Educação
| RGB-LED-Photometer and the digital image-based method using a smartphone for chemistry and physics teaching |
|
Vagner B. dos SantosI,*; Wevylla M. S. de OliveiraI; João Paulo B. de AlmeidaI; Marcos V. FoguelI; Willian T. SuarezII; Josiane L. de OliveiraII
I. Departamento de Química Fundamental, Universidade Federal de Pernambuco (UFPE), 50740-560 Recife - PE, Brasil Recebido em 23/01/2023 *e-mail: vagner.bsantos@ufpe.br This work describes the analysis of molecular spectroscopy in the UV/Vis region, as well as the study of transmittance, absorption, and reflectance phenomena in an undergraduate class using an LED (light emitting diodes)-photometer and digital image-based (DIB) method using a smartphone to compare with a benchtop spectrophotometer and literature data employing paired T-test for 95% confidence. The novelties of this work are focused on the use of simple, low-cost, and portable analytical instrumentation, based on an RGB-LED photometer and DIB using a smartphone, for teaching Chemistry to undergraduate classes. Moreover, discussions were held on concepts related to the Beer-Lambert law, spectroscopy, pH, equilibrium shift, and calculation for determining the equilibrium constant and pKa of bromothymol blue, which was used as proof of concept. The advantages are the use of low-cost instrumentation for teaching Chemistry, students' access to concepts, such as radiation source, photodetector, transmittance calculation from analog data, regulation of the power of the light emitted by the LED, and details to understand the interaction between matter and light were described by undergraduate students in Physics and Chemistry throughout the course of Experiments in Thermodynamic and Chemical Equilibrium. The better understanding of theoretical concepts and the possibility of taking the RGB-LED-photometer and DIB instrumentation to schools, exhibitions, science fairs, and Chemistry teaching meetings were pointed out by students as the main advantages of the instrumentation and methods described. INTRODUCTION In this work, an experimental activity was developed focused on the use of portable, low-cost, and accessible spectroscopic instrumentations based on RGB-LED-photometer and smartphone, which were applied to a classic acid-base equilibrium to obtain the Ka and pKa. As some education institutions do not have access to more sophisticated spectrophotometers,1-6 students' learning about analytical instrumentation is hampered, which can lead to a deficiency in their learning. Spectroscopy is a general term for science that studies the interaction of different types of radiation with matter by emission, scattering, or absorption phenomena. Among the various spectral regions of electromagnetic radiation, the ultraviolet (UV), visible (Vis), and infrared (IR) regions can be highlighted. UV/Vis spectrophotometry is based on measurements of the absorption of electromagnetic radiation in the ultraviolet and visible regions of the spectrum, in which the amount of radiation transmitted or absorbed is measured. The absorption of visible and ultraviolet radiation depends mainly on the number and arrangement of electrons and their energy levels in the absorbing molecules or ions. As a result, the absorption band can be correlated with the type of binding that exists in the chemical compounds under study.1,2 Molecular absorption spectrophotometry is based on measuring the transmittance or absorbance of molecules with some chromophore to be determined. A chromophore is a specific group of atoms banded with an excess of free or easily excited electrons in which photon absorption is in the visible region of the electromagnetic spectrum. When a radiation source of intensity (I0), with a certain wavelength (λ0) passes through a cuvette containing the chromophore, part of this energy is absorbed by the molecules resulting in the light intensity (I), and thus, I0 > I.1 Thus, the transmittance (T) can be calculated by the relationship shown in Equation 1:  Absorbance (A) can be defined as the negative logarithm to base 10 of the transmittance, according to Equation 2: 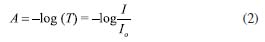 The mathematical relationship widely used within spectrophotometry is the Beer-Lambert law (Equation 3), which states that the absorbance is directly proportional to the concentration of the absorbing species (c) in mol L-1 and to the optical path (b) in cm using monochromatic radiation for low concentration of absorbing species. Molar absorptivity (ε) can be defined as a constant of proportionality between absorbance and concentration. Moreover, using only diluted bromothymol blue solutions, the coefficient of ionic strength approaches 1, thus the activity of the solution can be considered equal to its concentration. For this reason, only concentration at mol L-1 was used in Equations from 3 to 5 used here.  Absorbance is characteristic of a substance in a given medium and indicates the amount of radiation absorbed at a given wavelength. The same principles are employed in photometry. In photometry, optical filters have been used to select a band from the electromagnetic spectrum emitted by a radiation source. In one band, several wavelengths are selected, not a monochromatic beam, and thus, the linearity of the Beer-Lambert law can be reduced. However, photometers are simpler equipment than spectrophotometers, because to obtain a monochromatic beam in a spectrophotometer, a monochromator-based on diffraction grating, specific mirrors, slits, and other optical devices are needed, and the cost is higher than compared to the photometers.1-6 In this context, LED (light emitting diodes)-photometers presented a good cost/benefit ratio and the LEDs presented a wide variability of models, wavelengths, and intensity. Modern LEDs presented a narrow band (± 15 nm), high stability, durability, and low-cost.3,4 Spectrophotometry and photometry were performed to find the equilibrium constant of the bromothymol blue (BTB) indicator, whose color is dependent on the pH of the medium. In an acidic solution, BTB has a yellow color, while a basic solution has a blue color.6 This color change is closely linked to the concentration of H3O+ ions and it is related to the acidity constant (Ka) by Equation 4.  