farming soil also shows little signals at the dimethoate and simazine retention time segment. For the purpose of validation, this soil was used in the fortification studies and the uncultivated soil was employed as a blank soil matrix. Soil extraction with and without the clean-up step The uncultivated soil (blank soil matrix) was extracted by the citrate buffered solid phase dispersion technique, with and without the purification step with primary and secondary amine sorbent (PSA). From the results was no appreciable difference between the blank soil extract with or without purification (supplementary information). So we concluded that it was not necessary to perform the purification step with adsorbents, but in order to avoid introducing fine soil particles in the GC/MS instrument, the samples were filtrated by silanized glass wool and, anhydrous sodium sulphate was used to eliminate any remaining water. Matrix effect For this study the solvent and the soil matrix blank were extracted by QuEChERS. Then, calibration curves were prepared spiking both extracts (in triplicate), independently, with different concentrations of pesticides. The pesticide matrix effect (ME) was determined using the statistical comparison between the average solvent curves slope and the average slope of the blank soil matrix extract. From the results (Table III), all the pesticides had ANOVA P-values < 0.05, except for simazine; point out the influence of the matrix soil in 87.5% of the analyzed pesticides. The extent of the matrix effect was also calculated. From all of the pesticides with matrix effect, 75% presented negative matrix effect influence. Some authors attribute this matrix phenomenon to a pesticide interaction and co-elution with the matrix compounds, which are not at trace levels, resulting in a rise of the chromatogram background and a subsequent suppressing of the pesticides signal.38 As more than 50% of the analyzed pesticide showed the matrix effect, the matrix match calibration procedure should be used for quantification; and the pre-concentration of the final extract avoided.
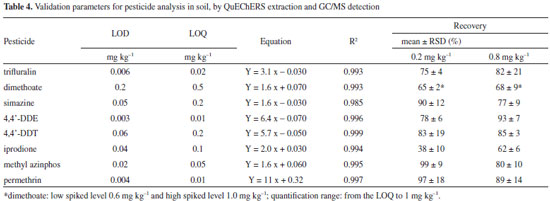
Detection and quantification limits The detection limit (LOD), for each pesticide, was calculated as the concentration corresponding to three times of the standard deviation (3S) of ten independent soil matrix blanks and; the quantification limit (LOQ) was based on ten times the standard deviation (10S). The detection and quantification method limit was calculated considering the internal standard and the matrix match calibration slope. The obtained LOD and LOQ values for trifluralin, dimethoate, simazine, 4,4'-DDE, 4,4'-DDT, iprodione, methyl azinphos and permethrin are presented in Table IV with other validation results. LOD values ranged from 0.003 to 0.05 mg kg-1 in accord with Lesueur2 (0.003 and 0.09 mg kg-1), and in general with the values for GC/MS detection reported by Vera et al.39 except for dimethoate 0.2 mg kg-1.
