Artigo
| Slow release drug based on hydroxyurea/chitosan cross-linked matrix |
|
Seta AzadI; Layla Jasim AbbasII,*
I. Department of Pharmaceutical Chemistry, College of Pharmacy, University of Basrah, 61004 Basrah, Iraq Recebido em: 28/04/2023 *e-mail: layla.abbas@uobasrah.edu.iq Hydroxyurea (HU), also known as hydroxycarbamide, is an effective anticancer drug. The present work is based on partially cross-linked chitosan with glutaraldehyde in the presence of hydroxyurea. The obtained embedded HU in the cross-linked matrix was evaluated as a controlled-release drug based on the swelling mechanism. The swelling study was carried out in simulated gastric fluid (SGF), simulated intestinal fluid (SIF), and deionised water. The % of swelling at equilibrium for the 0.25% crosslinking density after 24 h of immersion was: 200, 150, and 100% in SGF, H2O and SIF medium, respectively. The concentration of the released drug HU from the chitosan matrix in the different media was determined by UV spectroscopy at λmax at 208.5 nm as a function of time. The release rate of HU from the matrix increases with the swelling rate of the partially cross-linked chitosan. The loading capacity of the prepared gel was 33.3% by weight. The loading capacity and the rate of HU release were found to be inversely affected by the high degrees of crosslinking and increased with increasing the degree of hydrogel swelling. In addition, the rate of HU release was higher at lower pH levels. The study showed that the HU released from the hydrogel matrices in SGF reached 75 and 88.3% of the loaded HU within 8 and 24 h, respectively. This is almost twice the reported value in the literature based on polyphosphate-anion-cross-linked chitosan microspheres. INTRODUCTION Hydroxyurea (HU) is one of the essential drugs commonly used for various cancer treatments, mainly breast cancer. Cancer is the next most common cause of death after heart disease. Cancer-related deaths are projected to increase by 2030.1 The World Health Organization (WHO) list hydroxyurea (HU) as an essential medicine for cancer treatment due to its most effective therapeutic potential in health care systems.2 HU is an effective anticancer drug broadly used in treating head and neck cancer, chronic xylogenous leukemia, sickle-cell disease, cervical cancer, breast cancer, polycythemia vera and others.2,3,4,5 However, HU can have severe side effects, especially at high doses of pure substance or in combination with other drugs and treatments because it inhibits DNA production;6 causes acute chest syndrome, potential adverse and toxic side effects, nausea, liver toxicity, vomiting, diarrhea, hair loss, constipation, mucositis, increase blood urea, leukopenia and abnormal erythrocytes3 and rashes, leg ulcers.7 Due to these side effects and toxicity; extensive research studies have been fulfilled to overcome the side effects of HU, focusing on developing new drugs based on the controlled release formulations, suitable drug delivery systems for the HU at a predetermined dosage8 and developing suitable drug delivery vehicle for HU drug.9,10 Controlled-release drugs often involve the combination of the drug with other safe materials, such as organic polymers; natural or synthetic polymers, like chitosan (CH), sugars, starch, cellulose, proteins, glycolic esters, amides, polyvinyl alcohol, and others.11,12 Chitosan and its derivatives are one class of the most attractive polymers for the synthesis and formulation of controlled released drugs, favorable pharmaceutical material additives and medical and industrial applications. Chitosan and its derivatives have emerged as attractive polymers for the