Combining Equations 3 and 4 with Henderson-Hasselbalch equation, Equation 5 is obtained. It is noteworthy that the pKa for this acid-base equilibrium is reached on average at 7, and thus, in neutral solution, [alkaline form] = [acid form]. The absorptions at 616 nm and 453 nm are the λmax (nm) for BTB respectively according to Klotz et al.6 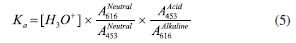 Where and are the absorbances for BTB in water measured at 616 nm and 453 nm, respectively. and are for BTB in alkaline and acid solutions measured at 616 nm and 453 nm, respectively, being the [H3O+] related to pH of the solution of BTB. However, as the wavelength at maximum absorption (λmax (nm)) is dependent on the pH, the absorption spectra for the neutral, acid and alkaline forms of BTB could be different and the λmax needs to be found for each BTB solution, Figure1. Finally, to calculate the pKa of bromothymol blue, a simple application of the negative logarithm of Ka is performed. The color tonality of a solution refers to the reflected radiation by a white radiation source and can be explained by the principle of complementary colors,6 where the directly opposite colors in the chromatic circle are called complementary. This phenomenon is also the basis of modern methods based on digital images using smartphones as analytical instruments.
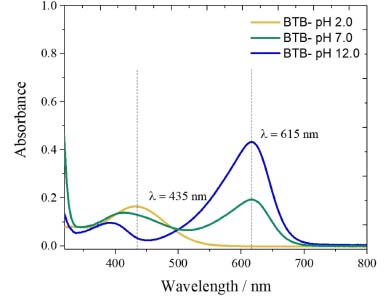 Figure 1. UV-Vis spectra of the BTB solutions in phosphate solution pH 2.0; 7.0 and 12.0. The maximum absorption wavelengths (λmax) represented by the dash lines are referred to the acid form of bromothymol blue (435 nm) and the basic form of bromothymol blue (615 nm). The bromothymol blue (615 nm). The bromothymol blue in neutral pH 7.0 presents 50% of each one
Digital image-based analysis (DIB)3,7-9 is a powerful and flexible tool that can be applied in several areas.10 It is an effective, easy, and low-cost method compared to conventional spectrophotometers. After the images are digitized, the color information is converted to RGB data at a range from 0 to 255, these values are used for quantification purposes.11,12 The application of the DIB method is carried out using devices such as webcams, cameras, scanners, and smartphones to capture the images. Through software and applications,9,13 the images obtained by the devices are decomposed into color values using color systems such as RGB (Red, Green and Blue), CMYK (Cyan, Magenta, Yellow, Key), HSL (Hue, Saturation, Lightness), HSV (Hue, Saturation, Value), among others. The commonly used color system is RGB, being the simplest model. The intensities of the generated colors are stored in 256 levels, on a scale from 0 to 255 for each color channel (Red, Green and Blue), where the combination of these three channels for each color level will be equivalent to a specific color of the electromagnetic spectrum.7 The value 0 for all channels represents the black color, while the value 255 for all channels represents the white color.7,14 To use the DIB method, it is necessary to use platforms to accommodate the solutions containing the analyte. For this, materials such as paper,15,16 conventional cuvettes,17 ceramic plates,18 polystyrene microplates,7 and polylactic acid (PLA)12 are among the materials most used for this purpose. In the DIB method, the control or homogenization of the radiation for capturing the digital image, in order to avoid spurious radiation, is of fundamental importance, since the negligence of this control can lead to low accuracy and precision.11,19 Thus, a careful procedure is required to overcome these drawbacks, and thus, a chamber is commonly used in this method, in order to avoid both excess light and shadow.11,12,20 The DIB method is based on the reflectance phenomenon,19,21,22 while the spectrophotometric or photometric methods are based on transmittance or absorbance.3,23 In reflectance, the measurement is performed with the wavelengths that were reflected from a radiation source, after interaction with the sample, unlike transmittance, in which the beam emitted by the radiation source passes through an absorbent sample and the attenuated radiation is measured in a detector located at an angle of 180º to the radiation beam.1,3 Thus, this work aims to fill this gap and contribute to the enrichment of the formation of undergraduate students in Chemistry, Physics, and related areas with complementary concepts of absorption and reflection phenomena and to find an equilibrium constant using portable instrumentation, such as RGB-LED-photometer or smartphone with the effective participation of the students in the experiments. Thus, with the participation of the students in carrying out the experiment, some relevant information regarding the teaching-learning relationship, its impacts on the assimilation of theoretical concepts and student satisfaction, among other important factors were properly obtained.