Linearity Determination coefficient (R2) values for each calibration curve (reported in Table III) indicates a linear relationship between areas and concentrations values. The ANOVA P-values for the regressions were less than 0.05 at 95% of confidence level, for the linear concentration range from the LOQ of each pesticide to 1 mg kg-1. Intermediate precision and repeatability The mean intermediate precision and repeatability were expressed as relative standard deviation percentage (RSD). The RSD were obtained by three measurements performed per day (repeatability) and in 3 different days (intermediate precision), with newly organic farming soil sample spiked with 0.20 mg kg-1 (0.6 mg kg-1 for dimethoate) of a multi-standard pesticide solution. Samples were aged for 48 hours and then extracted by QuEChERS after adding TPP (internal standard). Repeatability had values < 20% for all the analytes, similar to other reported soil studies.2 The intermediate precision also had values less than 20%, with the exception of simazine with 23% of RSD. Recovery Recovery percentage was determined by spiking organic farming soil with 0.20 mg kg-1 and 0.80 mg kg-1, as shown Table IV, except for dimethoate which was evaluated at 0.6 and 1.0 mg kg-1. As can be seen, the pesticides iprodione and dimethoate showed recovery values lower than 70%. This percentage varied on dependence of the pesticide and its concentration level. For the evaluated concentration levels, all the analytes had recoveries between 62 to 99%, except for iprodione which had low recovery (38%) at 0.2 mg kg-1 of concentration level, near its quantification limit; it can be associated with stronger matrix/analyte interactions and co-elution of interferents, decreasing its detectability. The range of those results was in accord with other reported recovery values for soil pesticide analysis using different variations of the QuEChERS methodology. Some authors reported recoveries between 60 to 93% for chlorinated compounds employing ethyl acetate40 and; with water/dichloromethane partition from 60 to 100%.36 Using clean-up by liquid/liquid partition with hexane, the informed recoveries for organochlorides reached 70 to 100%.20 Pesticides from organophosphate, triazines and pyrethroid chemical families had been analyzed by solid phase dispersion using 10 grams of soil and PSA for cleanup, reaching recoveries between 70 to 110%; while a median recovery 65.7% was obtained using the European Norm DIN 12393.2
CONCLUSIONS For the multi-residues method 87.5% of the studied pesticides were affected by the presence of the soil matrix; therefore the quantitation must be carried out in the presence of the matrix. 75% showed negative matrix effect (detection signal suppression), while dimethoate enhanced its chromatography response. Only the herbicide simazine was not influenced by the matrix of soil. The extraction by buffered citrate solid phase dispersion methodology had acceptable recoveries between 62 to 99%, except for iprodione with low recovery at concentration near its quantification limits. The clean-up step with absorbents was not necessary. Intermediate precision values were in general < 20%. LOD values range from 0.003 (4,4'-DDE) to 0.2 mg kg-1 (dimethoate) and LOQ goes from 0.01 to 0.5 mg kg-1. Dimethoate, simazine, 4,4'-DDT and iprodione had the higher quantification limits > 0.1 mg kg-1. The method was shown to be effective for extraction and quantification of pesticide from soil matrix with different physico-chemical properties, except for iprodione. But this compound could be quantified at concentrations higher than 0.2 mg kg-1 using the matrix match calibration procedure.
SUPPLEMENTARY MATERIAL Figure 1S presents SIR chromatograms of the uncultivated soil extracted by solid phase dispersion with and without clean up step. The supplementary material can be freely accessed at http://quimicanova.sbq.org.br in pdf format.
ACKNOWLEDGEMENTS The authors want to thank CONICYT for their financial support through the Integration Project No. 79095004-2010 and Fondef Project No. D09I1070; also to the DGIP- UTFSM for their support in the course of the Internal Project No. 131434; the collaboration of Dra. Beatriz Cámara, Dr. Mario Ollino, and Alison Sherman for this study edition; and organic farmer Mr. Daniel Rabb.