synthesis and formulation of controlled-released drugs and favorable pharmaceutical material additives due to their biocompatibility, biodegradability and ability to be easily modified and cross-linked as natural and modified polymer, nontoxic, antimicrobial, multi-functional, biodegradable, stable for sterilization, can be easily modified and linked to drugs and cross-linked partially to form hydrogels by various cross-linking agents. Chitosan has gained significant attention in various fields due to its unique properties as a biopolymeric, natural, non-toxic and sterilizable, plus other properties.13,14,15 Chitosan exhibits excellent biocompatibility with living tissues accordingly and can be used in a wide range of medical applications such as wound healing tissue engineering and drug delivery.16 Chitosan has antimicrobial properties due to its cationic nature and thus interacts with the membrane of the negatively charged microorganism. This makes chitosan applicable in food preservation, medical implants and wound dressing.17 Chitosan is biodegradable and sustainable, having advantages over synthetic polymers obtained from fossil fuels.18,19 Chitosan also has low immunogenicity20 and excellent water absorption and retention, making it an important natural hydrogel medium,21 with enhanced heavy metal binding properties due to its high amino group content.22 A number of researchers have extensively studied the polymers/ HU matrix.23 Chemical bonds between HU and a number of polymers, including tetramethylol urea, polyvinyl alcohol, carboxymethyl cellulose, and pectin. They studied the release of HU at various pH levels24 as well as a cross-linked chitosan matrix with sodium tripolyphosphate and sodium hex metaphosphate. They obtained maximum loading capacity at pH = 3 and 4.25 Successfully linked HU to albumen cross-linked with glutaraldehyde and found that the created particles were stable over time, thus extending the drug's effects26 as a novel vehicle for cancer therapy, they loaded HU into a blend of chitosan/starch and investigated the slow release of HU, and they found that the release rate was higher at pH = 2.27 They also studied the slow release of HU loaded on a blend of starch nanocrystals/gum Arabic, and they concluded that the rate of HU release in this system was higher at pH = 7.4 than pH = 4 and the blend enhanced the HU release rate. Based on the literature survey, the controlled release of HU from the partially cross-linked chitosan based on the swelling mechanism is not investigated. The swelling mechanism has the advantages of higher loading capacity and improved controlled drug release compared with drugs chemically bonded to the polymers category; accordingly, this research was planned and fulfilled.
EXPERIMENTAL SECTION Materials and methods Chitosan, with more than 85% degree of N-deacetylation, practical grade (from crab shells), and acetic acid, (analytical grade) were obtained from Sigma-Aldrich (USA). Glutaraldehyde was purchased as a 50% stock solution from Merck Chemical Co. (Germany). HU (pharmaceutical grade) was sourced from the Iraqi pharmaceutical industry. Instrumentation The instruments used for the characterization and evaluation of the HU release from the formulated matrix: UV absorption spectrophotometer UV-1100 PerkinElmer was used in this study with 3.5 mL quartz cells between 200-700 nm. All the measurements were carried out at a controlled temperature (27 ± 2 °C) Bruker FT-IR Affinity-1 spectrophotometer (at the College of Pure Sciences, Thi Qar University) was used for the characterization of the products. X-ray diffractometer (Analytical X'Pert Pro MPD), Netherlands (at