Artigo
| Sequential determination of Zn, Fe, Mg, Ca, Na and K in synthetized babassu fame by high resolution continuum source flame atomic absorption spectrometry |
|
Ana Carla S. BoeiraI; Leandro KollingI; Samuel J. SantosII I. Centro de Combustíveis, Biocombustíveis, Lubrificantes e Óleos, Instituto de Química, Universidade Federal do Rio Grande do Sul, 91501-970 Porto Alegre - RS, Brasil Received: 09/13/2023 *e-mail: lmazzini@uol.com.br; mmsilva@iq.ufrgs.br In this work, babassu FAME samples were synthesized by transesterification reaction under alkaline catalysis with sodium glyceroxide and by two steps process with KOH followed by H2SO4 as catalysts. A sequential multi-element method for determination of Zn, Fe, Mg, Ca, Na and K in synthesized babassu FAME by high resolution continuum source flame atomic absorption spectrometry (HR-CS FAAS) was developed. The proposed method is based on a microemulsion formation by mixing the babassu FAME samples with 1-propanol and aqueous phase. The mass proportion of the added components was evaluated through a ternary diagram. External and matrix-matching calibration techniques with inorganic and organic standard were investigated. In the five-level spike-recovery test, satisfactory results were obtained by performing the matrix-matching calibration along with inorganic standard solutions. By using the optimized conditions, limits of detection in the range of 0.002-0.12 mg kg-1 were obtained. Accuracy was attested by the agreement of the results obtained in the analysis of three certified reference materials. By applying the microemulsion system and HR-CS FAAS, the sequential determination of six elements was achieved. Therefore, the proposed method was a suitable alternative for metal determination in babassu FAME samples, with good sensitivities, accuracy, and a wide linear working range. INTRODUCTION Triglycerides present in the traditional oils and fats from vegetable or animal sources as those from soy, corn, sunflower, tallow, and lard are predominantly comprised of palmitic (C16:0), oleic (C18:1), and linoleic (C18:2) acids. The fats extracted from the kernel of palm tree fruits, in contrast, are rich in triglycerides containing short and medium chain fatty acids, specially lauric (C12:0) and myristic (C14:0) and, the fatty esters derived from them are more volatile and less viscous than those obtained from the common commercial oils and fats.1-4 Due to these features, the exploitation of such sources as feedstock for the biodiesel production has been intensively studied, and babassu (Attalea speciosa) is a prominent example.4-9 Moreover, biodiesels obtained from palm fats have been also highlighted as precursors for the production of biokerosene, since the medium-chain fatty acid esters and jet fuel distillation ranges are very similar. However, in order to meet all the specifications of a jet fuel, some biodiesel properties must be improved, which requires its composition modification by chemical process. One recent example is the catalytic deoxygenation of the fatty acid methyl esters (FAME) derived from the licuri (Syagrus coronate) oil, as proposed by Araújo et al.10 Other authors have suggested blending JET-A1 with light biodiesel, i.e., a mix of short and middle chain FAME produced by the distillation of biodiesels derived from babassu (Attalea speciosa),11,12 palm kernel (Elaeis guineensis),2,3,12 macauba (Acrocomia aculeata),2 and gueiroba (Syagrus oleracea)13 fats. In any context, continuous research and development are necessary to support the production and commercialization of these biofuels since regulatory requirements are very strict. Thus, quality control by monitoring the concentration of inorganic contaminants is essential to guarantee a product that complies with current legislation in the country, allowing its large-scale commercialization. The presence of metallic species in biofuels can occur through raw material or by incorporation during the production process, mainly as traces of catalysts.14,15 Alkali and alkaline earth metals such as Na, K, Ca and Mg can lead to deposits formation and corrosion of engine parts (injectors, pumps, rings and pistons).14,16 Transition metals including Fe and Zn, even when present in low concentrations, can catalyze oxidation reactions,14,17 affecting the stability of biofuel and promoting its degradation.17,18 Methods for the determination of metals in biofuels have been widely discussed in the literature,14,18,19 in view of the complexity of the samples in terms of organic content, viscosity and water immiscibility, which may cause several inconveniences during analysis when an adequate pre-treatment is not applied. The use of microemulsions is an interesting alternative in biofuel sample preparation for elemental analysis, due to it requires low amount of organic solvents, in addition to the high stability of the analytes in the system, since inorganic acids can be added to aqueous phase.16,20,21 The reduction of sample viscosity, allowing its introduction in equipment by using pneumatic nebulizers, and the possibility of using aqueous standard solutions in the microemulsion for calibration are other attractive advantages of this method.21,22 Different analytical techniques have been used for elemental determination in biofuel samples.18,19,23 Based on data recently published by Martínez, et al.,19 inductively coupled plasma optical emission spectrometry (ICP OES) stands out with the highest number of publications referring to this application, followed by inductively coupled plasma mass spectrometry (ICP-MS) and flame atomic absorption spectrometry (FAAS). An alternative technique for monitoring the concentration of metals in biofuels is the high-resolution continuum source flame atomic absorption spectrometry (HR-CS FAAS), which was commercially introduced in 2004. The xenon short-arc lamp, used as continuum source, allows the sequential determination of a wide range of elements. In addition, a detector consisting of a linear array of charge-coupled devices (CCD) operating with 588 pixels, makes it possible to visualize the spectral environment of 0.2 nm around the analytical line, providing a series of information about the region. The equipment's software allows full reprocessing of data after the measurements and has a reference spectra library to correct fine-structured background interferences, which are normally generated by diatomic molecules.24 Due to its characteristics, the HR-CS FAAS is especially useful in analysis of complex samples such as biofuels. It is possible to find in the literature some works that use this technique for trace analysis in biodiesel.21,25 However, multi-element determination in short and medium chain FAME has not yet been reported. Due to the different physical-chemical properties of babassu FAME in relation to traditional biodiesels, especially viscosity, as mentioned above, it is necessary to investigate the need of a new method to analyze it, since these properties can directly influence the sample introduction and nebulization process in HR-CS FAAS. In this work, a method for the sequential multi-element determination of Zn, Fe, Mg, Ca, Na and K in synthetized babassu FAME by HR-CS FAAS, using microemulsions as sample preparation procedure, was proposed. The formation of microemulsions, through a simple mixture of babassu FAME, aqueous phase and 1-propanol, was investigated by constructing a ternary phase diagram. The method accuracy was evaluated by analyzing certified reference materials of biodiesel and lubricating oil.