REFERENCES 1. Kummu, M.; Varis, O.; Applied Geography 2011, 31, 495. DOI: http://dx.doi.org/10.1016/j.apgeog.2010.10.009 2. Lesueur, C.; Gartner, M.; Mentler, A.; Fuerhacker, M.; Talanta 2008, 75, 284. DOI: http://dx.doi.org/10.1016/j.talanta.2007.11.031 PMID: 18371880 3. Whitehorn, P. R.; O'Connor, S.; Wackers, F. L.; Goulson, D.; Science 2012, 336, 351. DOI: http://dx.doi.org/10.1126/science.1215025 PMID: 22461500 4. Stokstad, E.; Science 2013, 340, 674. DOI: http://dx.doi.org/10.1126/science.340.6133.674 PMID: 23661734 5. González, N.V.; Molinari, G.; Soloneski, S.; Larramendy, M. L.; Theoria 2008, 17, 27. DOI: http://dx.doi.org/10.1111/j.1755-2567.1951.tb00229.x 6. Martínez, C.; Gómez, S.; Rev. Int. Contam. Ambient. 2007, 23, 185. 7. Oosthoek, S.; Nature 2013, doi:10.1038/nature.2013.13214. DOI: http://dx.doi.org/10.1038/nature.2013.13214 8. Kookana, R.; Holz, G.; Barnes, C.; Bubb, K.; Fremlin, R.; Boardman, B.; J. Environ. Manage. 2010, 91, 2649. DOI: http://dx.doi.org/10.1016/j.jenvman.2010.07.037 PMID: 20727665 9. Struger, J.; Fletcher, T.; J. Great Lakes Res. 2007, 33, 887. DOI: http://dx.doi.org/10.3394/0380-1330(2007)33[887:OOLCAA]2.0.CO;2 10. Belmonte, A.; Garrido, A.; Martínez, J. L.; Anal. Chim. Acta 2005, 538,117. DOI: http://dx.doi.org/10.1016/j.aca.2005.02.003 11. Andrade, M. L.; Fernández, E.; Alonso, M. F.; Rev. Pilquen. secc. cienc. soc. 2005, VII(7), 1. 12. Velki, M.; Hackenberger, B. K.; Soil Biol. Biochem. 2013, 57, 100. DOI: http://dx.doi.org/10.1016/j.soilbio.2012.09.018 13. Reinecke, S. A.; Reinecke, A.; Ecotoxicol. Environ. Saf. 2007, 66, 244. DOI: http://dx.doi.org/10.1016/j.ecoenv.2005.10.006 PMID: 16318873 14. Hussain, S.; Siddique, T.; Saleem, M.; Arshad, M.; Khalid, A.; Adv. Agron. 2009, 102, 159. 15. Chowdhury, A.; Pradhan, S.; Saha, M.; Sanyal, N.; Indian J. Microbiol. 2008, 48, 114. DOI: http://dx.doi.org/10.1007/s12088-008-0011-8 PMID: 23100705 16. Lo, C.C.; J. Environ. Sci. Health, Part B 2010, 45, 348. DOI: http://dx.doi.org/10.1080/10934520903467873 17. Darko, G.; Akoto, O.; Oppong, C.; Chemosphere 2008, 72, 21. DOI: http://dx.doi.org/10.1016/j.chemosphere.2008.02.052 PMID: 18397799 18. Wang, D. Q.; Yu, Y. X.; Zhang, X. Y.; Zhang, S. H.; Pang, Y. P.; Zhang, X. L.; Yu, Z. Q.; Wu, M. H.; Fu, J. M.; Ecotoxicol. Environ. Saf. 2012, 82, 63. DOI: http://dx.doi.org/10.1016/j.ecoenv.2012.05.010 PMID: 22673124 19. Tejada, M.; Gómez, I.; del Toro, M.; Ecotoxicol. Environ. Saf. 2011, 74, 2075. DOI: http://dx.doi.org/10.1016/j.ecoenv.2011.07.005 PMID: 21813178 20. Rashid, A.; Nawaz, S.; Barker, H.; Ahmad, I.; Ashraf, M.; J. Chromatogr. A 2010, 1217, 2933. DOI: http://dx.doi.org/10.1016/j.chroma.2010.02.060 PMID: 20303092 21. Wong, F.; Alegria, H. A.; Bidleman, T. F.; Environ. Pollut. 