the College of Sciences, Basrah University). Electron microscopy (FEI Nova Nano SEM 450), Netherland (at the college of sciences, Basrah University). Procedure Preparation of the cross-linked chitosan matrix A 50 mL round bottom reaction vessel fitted with a condenser, dropping funnel, and magnetic stirrer was charged with 1 g of chitosan and 10 mL of 2% acetic acid. The contents were mixed with a magnetic stirrer for 24-48 h or until a homogenous solution was obtained. Then 3 mL of 0.25% solution of glutaraldehyde in water were added dropwise within 20 min. Mixing was continued for 1 h. At the end of the reaction, sodium hydroxide solution (10%) was added dropwise with continuous mixing to neutralize the reaction medium. As a result, a viscous, partially cross-linked gel product was formed. The product was dried at 50 °C for 48 h, then the samples were characterized by IR and used in the evaluation of swelling experiments. The reaction scheme was repeated using different weight ratios of glutaraldehyde/chitosan by changing the concentration of the added glutaraldehyde solution (0.25, 0.5, 0.75 and 1%) to obtain different degrees of crosslinking in order to evaluate the relationship between water absorption and degree of crosslinking. Preparation of the hydroxyurea/chitosan cross-linked matrix The cross-linked neutralized gel obtained in the previous section (before the drying step) was loaded with 500 mg of HU with continuous mixing for a further 30 min. The obtained gel/HU matrix in chitosan was dried at 50 °C for 48 h, then it was characterized by IR and used in the controlled release study. Preparation of simulated gastric fluid (SGF) solution and simulated intestinal fluid (SIF) The solutions were prepared according to published literature.28 Swelling experiments The swelling ability of the non-cross-linked and cross-linked chitosan hydrogels with different degrees of crosslinking were evaluated by determining the percentage of swelling (S), Equation (1).29 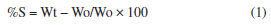 where, %S is the percentage of the swelling ratio; Wo is the weight of the dried chitosan sample. Wt is the weight of the chitosan swollen sample immersed in saline buffer at time t. Wo (g) of the dry sample was immersed for a definite period of time (t) in buffer solution at a specified pH. The sample was wiped with filter paper and weighed (Wt). The weight at time interval was measured (Wt). The swelling studies of the cross-linked chitosan in SGF at pH 1.2, SIF pH 7.2, and H2O pH 7 were carried out by adopting the same procedure. The same procedure was applied to several samples of chitosan with different degrees of crosslinking. The sample with the lowest degree of crosslinking (crosslinking density 0.25), which gave a higher degree of swelling, was used in the controlled release study. In vitro evaluation of HU controlled release 300 mg dry sample of partially cross-linked chitosan hydrogel (crosslinking density 0.25) loaded with 33% HU was immersed in 50 mL of extracting medium (SGF, H2O and SIF) at a controlled temperature 27 ± 2 °C. Samples were taken into 3.5 mL quartz cells at several time intervals of 1-24 h. The UV absorbance was measured at λmax at 208.5 nm, and the concentration of the released HU was determined from the calibration curve of absorbance vs. concentration of pure HU in the same extraction medium. The concentration of the released HU as a function of time is shown in Figure 5.
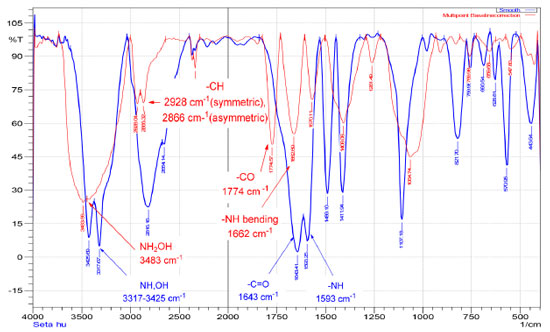 Figure 2. FTIR spectrum of hydroxyurea (blue), hydroxyurea embedded in the cross-linked chitosan matrix (red)