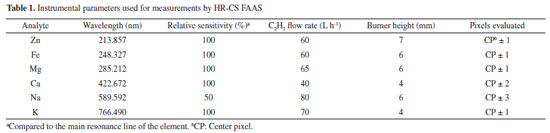
EXPERIMENTAL Instrumentation All measurements were carried out using a ContrAA 300 high-resolution continuum source flame atomic absorption spectrometer (Analytik Jena AG, Germany), equipped with a xenon short-arc lamp (which emits a continuous spectrum between 190 and 900 nm), operating in a hot-spot mode. The high resolution was obtained by using a double echelle monochromator (DEMON) and a detector consisting of a linear array of charge-coupled devices (CCD) with 588 pixels, 200 of which were used for analytical purposes. An air-acetylene flame was used for the determination of Zn, Fe, Mg, Ca, Na and K. The compressed air was supplied by an air compressor (Fiac CD TOP 50 V, Maringá, Brazil) operating with a flow rate of 470 L h-1. High-purity acetylene (99.0% v/v, White Martins, São Paulo, Brazil) was used as fuel gas. The optimized instrumental parameters for each analyte are detailed in Table 1. A flow injection valve SFS 6 (Analytik Jena) was used to introduce the solutions into the equipment. The total sample volume injected to measure the six analytes was 1.5 mL, considering the following optimized conditions: aspiration rate of 2.1 mL min-1, injection time of 1.0 s and capillary filling time of 20 s. The reading time was 10 s for each analyte and all measurements were performed in quadruplicate. As the signal generated were transient, integrated absorbance (Aint) of the center pixel (CP) and the adjacent pixels, as specified in Table 1, were measured for all elements. An analytical balance model ATX224 (Shimadzu, Kyoto, Japan) with a resolution of 0.0001 g was used to weigh samples and reagents.
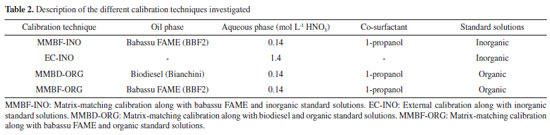
Reagents and solutions In this work, babassu FAME samples were produced by using commercial babassu oil (Campestre, São Bernardo do Campo, Brazil). Sodium hydroxide P.A. (Dinâmica, Indaiatuba, Brazil), ethanol (Êxodo, Sumaré, Brazil) and glycerol (Vetec, Rio de Janeiro, Brazil) were employed in the synthesis of sodium glyceroxide, which was used as catalyst in homogeneous basic transesterification, as described in the supplementary material. Potassium hydroxide (Vetec, Rio de Janeiro, Brazil) and sulfuric acid (FMaia, Cotia, Brazil) were used as catalysts in a modified transesterification double step process (TDSP), according to the literature.26 Methanol P.A. (Êxodo, Sumaré, Brazil) was employed in both synthetic routes. Analytical grade reagents were exclusively used during the sequential multi-element method development step. Deionized water with a specific resistivity of 18.2 MΩ cm from a Milli-Q water purification system (Millipore, Bedford, USA) was used for the preparation of standards and samples. All containers and glassware used in the preparation and storage of solutions were submitted to a cleaning and decontamination process. The materials were previously washed with a 1.0% (v/v) solution of Triton® X-100 (Dinâmica, Indaiatuba, Brazil) and deionized water. Subsequently, it was introduced in 1.4 mol L-1 HNO3 for 48 h and rinsed with deionized water before use. Nitric acid (Química Moderna, Barueri, Brazil) and 1-propanol (Fisher Scientific, New Jersey, USA) were employed in the microemulsions preparation. The nitric acid was purified by sub-boiling distillation in a quartz distiller (Marconi, São Paulo, Brazil). The 1-propanol was distilled under a heating mantle at 97 ºC. Inorganic standard solutions of 100 mg L-1 were prepared by appropriate dilution of the stock standard solutions 1000 mg L-1 of Zn, Fe, Cu, Ca and Na from Specsol® (Quimlab, Jacareí, Brazil) and Mg and K (SCP Science, Quebec, Canada) in 1.4 mol L-1 HNO3. Organic standard solutions were prepared by dilution of the stock standards 1000 mg kg-1 of Zn and K Specsol® (Quimlab), Fe (Merck, Darmstadt, Germany) and Mg, Ca and Na Conostan® (SCP Science) in soybean oil biodiesel (Bianchini, Canoas, Brazil). Certified reference materials (CRMs) of biodiesel: BDM1 (Ref: 150-441-095) containing 50 mg kg-1 of Ca and Mg; and BDM2 (Ref: 150-441-060) containing 50 mg kg-1 of K and Na, both from Conostan® (SCP Science), and the standard reference materials (SRM): 1084a (Wear-metals in lubricating oil (NIST, Gaithersburg, USA), containing 100 mg kg-1 of Fe and Mg), and 1083 (Wear-metals in lubricating oil (NIST), with low analyte content) were analyzed. An organic standard solution of 1000 mg kg-1 Zn from Specsol® (Quimlab) was used to fortify the biodiesel CRMs. Babassu FAME samples Two samples of babassu FAME (BBF1 and BBF2) were prepared as described by Santos et al.27 (method A: homogeneous alkaline transesterification using sodium glyceroxide as catalyst), and by Guzatto et al.26 (method B: modified transesterification double step process - TDSP). The procedures are described in the supplementary material. The babassu FAMEs were characterized as follow. The fatty esters content,26 and profile,28 the methanol content,29 iodine30 and saponification31 values were determined by 1H NMR. The spectra were acquired in a Varian Mercury 400 MHz spectrometer (Palo