2010, 158, 749. DOI: http://dx.doi.org/10.1016/j.envpol.2009.10.013 PMID: 19910095 22. Sun, L.; Lee, H. K.; J. Chromatogr. A 2003, 1014, 165. DOI: http://dx.doi.org/10.1016/S0021-9673(03)00574-0 PMID: 14558622 23. Merdassa, Y.; Liu, J.F.; Megersa, N.; Talanta 2013, 114, 227. DOI: http://dx.doi.org/10.1016/j.talanta.2013.04.035 PMID: 23953464 24. Popp, P.; Keil, P.; Möder, M.; Paschke, A.; Thuss, U.; J. Chromatogr. A 1997, 774, 203. DOI: http://dx.doi.org/10.1016/S0021-9673(96)00673-5 25. Hoai, P. M.; Sebesvari, Z.; Minh, T. B.; Viet, P. H.; Renaud, F. G.; Environ. Pollut. 2011, 159, 3344. DOI: http://dx.doi.org/10.1016/j.envpol.2011.08.044 26. Naeenia, M. H.; Yamini, Y.; Rezaee, M.; J. Supercrit. Fluid 2011, 57, 219. DOI: http://dx.doi.org/10.1016/j.supflu.2011.03.005 27. Nanita, S. C.; Pentz, A. M.; Grant, J.; Vogl, E.; Devine, T. J.; Henze, R. M.; Anal. Chem. 2009, 81, 797. DOI: http://dx.doi.org/10.1021/ac8020642 PMID: 19063674 28. Anastassiades, M.; Lehotay, S. J.; Stajnbaher, D.; Schenck, F. J.; J. AOAC Int. 2003, 86, 412. PMID: 12723926 29. Bragança, I.; Plácido, A.; Paíga, P.; Domingues, V. F.; Delerue, C.; Sci. Total Environ. 2012, 433, 281. DOI: http://dx.doi.org/10.1016/j.scitotenv.2012.06.035 PMID: 22796726 30. Tomasini, D.; Sampaio, M. R. F.; Caldas, S. S.; Buffon, J. G.; Duarte, F. A.; Primel, E. G.; Talanta 2012, 99, 380. DOI: http://dx.doi.org/10.1016/j.talanta.2012.05.068 PMID: 22967568 31. Norli, H.R.; Christiansen, A.; Deribe, E.; J. Chromatogr. A 2011, 1218, 7234. DOI: http://dx.doi.org/10.1016/j.chroma.2011.08.050 PMID: 21899854 32. Padilla, J. A.; Plaza, P.; Romero, R.; Garrido, A.; Martínez, J. L.; J. Chromatogr. A 2010, 1217, 5724. DOI: http://dx.doi.org/10.1016/j.chroma.2010.07.004 33. Camino, F. J.; Zafra, A.; Ruiz, J.; Bermúdez, R.; Ballesteros, O.; Navalon, A.; Vílchez, J. L.; J.Food Comp. Anal. 2011, 24, 427. DOI: http://dx.doi.org/10.1016/j.jfca.2010.11.009 34. Mejías, J.; Jerez, J.; In Guía para la toma de muestras de residuos de plaguicidas. Aguas, sedimentos y suelos; Boletín 154, Ministerio de Agricultura: Temuco, 2006, 30. (ISSN-0717-4829). 35. http://phx.phenomenex.com/lib/tn97851012_W.pdf, accessed September 2013. 36. Rouvière, F.; Buleté, A.; Cren, C.; Arnaudguilhem, C.; Talanta 2012, 93, 336. DOI: http://dx.doi.org/10.1016/j.talanta.2012.02.048 PMID: 22483920 37. http://www.pir.sa.gov.au/pirsa/more/factsheets/fact_sheets/salinity/testing_for_soil_and_water_salinity, accessed September 2013. 38. Hajslová, J.; Holadová, K.; Kocourek, V.; Poustka, J.; Godula, M.; Cuhra, P.; Kempny, M.; J. Chromatogr. A 1998, 800, 283. DOI: http://dx.doi.org/10.1016/S0021-9673(97)01145-X 39. Vera, J.; Correia-Sá, L.; Paíga, P.; Bragança, I.; Fernandes, V. C.; Domingues, V. F.; Delerue-Matos, C.; Sample Preparation 2013, 54. Doi: 10.2478/sampre-2013-0006. DOI: http://dx.doi.org/10.2478/sampre-2013-0006 40. Garcia, C.; Fernandez, M. E.; Herrero, S.; Casas, A. M.; Perez, J. L.; Moreno, B.; Talanta 2010, 81, 385. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access