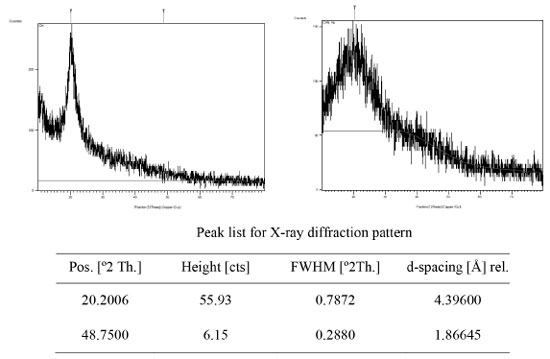 Figure 3. X-ray diffraction pattern of the cross-linked chitosan (left), and HU embedded in the chitosan matrix (right)
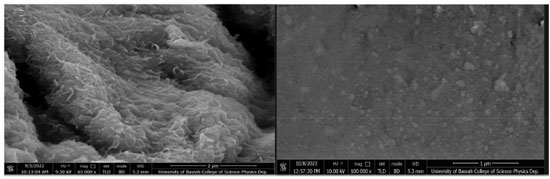 Figure 4. SEM image of (left) partially cross-linked chitosan with glutaraldehyde and (right) HU embedded in the chitosan matrix
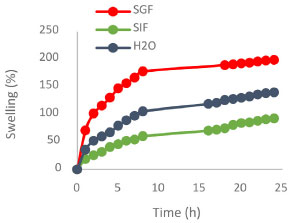 Figure 5. Swelling of the chitosan cross-linked network at different pH as a function of time
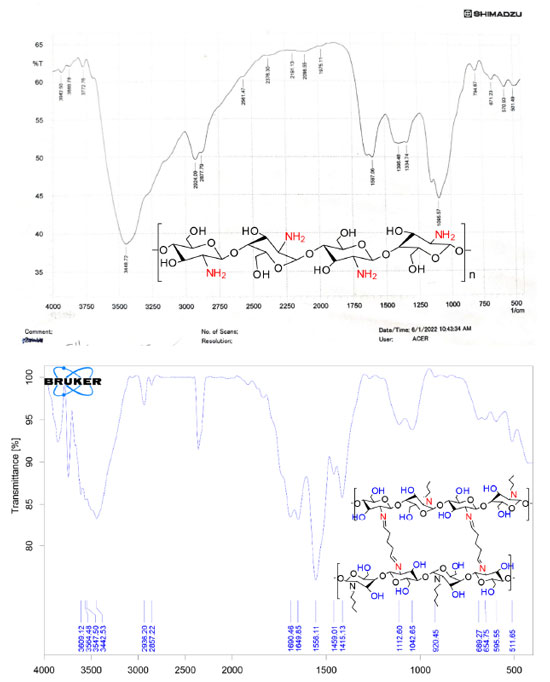
RESULTS AND DISCUSSION Characterization of the prepared hydrogel drug matrix by FTIR Infrared spectra of the prepared hydrogels were analyzed using Bruker FT-IR Affinity-1 spectrophotometer in KBr discs from 4000 to 400 cm-1. The FTIR study was carried out to identify the functional groups that are present in the hydrogels. The infrared spectrum of chitosan, Figure 1, showed a band at 3448 cm-1 related to the overlapping N-H and O-H stretching and the intramolecular hydrogen bonds. The vibration at 2924 and 2877 cm-1 can be related to C-H symmetric and asymmetric stretching, respectively. There is a third distinct band for typical N-acetyl groups, and it is possibly overlapped by other bands; the band at 1597 cm-1 is related to the N-H bending of the primary amine. The CH2 bending and CH3 symmetrical distortions were confirmed by the presence of bands at 1396 and 1334 cm-1, respectively. The bands at 1095 cm-1 correspond to C-O stretching. The spectrum of the cross-linked chitosan with glutaraldehyde showed absorption bands at 2857 and 1459 cm1, reflective of the glutaraldehyde-cross-linked chitosan. The bands were relatively strong due to the absorption of aliphatic C-H. The new peak at 1662 cm-1 is the shifting peak of 1643 cm-1 that characterizes imines in Schiff's base formed by the reaction of chitosan (amine) and glutaraldehyde (aldehyde). The FTIR spectrum of the HU shown in Figure 2, exhibited O-H and N-H stretching vibration, which overlaps at 3425, 3317, and 2821 cm-1, the band at 1643 cm-1 is related to the C=O group, the bands at 1593, 1489, and 1411 cm-1 are related to N-OH and stretching vibration of C-N group at 1107 cm-1. Similar absorption bands are observed in the spectra of the HU embedded in the matrix of the cross-linked chitosan with glutaraldehyde shown in Figure 2 (red spectrum) with some shifts to higher wavelength as both consist of similar functional bonds as detailed in the previous section for the cross-linked matrix for the functional groups: amino, hydroxyl, carbonyl, hydrogen-bonded OH and NH groups. The discussed characteristic bands in the FTIR spectra are in good agreement with literature.29 The X-ray diffraction pattern (XRD) of the produced partially cross-linked chitosan with glutaraldehyde is shown in Figure 3 (left), which reveals two significant peaks at 2θ = 20.2 and 48.75 which are typical for semi-crystalline chitosan. These peaks are related to the crystal structure of chitosan and are attributed to the high degree of crystallinity. The high crystallinity development within the polymer can be due to its structural regularity, polarity, presence of hydrogen bonding, and also to packing capacity of the polymer chains. On the other hand, the X-ray pattern of the HU/cross-linked matrix that is presented in Figure 3 (right) showed a broadening diffraction pattern at 2θ = 20.2 and 48.75. The broadening peak of the X-ray spectrum indicates some degree of disruption to the growth of the crystalline structures due to the new strong hydrogen bonding formed between HU and the partially cross-linked chitosan that is shown in Scheme 1 (represents the suggested structure of hydroxyurea embedded in the polymer matrix of chitosan linked with glutaraldehyde).