Alto, USA) in CDCl3 (D 99.8%, 0.1% TMS, Cambridge, Andover, USA), and are presented in the supplementary material. The specific mass (ρ) at 20 ºC, the kinematic viscosities at 40 ºC (ν), and the pour point (PP) were determined as described in the ASTM D1298-12 (hygrometer, 0.7 to 1 g mL-1, Incoterm, model 5598, Porto Alegre, Brazil),32 ASTM 445-06 (Cannon-Fenske 75 tube, Laborglass, São Paulo, Brazil, k 0.0066105 m2 s-2),33 and ASTM D97-17 (PP Meter, IBP, Porto Alegre, Brazil),34 respectively. Microemulsion preparation Microemulsions were obtained by mixing babassu FAME, aqueous phase (0.14 mol L-1 HNO3) and 1-propanol in polypropylene flasks Corning® (New York, USA). After the addition of all components, the containers were closed, and the system was shaken manually for a few seconds. This procedure was performed at 25 ºC, maintained by the laboratory's refrigeration system. Different proportions of babassu FAME, diluted nitric acid and 1-propanol were mixed, and the formation of microemulsion was evidenced when a system with a clear, transparent, and stable appearance was observed. Through the different proportions of mixed reagents, a ternary phase diagram was constructed, which will be presented and discussed later. The composition adopted in the preparation of the microemulsions was 3.5 g of babassu FAME, 1.02 g (1.0 mL) of nitric acid 0.14 mol L-1 and 4.0 g (5.0 mL) of 1-propanol. The final volume of the microemulsion was 10 mL. All samples were prepared in triplicate. Preparation of analytical curves Four calibration techniques were investigated in order to evaluate which was the most suitable for the analyses: (i) matrix-matching calibration along with babassu FAME and inorganic standard solutions (MMBF-INO); (ii) external calibration along with inorganic standard solutions (EC-INO); (iii) matrix-matching calibration along with biodiesel and organic standard solutions (MMBD-ORG) and (iv) matrix-matching calibration along with babassu FAME and organic standard solutions (MMBF-ORG). The analytical curves were prepared in different media, as described in Table 2. The BBF2 babassu FAME sample and soybean oil biodiesel (Bianchini), that were previously analyzed and did not show significant analytes content, were used as the oil phase in matrix-matching calibration microemulsions. Standard solutions were prepared by dilution of the stock standard solutions in inorganic or organic media, as described above, and were added to the aqueous phase (inorganic standards) or to the oil phase (organic standards) of the microemulsions. The following concentration ranges in mg L-1 were used: 0.1-0.9 for Zn, 0.6-5.4 for Fe, 0.1-0.5 for Mg and K and 0.2-1.2 for Ca and Na.
Recovery tests and certified reference materials analysis Recovery tests at five analytes concentration levels were used to evaluate the matrix effects in different calibration techniques investigated. The BBF2 sample were spiked with organic standards resulting in the following concentrations: spike 1: 0.1 mg L-1 of Zn, Mg and K, 0.6 mg L-1 of Fe and 0.3 mg L-1 of Ca and Na; spike 2: 0.2 mg L-1 of Zn, Mg and K, 1.3 mg L-1 of Fe and 0.5 mg L-1 of Ca and Na; spike 3: 0.3 mg L-1 of Zn, Mg and K, 2.0 mg L-1 of Fe and 0.8 mg L-1 of Ca and Na; spike 4: 0.4 mg L-1 of Zn, Mg and K, 2.6 mg L-1 of Fe and 1.1 mg L-1 of Ca and Na; spike 5: 0.6 mg L-1 of Zn and K, 0.5 mg L-1 of Mg, 3.4 mg L-1 of Fe, 1.4 mg L-1 of Ca and 1.3 mg L-1 of Na. Afterwards the spiked sample aliquots were prepared for analysis by the microemulsification proposed method as described above. Certified reference materials of biodiesel (BDM1 and BDM2) and NIST 1084a lubricating oil standard reference material were also analyzed. Since CRMs BDM1 and BDM2 have the same matrix (biodiesel) and different elements, both were prepared together, in the same microemulsion, in order to perform a multi-element analysis. Furthermore, as there was no certified value of Zn in these materials, biodiesel CRMs were spiked with Zn organic standard solution, so that all investigated analytes could be determined. Thus, BDM1 and BDM2 aliquots and the Zn standard solution were diluted to a final mass of 15 g with soybean oil biodiesel (Bianchini). The mass of CRMs and Zn standard solution used were calculated so that the analytes concentration was in the instrumental working range at the final dilution (microemulsion). In a second moment, SRM NIST 1084a analysis was carried out. Due to the analytes high concentration in this material, a dilution with soybean oil biodiesel (Bianchini) was also performed, and from this, microemulsions were prepared. In order to investigate possible matrix effects in SRM NIST 1084a analysis, recovery tests were performed using SRM NIST 1083, which is a diluent base oil for SRM 1084a with low analyte content (concentration below the limit of detection - LOD for all investigated elements). For the analysis, inorganic standard solutions with concentrations referring to the midpoint of the calibration curves were added to the microemulsions aqueous phase prepared with the SRM 1083 (oil phase).