MATERIALS AND METHODS Materials and reagents To carry out the experiment, 10 mL volumetric flasks, 10 mL beaker, 20 to 200 μL micropipette Kasvi (Brazil), and deionized water (resistivity > 18.0 MΩ cm) obtained from a Millipore Milli-Q (USA) system were used. The phosphate solutions at pH 2.0; 7.0 and 12.0 were prepared and used to obtain BTB in acid, neutral and basic forms. For this, 0.5 mol L-1 phosphoric acid and 0.5 mol L-1 dihydrogen phosphate of potassium solutions were prepared. 1.0 mol L-1 hydrochloric acid and 1.0 mol L-1 sodium hydroxide were used to adjust the pH of the solutions. All reagents were of analytical grade and purchased from Dinamica (Brazil). Bromothymol blue solutions A 1.6 mmol L-1 bromothymol blue (BTB) aqueous solution was prepared and 50 μL of this solution was inserted in each one of three 10 mL volumetric flasks. Each phosphate solution was inserted into each of the volumetric flasks to prepare BTB solutions at pH 2.0; 7.0 and 12.0. For more accurate pH data, a HI-222 pHmeter of Hanna (Brazil) with a glass electrode combined with an Ag/AgCl reference electrode with 3.0 mol L-1 KCl was previously calibrated using solutions at pH 4.0; 7.0 and 10.0. The UV-Vis spectra of the BTB in solutions at pH 2.0; 7.0 and 12.0 were obtained using a Perkin Elmer Lambda 650 spectrophotometer with a scanning from 300 to 800 nm with a resolution of 1 nm using a quartz cuvette with 10 mm optical path, Figure 1. The phosphate buffer solution at pH 7.0 was used as a blank solution. LED-chamber for DIB The LED-chamber used for the analysis consisted of four ultra-bright white LEDs (Bluex, Aliexpress), with a voltage of 12 V and a power of 1 W, which were used to illuminate the chamber. At the top of the chamber, there is a small opening for the objective or lens of the cell phone camera (Figure 2) and four variable resistors of 10 kΩ, which were used to supply an adequate electric current to the LEDs and to provide a homogenous light in the chamber. An On/Off switch and two connectors for the battery charger were also employed. In the lower compartment of the chamber, the cuvettes with the analytes of interest were arranged, as well as a rechargeable battery, Unipower 12 V with 1.3 A h-1 for powering the LEDs, Figure 2.