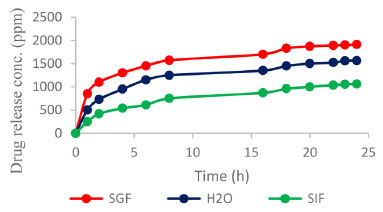 Figure 6. Release of HU from the cross-linked chitosan matrix in SGF, SIF and water media
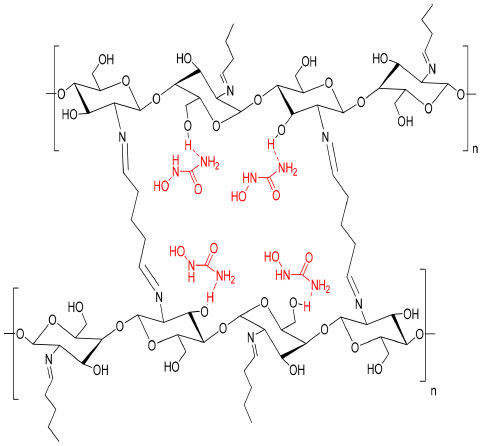 Scheme 1. The suggested structure of hydroxyurea embedded in the cross-linked chitosan matrix
The SEM image shown in Figure 4 reveals that the partially cross-linked chitosan particles have a needle-like crystalline structure with a smooth surface (left). The SEM image of the HU embedded in the chitosan matrix shown in Figure 4 (right) indicates uniform and well-distributed HU within the polymer matrix. The swelling and control release results The obtained % swelling as a function of time at different pH media is presented in Figure 5. The obtained results showed that the highest swelling ratio was observed with chitosan gel at the lowest degree of crosslinking (crosslinking density of 0.25). Figure 4 shows the effect of time on the % of swelling in different media. The % of swelling at equilibrium for the 0.25% crosslinking density after 24 h of immersion was: 200, 150, and 100% in SGF, H2O and SIF medium, respectively. The swelling ratio of the cross-linked chitosan decreases with pH (higher in an acidic medium). This can be related to the increased hydrogen bonding between H2O molecules with amine and the hydroxyl groups of the chitosan, leading to an increase in the swelling ratio. The effect of crosslinking density on the swelling ratio of chitosan with glutaraldehyde has been investigated, and several kinetic parameters were reported.29 The obtained results on the effect of pH on the swelling ratios of the cross-linked chitosan are in good agreement with the reported data. The % swelling at equilibrium found in this study is much higher than the reported values in the literature,29 this could be related to the source of chitosan, molecular weight, degree of de-acetylation and the degree of crosslinking (crosslinking density). The swelling ratio of the samples consisting of HU embedded in the cross-linked chitosan matrix was found to be pH-dependent. Figure 6 shows the HU release as a function of immersion time in different media. The HU release at equilibrium was in the following order: SGF > H2O and > SIF media. This behavior can be attributed to an increased positive charge in chitosan output due to converting the amine group to cations in the acidic media, enhancing the repulsion forces between the cationic charges and subsequently expanding the polymeric chains due to the electric repulsion.30 As a result, the swelling ratio in SGF, SIF, and H2O media increases with time and then stabilizes at the optimum absorption of H2O, as shown in Figure 5. The slow release of HU from the cross-linked chitosan matrix in SGF, SIF and deionized water was investigated using UV-Vis. spectroscopy by measuring the absorption at λmax at 208.5 nm, adopting standard procedure.23 The total loaded HU on chitosan gel was 33.3% by weight. The results shown in Figure 6 indicate that 75% of the HU is released within the first 8 h in the SGF, which is very interesting from a medication point of view as 8 h is the common dosage interval of most