RESULTS AND DISCUSSION Babassu FAME characterization Babassu FAME samples were prepared by two different methods in order to obtain distinct samples with possible different contaminants; BBF1 was isolated in the presence of a basic catalyst, while BBF2, under acidic conditions. The samples composition and physical properties are presented in the Table 3. As can be seen, the transesterification process does not affect the main attributes of babassu FAME. The 1H NMR spectra of samples BBF1 and BBF2 can be seen in Figures 1S and 2S (at supplementary material).
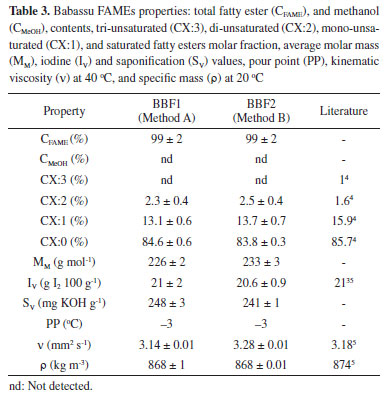
In both cases, babassu FAMEs were free from methanol, with fatty esters content of 99%. The 1H NMR spectrum gives all the necessary information to calculate the average molar mass, from what iodine and saponification values were estimated as 21 g I2 100 g-1 and approximately 245 mg KOH g-1, respectively. As expected, babassu FAMEs are mainly comprised of saturated chains, 84-85%, thus justifying such low values of iodine. The babassu fat is known to have short and middle acyl groups, mainly formed by 12 and 14 carbons, in agreement with the high saponification value, and with the low kinematic viscosity (ν) and specific mass (ρ). Soy FAME, for instance, in which short and medium chain fatty esters are negligible, shows a higher specific mass, 880 kg m-3, and viscosity, 4,29 mm2 s-1.27 Babassu FAMEs composition, and physical properties are in accordance with those described in the literature, as presented in Table 3.4,5,35 Microemulsion formation Mixtures of different proportions of babassu FAME, 0.14 mol L-1 nitric acid and 1-propanol were investigated, with the aim of obtaining a stable microemulsified system to be used as a sample preparation method for the determination of metals in babassu FAME. This methodology was chosen in view of the various advantages reported in the literature, such as its practicality and speed.16,21,36-38 The microemulsion formation region was obtained through the construction of a ternary phase diagram (Figure 1), consisting of three axes, which represent the mass fraction of each component (babassu FAME, 1-propanol, and nitric acid). Each point inside the diagram corresponds to a mixture in which the sum of the fractions is equal to 1. The different mixtures obtained (emulsified or microemulsified) have its own physicochemical characteristics. However, they could be visually distinguished, since emulsions have a turve appearance while microemulsions have a clear, transparent, and stable aspect, often being confused with solutions.
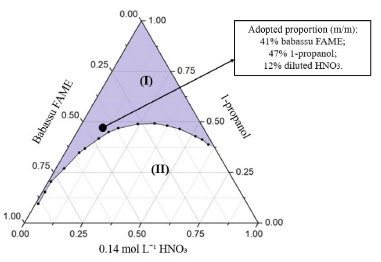 Figure 1. Ternary phase diagram for babassu FAME; 0.14 mol L-1 HNO3 and 1-propanol, mixed at 25 °C. Region (I): microemulsion; region (II): emulsion
For this study, sample BBF1 was used and the investigation was initially based on the experimental condition proposed by Antunes et al.,38 where microemulsions were used as a sample preparation method for the determination of Fe, Cu, Zn, Al and Cr in biodiesel by FAAS. Subsequently, points referring to the border between the microemulsion and emulsion regions were plotted by fixing the sample mass and 1-propanol volume and by changing the amount of diluted HNO3, which was added until the visualization of an emulsified system. This procedure was performed for different sample masses, until the entire border region was delimited. In Figure 1, two distinct regions are visualized: region (I), where the formation of clear and stable mixtures (microemulsion) was observed, and region (II), which represents mixtures that form emulsions. Comparing the phase diagram obtained in the present work for babassu FAME with the diagram presented by Antunes et al.38 for biodiesel, it was noticed that the regions of microemulsion formation are similar, since the samples in question present a certain similarity in its chemical structure. The adopted composition for microemulsions used in this work was 3.5 g of babassu FAME, 4.0 g of 1-propanol and 1.02 g of HNO3 0.14 mol L-1, which corresponds to the following mass proportion: 41% of sample, 47% 1-propanol and 12% diluted HNO3. This condition was chosen because even if there is a small variation in the system composition, the microemulsion will not be destabilized. In addition, a high amount of sample was adopted, in order to improve the limits of detection. Maximum amount of acidified water is also desirable since it allows the use of standards in aqueous medium for calibration and increases the analytes stability in the microemulsion due to the presence of acid. The system employed in this work can be classified as water-in-oil (w/o) microemulsion, since the hydrophobic percentage is greater than the aqueous one, and the water droplets are dispersed in oil phase. Optimization of instrumental parameters The optimization of the spectrometer experimental parameters for sequential multi-element analysis was carried out with a babassu FAME sample (BBF2) enriched with an inorganic standard solution containing all the analytes, prepared as microemulsion. Flame composition and burner height were automatically optimized by the software (Aspect CS 2.1.2.0 – Analytik Jena) using continuous flow mode, taking maximum absorbance as a criterion. The aspiration rate was manually adjusted to the maximum possible (2.1 mL min-1), since later an injection valve would be used to introduce the samples into the equipment. For the elements Zn, Fe, Mg, Ca and K, the main absorption lines were used, due to the absence of spectral interferences and the maximum relative sensitivity. The 589.592 nm line (50% relative sensitivity) was used for Na, chosen based on desired working range (from 0.1 to 1.2 mg L-1). After optimizing the instrumental conditions using continuous flow mode, the use of a flow injection valve (SFS 6) was evaluated. Its use allows controlling the injection of solutions, passing from the sample