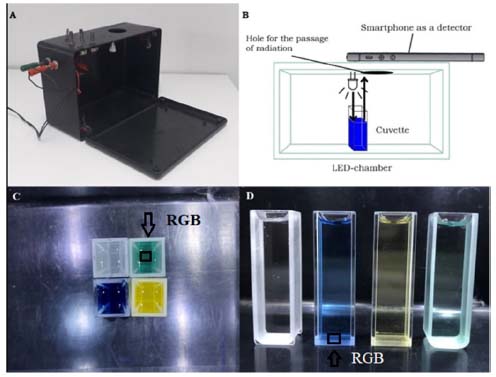 Figure 2. LED-chamber used for DIB analysis (A); scheme of the chamber with the sample and the smartphone (B); image of the top (C) and front (D) views. The blank solution (phosphate pH 7.0), BTB at pH 12.0 (blue), BTB at pH 2.0 (yellow), BTB at pH 7.0 (green) shown from left to right in (D). The RGB values are obtained by the treatment of the region of interest (ROI), (C) and (D), respectively
RGB-LED-photometer The RGB-LED-photometer employs an RGB ultrabright light emitting diodes (LED) (Wavgat, Aliexpress) as a radiation source at a wavelength of 463, 525, and 645 nm (λmax), with bandwidths (w1/2) of 20, 30, and 20 nm, respectively. The spectra of the RGB-LED are presented in the supplementary material, Figure 1S. A spectrophotometer Ocean Optics model USB 2000 (USA) equipped with an optical fiber model ps50-2 was used to measure the emission spectra of RGB-LED. The TSL257 supplied by TAOS was used as a photodetector. A quartz cuvette with a 10 mm optical path commonly used in spectrophotometer was employed. A PIC18F4550 microcontroller (Microchip, USA) was used as a central processing unit (CPU) for data acquisition and processing. On the other hand, an Arduino microcontroller board could be used.24 Due to its multifunctionality, the RGB-LED-photometer used here was previously employed as a turbidimeter in a previous work.4 However, here a RGB-LED with 463, 525 and 645 nm was used instead of a single LED at 405 nm used for turbidimetry.3,4 In fact, the optical configuration of the RGB-LED-photometer is similar to that of a LED-turbidimeter, the difference is in the studied phenomenon, which for the RGB-LED-photometer is transmittance and for the turbidimeter is scattered light with both measurements being performed at an 180º angle between the radiation source and detector. The electronic circuit and the optical compartment are separated to avoid risks of solution spillage in the circuit, Figure 3. Another modification was the use of Equations 1 and 2 in the algorithm developed in C language to convert data in absorbance. Moreover, 7 different functions were developed in the C language for this present work. Dark function (1) to teach the student about the instrumental noise in the absence of light. Blue blank function (2) to perform the analysis of the blank solution using the B-LED (463 nm). Green blank function (3) to measure the blank with the G-LED (525 nm). Red blank function (4) using the R-LED (645 nm). Function 5, 6, and 7 are the BTB solutions measured using the B-LED (463 nm), G-LED (525 nm) and R-LED (645 nm), respectively. For each analysis, the blank signal measured on the same LED is subtracted from the BTB solutions signals automatically. For all cases, a double pulse of the LED was used to avoid variation in the intensity of the beam emitted by the LED. The function is selected using three bottom-keys. In addition, the absorbance is shown on the RGB-LED-photometer display. The analog signal in volts is available using a multimeter, thus the students can calculate the transmittance using the values of I0 (V), and I (V), using phosphate buffer solution at pH 7.0 as a blank solution to measure the I0, and the BTB solutions to measure the I. Furthermore, the RGB-LED-photometer is powered by a rechargeable battery of 9 V at 450 mA h-1 for portability, and more details of the electronic circuit could be seen in previous work4 and in the Figure 2S in supplementary material. The source code in C language was inserted in the supplementary material. However, to avoid an extensive line of code, only the B-blue and A-blue functions are described. The other B-green and A-green, and B-red and A-red functions present similar algorithms. The C language has been used for several applications using microcontrollers to create portable, low-cost and automated analytical instrumentation.25,26
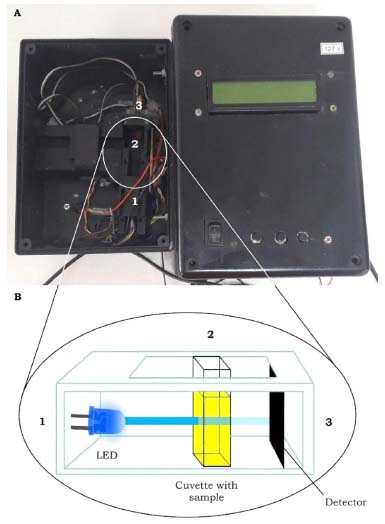 Figure 3. RGB-LED-photometer used to carry out the experiment. The optical compartment is opened (A) to show the cuvette aligned with the LED and the photodiode (B). From 1 to 3 are the LED, cuvette, and photodiode, respectively, with 180º optical alignment