drugs and 88.3% of the loaded HU is released within 24 h. The formed hydrogel was highly compatible with HU based on the obtained high loading capacity. The HU release in the SIF medium is much lower at 8 h immersion time the HU release is 30.3 and 37.9% at 8 and 24 h, respectively. In the neutral medium, deionized water, the HU release is 50.5 and 63.1%, at 8 and 24 h, respectively. The loading capacity of HU found in this study is almost in the same range reported in the literature for polyphosphate-anion-cross-linked chitosan microspheres.24 The rate of controlled release of HU is much higher almost twice the reported value mainly within the first 8 h. The rate of release in SGF is 75% compared with 38%. In conclusion, the concentration of HU (ppm) leached out from the cross-linked chitosan matrix at different pH as a function of time is in the following order in different media; SGF > deionized water > SIF. This can be related to the increase in the swelling ratio of the cross-linked matrix in different pH media. In other words, the rate of HU release depends on the rate of the matrix swelling as H2O enters the network and extrudes and expels the HU from the matrix. The enhanced release of HU from the matrix in acidic conditions can be due to the formation of positive ammonium ions in the acidic solutions, which increases hydrogen bonding with H2O. This phenomenon is a well-established principle in the controlled release of drugs according to the swelling mechanism.30,31
CONCLUSION The partially cross-linked chitosan with glutaraldehyde formed a hydrogel polymer with H2O. The highest swelling ratio obtained was 200%. The measured degree of H2O absorption depended on the degree of cross-linking. The formed hydrogel was highly compatible with HU. The rate of HU release was found to vary with the swelling rate. The rate of HU release was higher under acidic conditions, and the rate was in the following order in different media; SGF > deionized water > SIF.
ACKNOWLEDGEMENTS The authors appreciate the professional advice of Prof. Dr. Salah Shakir Hashim - The Department of Chemistry at the University of Basrah. No (scientific or financial) conflicts of interest to achieve this research paper in all its steps.
REFERÊNCIAS 1. Arya, G.; Gupta, N.; Nimesh, S. In Polysaccharide Nanoparticles; Venkatesan, J.; Kwon Kim, S.; Anil, S.; Rekha, P. D., eds.; Elsevier: Amsterdam, 2022, ch. 8. [Crossref] 2. Karakus, G.; Can, H. K.; Yaglioglu, A. S.; J. Mol. Struct. 2020, 1210, 127989. [Crossref] 3. Angona, A.; Bellosillo, B.; Larrán, A. A.; Avilés, L. M.; Camacho, L.; Pairet, S.; Fernández-Rodriguez, M. C.; Ancochea, A.; Besses, C.; Leuk. Res. 2013, 37, 917. [Crossref] 4. Barbui, T.; Birgegard, G.; Cervantes, F.; Finazzi, G.; Harrison, M. G. C.; Hasselbalch, H. C.; Hehlmann, R.; Hoffman, R.; Kiladjian, J. J.; Kröger, N.; Mesa, R.; McMullin, M. F.; Pardanani, A.; Passamonti, F.; Vannucchi, A. M.; Reiter, A.; Silver, R. T.; Verstovsek, S.; Tefferi, A.; J. Clin. Oncol. 2011, 29, 761. [Crossref] 5. Escribano, L.; Álvarez-Twose, I.; Sánchez-Muñoz, L.; Garcia-Montero, A.; Núñez, R.; Almeida, J.; Jara-Acevedo, M.; Teodósio, C.; García-Cosío, M.; Bellas, C.; Orfao, A.; J. Allergy Clin. Immunol. 