to the Milli-Q® water (cleaning solution) and vice versa, avoiding flame instability, since no air is aspirated between the solutions. In addition, there is a reduction in sample consumption,39 which is advantageous for the method developed in this work, considering that six elements were investigated. A manual optimization of the flame conditions for each analyte was carried out with SFS 6 valve, in order to verify if the automatically defined parameters in continuous flow mode were the most suitable when using flow injection. For this evaluation, not only the maximum absorbance was taken into account, but also the peak profile. The results demonstrated conditions of burner height and flame composition similar in both optimizations (automatic and manual). Thus, it was decided to use the automatically optimized parameters by the software. The conditions adopted for each analyte are shown in Table 1. Valve injection volumes were tested for the following times: 0.5; 1.0; 1.5 and 2.0 s, with 1.0 s chosen as a compromise condition for all analytes, since the obtained signals presented peak profiles closer to the Gaussian format, as shown for Mg in Figure 2. Longer injection times have not been investigated in order to avoid higher consumption of samples and to decrease analysis time. Considering the defined injection time (1 s), to measure a replicate of one analyte, the sample volume employing the SFS 6 valve was 35 µL. The total volume used to determine all analytes in quadruplicate was 1.5 mL, considering also the capillary filling time (20 s).
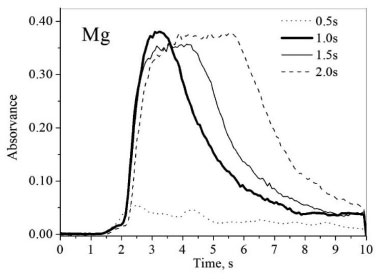 Figure 2. Overlaid transient signals for 0.4 mg L-1 Mg at different injection times, obtained from a microemulsion of BBF2 sample enriched with an inorganic standard solution
Calibration techniques study In order to avoid transport interferences in HR-CS FAAS, calibration solutions must have physical characteristics very similar to those sample, since changes in physical properties of aspirated solutions, such as viscosity and surface tension, can influence the nebulization process.18,38 Therefore, the use of microemulsions for calibration by matrix-matching method was investigated in this work. Inorganic and organic standard solutions were also evaluated. Babassu FAME (BBF2) and biodiesel samples, which were used as oil phase in the calibration microemulsions by MMBF-INO, MMBF-ORG and MMBD-ORG techniques were previously analyzed and did not show significant analytes content. Furthermore, the use of biodiesel to simulate the oil phase in calibration microemulsions was tested due to the physical and chemical similarity with babassu FAME samples and their availability. For comparison purposes, external calibration (EC-INO) was also evaluated. Angular coefficients and intercepts were compared by analysis of covariance (ANCOVA)40 to verify equivalence possibility between the four proposed calibration techniques. This statistical test employs multiple linear regression analysis through the use of dummy variables and allows evaluating, simultaneously, equality between regression coefficients when more than two calibration techniques need to be compared. ANCOVA test was performed using the Excel software at a 95% confidence level, and MMBF-INO calibration curve was used as a parameter. Linear regressions obtained by the different calibration techniques evaluated, which were statistically compared, are presented in Table 4.
Since the statistical results demonstrated that there is no equivalence between the four investigated calibration techniques for all analytes, new ANCOVA tests were carried out, with the purpose of comparing separately the linear regressions: (i) by fixing the type of standard solutions used (inorganic or organic) and (ii) by fixing matrix-matching calibration along with babassu FAME (MMBF). When establishing the use of inorganic standard solutions, external calibration (EC-INO) and matrix-matching along with babassu FAME (MMBF-INO) methods were compared. Results indicated that these investigated methods presented curves with equal sensitivities and intercepts only for the elements Zn, Mg and K. Furthermore, by fixing the use of organic standard solutions, matrix-matching along with biodiesel (MMBD-ORG) and matrix-matching along with babassu FAME (MMBF-ORG) methods were compared. The results showed that these two methods presented curves that are coincident only for Ca and K. As already expected, the medium in which the analytes are present significantly influence the measurements in most cases, which can be explained by the differences in physicochemical properties of the prepared calibration solutions, affecting their nebulization and transport until the flame. Thus, it is necessary to use a method in which the sample matrix is present, to avoid this type of interference. For this reason, matrix-matching along with babassu FAME (MMBF) was chosen for another ANCOVA test, and standard solutions employed (inorganic or organic) were compared. For this test, results demonstrated that for Fe, Ca and Na, the curves obtained by MMBF-INO and MMBF-ORG methods are coincident (equivalent sensitivities and intercepts), indicating that only for these three elements both standard solutions could be used. Since the methodology proposed in this work consists of a sequential multi-element analysis, employed calibration technique must be satisfactory for all investigated elements. Therefore, based only on ANCOVA tests results, it was not possible to conclude which calibration technique was the most suitable for the proposed analysis. Additionally, recovery tests were performed, with the purpose of evaluating possible matrix effects on the four investigated calibration curves. For this study, the BBF2 sample was enriched with organic standard solutions at five analytes concentration levels. Added values are described in the experimental section. Results are shown in Figures 3 and 4.