Determination of absorbance with the UV/Vis spectrophotometer The solutions containing BTB (neutral, acidic and basic) were transferred to different cuvettes, always handling them in the opaque part of the cuvette. With the Kasuaki spectrophotometer model IL-593 (190-1100 nm) already calibrated, the wavelength of 435 nm was selected. Then, a cuvette with any phosphate solution pH 7.0 was used as a blank and its absorbance was measured. Then, the acid solution (BTB in phosphate solution at pH 2.0) or alkaline solution (BTB in phosphate solution at pH 12.0) were also recorded. Measurements at a wavelength of 615 nm were performed using the same cuvettes with BTB in different solutions. Determination of absorbance with the RGB-LED-photometer Similarly, to the procedure described for the spectrophotometer, the RGB-LED-photometer was used, however, the blank measurement was recorded at 645 nm (Function 4). After, in Function 7, cuvettes containing BTB in acidic solution and BTB in alkaline solution, respectively, were measured and the blank signal was automatically discounted. Likewise, measurement at 463 nm (Function 2) was performed for the blank solution and Function 5 was selected to measure the same solutions containing BTB. Determination of reflectance data by digital image based method Photographs were taken of the front and top views of cuvettes containing blank solution, BTB in neutral solution, BTB in acidic solution, and BTB in alkaline solution. For the processing of digital images data (1500 × 1500 pixels), the Color Grab App (version 3.6.1, Loomatix©)13 for smartphone (Motorola One) developed for Android was used, in which regions of shadow and excess brightness of the digital images were avoided, then the most suitable regions for cuvette were selected, Figure 2(C) and Figure 2(D). For the DIB setup, only the standard configuration of the smartphone camera was used and the digital image was not treated for contrast and brightness correction. The flash mode was not used, because the homogeneous illumination was obtained from the white LEDs. Other smartphone cameras could be used, however, the configuration of each smartphone should not be changed throughout the application of the method.11,18 Other software would be used to obtain the RGB data such as Photometrix27 for mobile devices and ImageJ for computers.11,12,18 All data present in this paper were obtained in triplicate by a Physics undergraduate student in the 3rd semester (co-author of the work) along the Experiments in Thermodynamic and Chemical Equilibrium curricular component taught for physical and chemistry courses. The other qualitative data obtained were synthesized after dialogues with different students of the degree in physics and chemistry over 2 years, during which time the experiments were reproduced for 8 classes. This qualitative information was acquired from an experimental report released by the students after the execution of the experiments. For this, an experimental procedure script was developed as can be seen in the supplementary information section. The advantages of the use of the instrumentation developed here for Physical and Chemistry learning were highlighted and they are present in the results and discussion section.
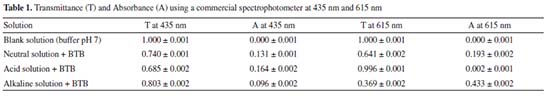
RESULTS AND DISCUSSION pKa calculation with the UV/Vis spectrophotometer The BTB shows a yellowish color in solutions with a pH value below 6.6 and blue color in a pH above 7.6, while in the pH range of 6.6-7.6, the solution usually presents a green color.6,28 The UV-Vis spectra for each BTB solution at pH 2.0, 7.0 and 12 are presented in Figure 1. After, the UV/Vis spectrophotometer used to carry out the experiments presented the results shown in Table 1. As the λmax is pH dependent, the absorption spectra for acid and basic forms of BTB were at 435 nm and 615 nm for pH 2.0 and pH 12, respectively. These λmax were different from those described by Klotz et al.6 (453 nm and 616 nm, respectively), mainly for BTB in acidic form. In fact, in the work described by Klotz et al.6 the BTB spectra were obtained in 1.0 mol L-1 HCl and 1.0 mol L-1 NaOH solutions. Thus, the λmax of the BTB acid form is more pH dependent than its basic form.
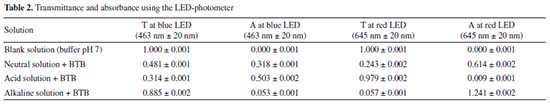
Table 1 shows that the solutions with the highest absorbance at the wavelength of 435 nm have a yellow color, i.e., BTB in phosphate solution at pH 2.00. At the wavelength of 615 nm, the solution with the highest absorbance was BTB in alkaline solution, which has a blue color. This result is associated with the concept of color and its complementary color as expected. To calculate the BTB equilibrium constant, Equation 5 was used. This equation requires the H3O+ concentration, whose value has already been measured by the pH value in the neutral buffer solution (pH = 7.0). Thus, the concentration of the hydronium ion was 1.0 × 10-7 mol L-1. 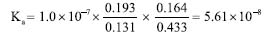 From the negative logarithm of the Ka, the experimental pKa of BTB was 7.25 (n = 3). pKa calculation with the RGB-LED-photometer The absorbance data obtained using the blue LED with emission at wavelength of 463 nm ± 20 nm and with the red LED with emission at 645 nm ± 20 nm are presented in Table 2. These results also corroborate the concept of complementary color discussed previously. The higher or lower absorbance signal of the RGB-LED-photometer compared to the absorbance measured by the spectrophotometer does not interfere with the determination of the equilibrium constant.