2009, 124, 514. [Crossref] 6. Jinna, S.; Khandhar, P. B.; Hydroxyurea Toxicity, StatPearls Publishing LLC: e-book, 2021. [Link] accessed in October 2023 7. Daoud, M. S.; Gibson, L. E.; Pittelkow, M. R.; J. Am. Acad. Dermatol. 1997, 36, 178. [Crossref] 8. Karagozlu, M. Z.; Kim, S. K.; Adv. Food Nutr. Res. 2014, 72, 215. [Crossref] 9. Chen, X.; Sun, Z.; Zhang, H.; Onsori, S.; Molecular Physics 2020, 118, 1. [Crossref] 10. Mortazavifar, A.; Raissi, H.; Shahabi, M.; J. Biomol. Struct. Dyn. 2019, 37, 4852. [Crossref] 11. Adepu, S.; Ramakrishna, S.; Molecules 2021, 26, 5905. [Crossref] 12. Khan, I.; Hossain, M. I.; Hossain, M. K.; Rubel, M. H. K.; Hossain, K. M.; Mahfuz, M. U. B.; Anik, M. I.; ACS Appl. Bio Mater. 2022, 5, 971. [Crossref] 13. Parhi, R.; Environ. Chem. Lett. 2020, 18, 577. [Link] accessed in October 2023 14. Anitha, A.; Rejinold, N. S.; Bumgardner, J. D.; Nair, S. V.; Jayakumar, R. In Chitosan-Based Systems for Biopharmaceuticals: Delivery, Targeting and Polymer Therapeutics; Sarmento, B.; das Neves, J., eds.; John Wiley and Sons: Hoboken, 2012, ch. 7. [Crossref] 15. Abdulelah, Z.; Ali, D.; Shakir, S.; Advanced Journal of Scientific Research 2016, 1, 28. [Link] accessed in October 2023 16. Muzzarelli, R. A. A.; Boudrant, J.; Meyer, D.; Manno, N.; DeMarchis, M.; Paoletti, M. G.; Carbohydr. Polym. 2012, 87, 995. [Crossref] 17. Rhim, J. W.; Ng, P. K. W.; Crit. Rev. Food Sci. Nutr. 2007, 47, 411. [Crossref] 18. Kardas, I.; Struszczyk, M. H.; Kucharska, M.; van den Broek, L. A. M.; van Dam, J. E. G.; Ciechańska, D. In The European Polysaccharide Network of Excellence; Navard, P., ed.; Springer: Vienn, 2012, p. 329. [Crossref] 19. Rabea, E. I.; Badawy, M. E. T.; Biomacromolecules 2003, 4, 1457. [Crossref] 20. Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O.; Eur. J. Pharm. Biopharm. 2015, 97, 417. [Crossref] 21. Meyers, H. K.; Prinyawiwatkul, W.; Xu, Z.; J. Food Sci. 2007, 72, R87. [Crossref] 22. Kumar, M. N. V. R.; React. Funct. Polym. 2000, 46, 1. [Crossref] 23. Hussain, K. A.; Abid, D. S.; Adam, G. A.; Journal of Basrah Researches 2015, 41, 130. [Crossref] 24. Gupta, K. C.; Jabrail, F. H.; J. Appl. Polym. Sci. 2007, 104, 1942. [Crossref] 25. Tazhbayev, Y.; Mukashev, O.; Burkeyev, M. Z.; Kreuter, J.; Pharmaceutics 2019, 11, 410. [Crossref] 26. Alwaan, I. M.; Ahmed, M.; Al-Kelaby, K. K. A.; Allebban, Z. S. M.; Pak. J. Biotechnol. 2018, 15, 947. [Link] accessed in October 2023 27. Alwaan, I. M.; Jafar, M. M. R. M.; Allebban, Z. S. M.; Biomedical Physics & Engineering Express 2019, 5, 1. [Crossref] 28. Johnson, L. R. G.; Gastrointestinal Physiology - Mosby Physiology Monograph Series, 8th ed.; Mosby: Amsterdam, 2013. 29. Akakuru, O.; Isiuku, B.; J. Phys. Chem. Biophys. 2017, 7, 1. [Crossref] 30. Abdelaal, M. Y. ; Abdel-Razik, E. A.; Abdel-Bary, E. M.; El-Sherbiny, I. M.; J. Appl. Polym. Sci. 2007, 103, 2864. [Crossref] 31. Remington, J. P.; Remington: The Science and Practice of Pharmacy, 21st ed.; Lippincott Williams & Wilkins: Philadelphia, 2016. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access