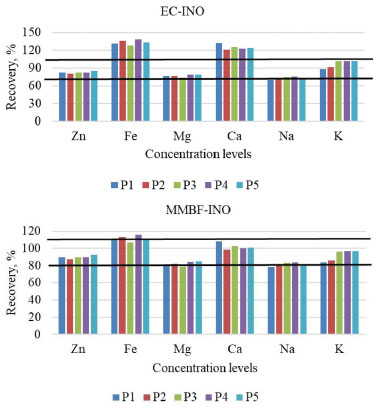 Figure 3. Recovery tests results at five analytes concentration levels, for the EC-INO and MMBF-INO calibration techniques. EC-INO: external calibration along with inorganic standard solutions; MMBF-INO: matrix-matching along with babassu FAME and inorganic standard solutions. P1: 0.1 mg L-1Zn, Mg and K; 0.6 mg L-1 Fe and 0.3 mg L-1 Ca and Na; P2: 0.2 mg L-1 Zn, Mg and K; 1.3 mg L-1 Fe and 0.5 mg L-1 Ca and Na; P3: 0.3 mg L-1 Zn, Mg and K; 2.0 mg L-1 Fe and 0.8 mg L-1 Ca and Na; P4: 0.4 mg L-1 Zn, Mg and K; 2.6 mg L-1 Fe and 1.1 mg L-1 Ca and Na; P5: 0.6 mg L-1 Zn and K; 0.5 mg L-1Mg; 3.4 mg L-1 Fe; 1.4 mg L-1 Ca and 1.3 mg L-1 Na. The black lines are the limits (80-110%) established by Instituto Nacional de Metrologia, Qualidade e Tecnologia (INMETRO)41 for recovery tests, according to the concentration range of the analytes
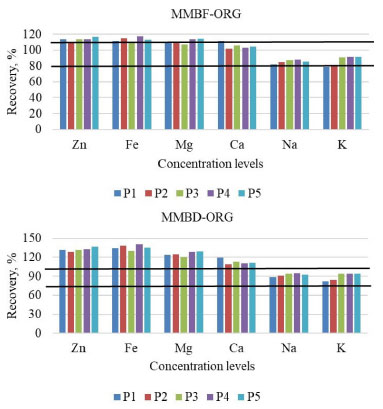 Figure 4. Recovery tests results at five analytes concentration levels, for the MMBF-ORG and MMBD-ORG calibration techniques. MMBF-ORG: matrix-matching along with babassu FAME and organic standard solutions; MMBD-ORG: matrix-matching along with biodiesel and organic standard solutions. P1: 0.1 mg L-1 Zn, Mg and K; 0.6 mg L-1 Fe and 0.3 mg L-1 Ca and Na; P2: 0.2 mg L-1 Zn, Mg and K; 1.3 mg L-1 Fe and 0.5 mg L-1 Ca and Na; P3: 0.3 mg L-1 Zn, Mg and K; 2.0 mg L-1 Fe and 0.8 mg L-1 Ca and Na; P4: 0.4 mg L-1 Zn, Mg and K; 2.6 mg L-1 Fe and 1.1 mg L-1 Ca and Na; P5: 0.6 mg L-1 Zn and K; 0.5 mg L-1 Mg; 3.4 mg L-1 Fe; 1.4 mg L-1 Ca and 1.3 mg L-1 Na. The black lines are the limits (80-110%) established by Instituto Nacional de Metrologia, Qualidade e Tecnologia (INMETRO)41 for recovery tests, according to the concentration range of the analytes
Based on results showed in Figure 3, it was possible to observe certain similarity between recoveries obtained for MMBF-INO and EC-INO methods. This can be explained by the calibration curves tendency to present closer sensitivities to each other when the same standard solutions (inorganic or organic) are used. Same similarity was also observed between MMBD-ORG and MMBF-ORG methods (Figure 4). An exception was noticed for K, in which similar recoveries were obtained for all investigated calibration techniques, since for this analyte the four curves presented very close regression coefficients. Among the studied calibration techniques, matrix-matching along with babassu FAME and inorganic standard solutions (MMBF-INO) presented satisfactory mean recoveries (81-111%) for all analytes, which are in accordance with the established range by Instituto Nacional de Metrologia, Qualidade e Tecnologia (INMETRO).41 Suitable recoveries were also obtained for MMBF-ORG method. However, this was already expected since calibration solutions were the same as those used for recovery tests. Based on these results, MMBF-INO method was chosen to carry out the analyses, considering that satisfactory recoveries were obtained, in addition to the easier preparation of calibration solutions when inorganic standards are used. Figures of merit After optimizing the instrumental conditions and defining the calibration strategy to be used, merit parameters were determined from the linear regression equations of calibration curves, obtained by MMBF-INO method, which are shown in Table 5.