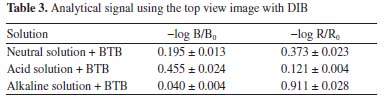
For the determination of the Ka of BTB employing the RGB-LED-photometer, the same calculation was performed using the data acquired with the spectrophotometer. However, Equation 5 was adapted: absorbance at 615 nm was measured using a R-LED (645 ± 20 nm) and absorbance at 435 nm by B-LED (463 ± 20 nm). Thus, Equation 5 was adapted as shown in Equation 6. 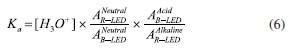 Substituting the absorbance values obtained experimentally in Equation 6, the Ka value was: 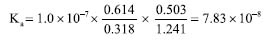 The pKa found experimentally was 7.11 (n = 3). Calculation of pKa by digital images Figures 1(C) and 1(D) show the images top of and front views of the cuvettes, respectively, which were processed by the Color Grab App to obtain the RGB data. By processing the images of the cuvettes, the reflectance data were obtained and converted to -log R/R0, -log G/G0 and -log B/B0, being the R0, G0 and B0, the signal from the blank solution, and R, G and B the signal from the BTB solutions (Table 3). This calculation should not be confused with absorbance, since the measurement for the DIB method is reflectance, measuring the radiation reflected by the solution according to the configuration presented in Figure 1. On the other hand, the attenuation of the radiation at 180º is measured in spectrophotometry or photometry.3,4,7 However, there is a correlation between reflectance and absorption, but the experimental data collected in DIB methods are reflectance with the setup employed here.
The determination of Ka was also performed using the adapted Equation 5, as shown in Equation 7, where R' and B' are R/R0 and B/B0, respectively. 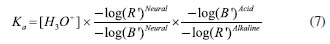 Thus, the Ka was 9.55 × 10-8 and the experimental pKa was 7.02 (n = 3) for the top view image. 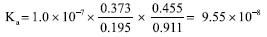 The same process was used for the front view of the cuvettes and Table 4 shows the values for each solution at blue (B) and red (R) regions using DIB, respectively.
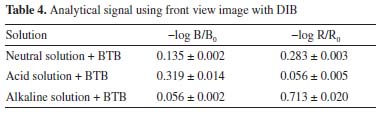
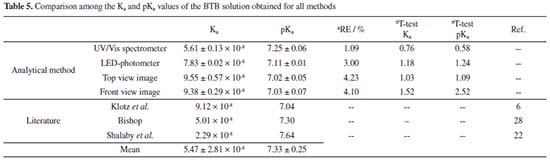
The Ka of BTB was calculated and a value of 9.38 × 10-8 was obtained, with a pKa of was 7.03 (n = 3). 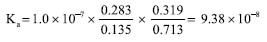 Comparison of the obtained values of Ka and pKa regards to literature for BTB A comparison regarding the Ka and pKa for the BTB found by methods proposed in this work and those reported in the literature is presented in Table 5. A paired T-test for a confidence level of 95% and relative errors (%) were calculated to verify the accuracy of the results using data from the literature as a reference.
Based on the theoretical value of 5.47 ± 2.81 × 10-8 (n = 3) for Ka, and the pKa of 7.33 ± 0.25 (n = 3), it can be concluded that the top view DIB method presented results closer than the literature. The RGB-LED-photometer showed satisfactory results as good as the spectrophotometer with a relative error of 3.0%. All Ka and pKa values were compared to the literature and a close agreement was obtained at a 95% of confidence level, thus, all developed methods presented similar results and can be used to find Ka and pKa values for BTB and other colorimetric equilibrium systems. On the other hand, taking account the Chemistry/Physic teaching, the commercial spectrophotometer does not allow access to instrumentation details or makes it difficult for the student to visualize the radiation source and photodetector, as well as it is not allowed to obtain the analogic data acquired from the photodetector in which students could calculate the transmittance, adjust the power of the light emitted by the radiation source and evaluate this effect in the experiments. Moreover, the arrangement of the optical path, and other reversal details to understand the interaction between matter and light were used, and for this, the RGB-LED-photometer was very interesting. Additionally, the experiments with DIB using smartphones allow students to create a discussion about the complementary color, absorption and reflection phenomena of light using their own smartphone with a free application, with a relative error lower than 4.3% making this method accurate, simpler, inexpensive and accessible compared to the digital method and others presented in Table 5. In fact, the digital image method reported by Shalaby and Mohamed22 and presented in Table 5 employed a professional digital camera with a higher cost than a simple smartphone, moreover, it needs a diffuser; a cell and camera holder based on wood and a conventional fluorescent lamp used as a light source to capture the digital image. Here only a LED-chamber in battery module was used to capture the digital image from a smartphone without the need to have a stable electrical network in a laboratory environment throughout the execution of the experiments. Besides, the quality of the smartphone used by the students here does not harm to find the Ka e pKa with accuracy as similarly reported by Benedetti et al.29 Thus, in this work, undergraduate students employed both instrumentations, an RGB-LED-photometer and a smartphone with DIB, and they described the experiments as ideal for teaching Chemistry, and also highlighted some advantages, such as the portability, low-cost, practicality, and the possibility to observe all parts of devices and the instruments. According to the students, a teaching-learning relationship was better assimilated, with the methodologies being evaluated with details, the main advantages being the ease, mastery of the content, observations of principles, correlation between theory and practice, and to pointing out advantages and disadvantages of each methodology. The use of analog data to calculate the transmittance was also reported by students as important, because sometimes this concept is given only in theory. The possibility of taking the RGB-LED-photometer and the DIB instrumentation for schools, exposition, science fairs, Chemistry teaching meetings, the possibility of using it in several other applications and the low-cost were pointed out by students as other advantages of the methods. In fact, spited to be used only for an acid-base equilibrium, the proposed instrumentations and methods could be employed for several other acid-base systems, such as Alizarin Red S (1,2-dihydroxyanthraquinone-3-sodium sulfonate) and anthocyanins.30 Thus, the main objective of carrying out such experiments is to facilitate the transmission of knowledge and greater participation of the students was successfully achieved by using alternative instruments developed here.
CONCLUSIONS The RGB-LED-photometer and the DIB method using a smartphone were applied by students of an undergraduate course in Chemistry and Physics to teach the principle of spectroscopy with modern and portable instrumentation to obtain the equilibrium constant of an acid-base system. Thus, students could visualize and handle the detector, the intensity of the radiation source based on LED, obtaining the analog data to calculate the transmittance and absorbance, or using digital data on the photometer display to record the absorbance. Moreover, it was possible to explore the RGB system for quantitative purposes, the reflectance phenomenon, and acquisition of similar results in relation to spectrophotometry/photometry were obtained based on the T-test applied for 95% of confidence and low relative errors found. Portability, ease, handiness, low-cost, and the possibility of taking the devices developed to a school to teach the principles of Chemistry or Physics were some advantages pointed out by the students. In addition to manipulating and controlling each instrumentation variable, be it the source, detector, optical arrangement, environment for obtaining the digital images, use of their own smartphone, and observing the impact of this on the response were highlights for them due to the few opportunities to carry out such activities. Thus, for the first time, all these themes are addressed in the same work, with a real application in the teaching carried out by the students, being a great differential of this present work.
SUPPLEMENTARY MATERIAL The supplementary material is available at http://quimicanova.sbq.org.br in pdf format, with free access.
ACKNOWLEDGMENTS This work was supported by CNPq (grant number: 421147/2018-0), FACEPE (APQ-0942-1.06/22, APQ-0413-1.06/21), NUQAAPE/FACEPE (grant APQ-0346-1.06/14) and FAPEMIG (APQ-01889-21). The authors are grateful for the CNPq, FACEPE and CAPES fellowships provided.