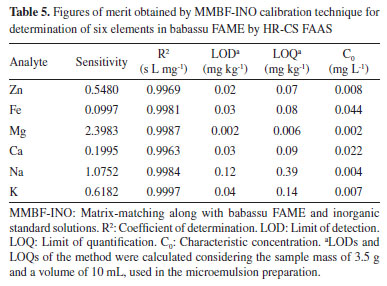
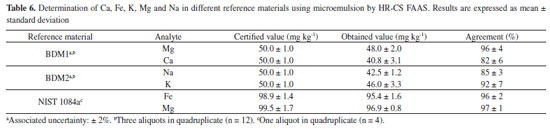
For all investigated elements, the calibration curves presented good linearity (R2 ≥ 0.996). Limits of detection (LOD) and quantification (LOQ) were calculated according to INMETRO recommendations,41 as three times and ten times, respectively, the standard deviation of 10 blank measurements, divided by the slope of the calibration curve. In order to estimate the LODs and LOQs of the method, the mass used in sample preparation (3.5 g of babassu FAME) and microemulsion final volume (10 mL) were considered. Although there is no specific legislation for oxygenated biokerosene, consisting of esters mixture, according to Agência Nacional do Petróleo, Gás Natural e Biocombustíveis (ANP) Resolution No. 856,42 the maximum allowed limit for all investigated elements in aviation biofuels synthesized through the certified methods by ASTM is 0.1 mg kg-1 per metal. In addition, among the analytes, there are only defined limits for the amounts of Na + K (2.5 mg kg-1) and Ca + Mg (2.5 mg kg-1) in biodiesel samples, according to ANP Resolution No. 920.43 Therefore, detection limits obtained in this work for all analytes were lower than or equivalent to the maximum value established by the Brazilian legislations. The characteristic concentrations (C0) obtained in this work are in agreement with the values reported by Welz et al.24 and Jesus et al.21 for the same technique (HR-CS AAS). Comparing the figures of merit obtained in this work with those reported by Jesus et al.,21 in general, although lower sensitivities were obtained, lower limits of detection were achieved, since a higher amount of sample was used in the microemulsions preparation. Furthermore, the difference in sensitivities is related to the defined conditions of sample injection time, since using flow injection mode the absorbance is measured in area. Method accuracy evaluation The accuracy of the proposed method was evaluated by analyzing two certified reference materials of biodiesel (BDM1 and BDM2) and a lubricating oil standard reference material (SRM NIST 1084a). As described in the Experimental section, due to the high concentration of the analytes in these materials, they were diluted in a biodiesel free of analytes before the preparation of the microemulsion. The results are reported in Table 6.
Since the analyzed materials did not contain certified values for Zn, an aliquot of organic standard of this element (at a concentration of 50 mg kg-1) was solubilized in the biodiesel CRMs solution, so that all analytes could be determined. The obtained concentration for Zn was 48.9 ± 2.3 mg kg-1, resulting in 97 ± 4% of agreement between added and found value. By using the Yuen-Welch's t-test (comparison of means with unequal variances),44 the obtained results were in agreement with the certified values for a 95% confidence level, as demonstrated by the p-values for all the evaluated analytes (p-values for CRMs BDM1 and BDM2: 0.49 for Zn, 0.06 for Mg, 0.16 for Ca, 0.22 for Na, and 0.32 for K; p-values for SRM NIST 1084a: 0.08 for Fe and 0.17 for Mg). An additional recovery test was performed, in order to investigate possible physical interferences in SRM NIST 1084a analysis, due to the matrix difference between lubricating oil and babassu FAME. The studies were carried out with the lubricating oil standard reference material SRM NIST 1083. As the concentrations for all investigated elements in SRM 1083 were below the LOD, inorganic standard solutions with concentrations referring to the midpoint of calibration curves were added to aqueous phase of the microemulsions prepared from it. Results showed satisfactory values ranging from 95 to 108%, indicating the absence of matrix effect in lubricants analysis for Zn, Fe, Mg, Ca, Na and K by the proposed method. Details can be seen in Table 1S (at supplementary material). Determination of Zn, Fe, Mg, Ca, Na and K in synthetized babassu FAME The babassu FAME, BBF1 sample, previously synthetized in this work, was analyzed by HR-CS FAAS using microemulsion as sample preparation. The results obtained by the proposed method are presented in Table 7. As already discussed in the calibration techniques study section, the sample BBF2, that were previously analyzed and did not show significant analytes content, was employed as the oil phase in calibration microemulsions of the technique chosen to the analysis (MMBF-INO). Therefore, this sample was not further analyzed by the proposed method. The sample BBF1 showed concentrations below the LOD for all investigated elements, except for Mg. Furthermore, the concentrations of Zn, Fe, Mg, Ca, Na and K in this sample were all below the maximum allowed value by Brazilian legislation for aviation biofuel (0.1 mg kg-1 per metal)42 and biodiesel (2.5 mg kg-1 for Na + K and Ca + Mg).43 These results showed that both methods of synthesis employed were suitable for obtaining babassu FAME samples without these metallic contaminants.
CONCLUSIONS As shown in this work, the sequential multi-element determination of six analytes in babassu FAME samples by HR-CS FAAS was possible. The use of a microemulsion system proved to be a simple and fast alternative for sample preparation, allowing the use of inorganic standard solutions for the calibration process and circumventing the use of concentrated inorganic acids throughout the pretreatment steps. Besides, there was a decrease in the generated residual sample volume by using the HR-CS FAAS along with the flow injection system. The accuracy of the proposed method was attested by the satisfactory results of the spike-recovery test and the agreement with the analytes concentrations of the certified reference materials. The detection limits achieved were lower than or equivalent to those established by Brazilian legislation for aviation biofuel and biodiesel, showing that the method can be used for routine analysis of medium-chain fatty acid methyl esters samples applied as biofuel.