REFERENCES 1. Skoog, D. A.; Holler, F. J.; Nieman, T. A.; Principles of Instrumental Analysis, 5th ed.; Bookman: Porto Alegre, 2002. 2. Ingle, J. D.; Crouch, S. R.; Spectrochemical Analysis, 1st ed.; Pearson: United States, 1988. 3. Suarez, W. T.; Pessoa-Neto, O. D.; dos Santos, V. B.; Nogueira, A. R. A.; Faria, R. C.; Fatibello-Filho, O.; Puyol, M.; Alonso, J.; Anal. Bioanal.Chem. 2010, 398, 1525. [Crossref] 4. dos Santos, V. B.; Guerreiro, T. B.; Faria, R. C.; Fatibello-Filho, O.; Quim. Nova 2012, 35, 802. [Crossref] 5. Harris, D. C.; Quantitative Chemical Analysis, 8th ed.; W. H. Freeman: United States, 2012. 6. Klotz, E.; Doyle, R.; Gross, E.; Mattson, B.; J. Chem. Educ. 2011, 88, 637. [Crossref] 7. de Oliveira, L. M. A.; dos Santos, V. B.; da Silva, E. K. N.; Lopes, A. S.; Dantas-Filho, H. A.; Talanta 2020, 206, 120219. [Crossref] 8. Filgueiras, M. F.; de Jesus, P. C.; Borges, E. M.; J. Chem. Educ. 2021, 98, 3303. [Crossref] 9. Böck, F. C.; Helfer, G. A.; Costa, A. B.; Dessuy, M. B.; Ferrão, M. F.; J. Chemom. 2020, 34, e3251. [Crossref] 10. Sonka, M.; Hlavac, V.; Boyle, R.; Image Processing, Analysis and Machine Vision, 4th ed.; Cengage Learning: New York, 2008, p. 11-44. 11. da Silva, E. K. N.; dos Santos, V. B.; Resque, I. S.; Neves, C. A.; Moreira, S. G. C.; Franco, M. O. K.; Suarez, W. T.; Microchem. J. 2020, 157, 104986. [Crossref] 12. de Almeida, J. P. B.; dos Santos, V. B.; do Nascimento, G. A.; Suarez, W. T.; de Azevedo, W. M.; Ferreira, A. F.; Maia, M. V.; Anal. Methods 2022, 14, 2631. [Crossref] 13. https://play.google.com/store/apps/details?id=com.loomatix.colorgrab&hl=pt_BR., accessed in May 2023 14. Capitán, V. L. F.; Ruiz, N. L.; Olmos, A. M.; Erenas, M. M.; Palma, A. J.; Anal. Chim. Acta 2015, 899, 23. [Crossref] 15. Mahato, K.; Chandra, P.; Biosens. Bioelectron. 2019, 128, 9. [Crossref] 16. Li, W.; Zhang, Xiaoyue, X.; Miao, C.; Li, R.; Ji, Y.; Anal. Bioanal. Chem. 2020, 412, 2805. [Crossref] 17. Gee, C. T.; Kehoe, E.; Pomerantz, W. C. K.; Penn, R. L.; J. Chem. Educ. 2017, 94, 941. [Crossref] 18. Resque, I. S.; dos Santos, V. B.; Suarez, W. T.; Chem. Pap. 2019, 73, 1659. [Crossref] 19. Paciornik, S.; Yallouz, A. V.; Campos, R. C.; Gannerman, D.; J. Braz. Chem. Soc. 2006, 17, 156. [Crossref] 20. Curbani, L.; Gelinski, J. M. L. N.; Borges, E. M.; Food Analytical Methods 2020, 13, 249. [Crossref] 21. Soares, S.; Rocha, F. R. P.; Microchem. J. 2021, 162, 105862. [Crossref] 22. Shalaby, A. A.; Mohamed, A. A.; RSC Adv. 2020, 10, 11311. [Crossref] 23. Shimada, T.; Hasegawa, T.; Spectrochim. Acta, Part A 2017, 185, 104. [Crossref] 24. Dantas-Neto, J. C.; dos Santos, V. B.; de Oliveira, S. C. B.; Suarez, W. T.; de Oliveira, J. L.; Anal. Methods 2022, 14, 1311. [Crossref] 25. dos Santos, V. B.; Fava, E. L.; Curi, N. S. M.; Faria, R. C.; Guerreiro, T. B.; Fatibello-Filho, O.; Anal. Methods 2015, 7, 3105. [Crossref] 26. dos Santos, V. B.; Fava, E. L.; Pessoa-Neto, O. D.; Bianchi, S. R.; Faria, R. C.; Guerreiro, T. B.; Fatibello-Filho, O.; Anal. Methods 2014, 21, 8526. [Crossref] 27. Franco, M. O. K.; Suarez, W. T.; dos Santos, V. B.; Resque, I. S.; dos Santos, M. H.; Capitán-Vallvey, L. F.; Spectrochim. Acta, Part A 2021, 253, 119580. [Crossref] 28. Bishop, E.; Indicators, 1st ed.; Pergamon: Oxford, 1972. 29. Benedetti, L. P. S; dos Santos, V. B.; Silva, T. A.; Benedetti Filho, E.; Martins, V. L.; Fatibello-Filho, O.; Anal. Methods 2015, 7, 4138. [Crossref] 30. Silva, W. R. F.; Suarez, W. T.; Reis, C.; dos Santos, V. B.; Carvalho, F. A.; Reis, E. L.; Vicentini, F. C.; J. Chem. Educ. 2021, 98, 1442. [Crossref] |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access