SUPPLEMENTARY MATERIAL Supplementary material describes the sodium glyceroxide synthesis and babassu FAMEs synthesis through methods A and B, and shows 1H NMR spectra of synthesized babassu FAMEs (Figures 1S and 2S) and recovery test results from SRM NIST 1083 (Table 1S). Supplementary material is available at http://quimicanova.sbq.org.br, as PDF file free of charge.
ACKNOWLEDGMENTS The authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - 136090/2019-2; 311269/2019-2; 308378/2019-9), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES - finance code 001) and Financiadora de Estudos e Projetos (FINEP – 0045/21) for the financial support.
REFERENCES 1. Pierezan, L.; Cabral, M. R. P.; Martins Neto, D.; Stropa, J. M.; de Oliveira, L. C. S.; Scharf, D. R.; Simionatto, E. L.; da Silva, R. C. L.; Simionatto, E.; Quim. Nova 2015, 38, 328. [Crossref] 2. Da Silva, J. Q.; Santos, D. Q.; Fabris, J. D.; Harter, L. V. L.; Chagas, S. P.; Renewable Energy 2020, 151, 426. [Crossref] 3. Llamas, A.; García-Martínez, M. J.; Al-Lal, A. M.; Canoira, L.; Lapuerta, M.; Fuel 2012, 102, 483. [Crossref] 4. Braun, J. V.; Santos, S. J.; Espíndola, G. C.; de Mattos, G. F.; Ongaratto, D. P.; de Oliveira, D. M.; da Silva, M. W.; Vendrusculo, V.; dos Santos, V. O. B.; Renner, R. E.; Naciuk, F. F.; Marques, M. V.; Fontoura, L. A. M.; Quim. Nova 2020, 43, 1246. [Crossref] 5. Nogueira, C. A.; Feitosa, F. X.; Fernandes, F. A. N.; Santiago, R. S.; de Sant'ana, H. B.; J. Chem. Eng. Data 2010, 55, 5305. [Crossref] 6. da Silva, R. B.; Lima Neto, A. F.; dos Santos, L. S. S.; Lima, J. R. O.; Chaves, M. H.; dos Santos, J. R.; de Lima, G. M.; de Moura, E. M.; de Moura, C. V. R.; Bioresour. Technol. 2008, 99, 6793. [Crossref] 7. de Moura, C. V. R.; de Castro, A. G.; de Moura, E. M.; dos Santos Júnior, J. R.; Moita Neto, J. M.; Energy Fuels 2010, 24, 6527. [Crossref] 8. da Rós, P. C. M.; Costa e Silva, W.; Grabauskas, D.; Peres, V. H.; de Castro, H. F.; Ind. Crops Prod. 2014, 52, 313. [Crossref] 9. de Oliveira, V. F.; Parente Júnior, E. J. S.; Manrique-Rueda, E. D.; Cavalcante Júnior, C. L.; Luna, F. M. T.; Chem. Eng. Res. Des. 2020, 160, 224. [Crossref] 10. Araújo, P. H. M.; Maia, A. S.; Cordeiro, A. M. T. M.; Gondim, A. D.; Santos, N. A.; ACS Omega 2019, 4, 15849. [Crossref] 11. Llamas, A.; Al-Lal, A. M.; Hernandez, M.; Lapuerta, M.; Canoira, L.; Energy Fuels 2012, 26, 5968. [Crossref] 12. Ranucci, C. R.; Alves, H. J.; Monteiro, M. R.; Kugelmeier, C. L.; Bariccatti, R. A.; de Oliveira, C. R.; da Silva, E. A.; J. Cleaner Prod. 2018, 185, 860. [Crossref] 13. Chagas, S. P.; Santos, D. Q.; Fabris, J. D.; Harter, L. V. L.; da Silva, J. Q.; J. Braz. Chem. Soc. 2023, 34, 390. [Crossref] 14. Lepri, F. G.; Chaves, E. S.; Vieira, M. A.; Ribeiro, A. S.; Curtius, A. J.; de Oliveira, L. C. C.; de Campos, R. C.; Appl. Spectrosc. Rev. 2011, 46, 175. [Crossref] 15. Arun, N.; Sharma, R. V.; Dalai, A. K.; Renewable Sustainable Energy Rev. 2015, 48, 240. [Crossref] 16. Lyra, F. H.; Carneiro, M. T. W. D.; Brandão, G. P.; Pessoa, H. M.; de Castro, E. V.; Microchem. J. 2010, 96, 180. [Crossref] 17. de Quadros, D. P. C.; Chaves, E. S.; Silva, J. S. A.; Teixeira, L. S. G.; Curtius, A. J.; Pereira, P. A. P.; Rev. Virtual Quim. 2011, 3, 376. [Crossref] 18. Sánchez, R.; Sánchez, C.; Lienemann, C. P.; Todolí, J. L.; J. Anal. At. Spectrom. 2015, 30, 64. [Crossref] 19. Martínez, S.; Sánchez, R.; Lefevre, J.; Todolí, J. L.; Spectrochim. Acta, Part B 2022, 189, 106356. [Crossref] 20. de Jesus, A.; Zmozinski, A. V.; Barbará, J. A.; Vale, M. G. R.; Silva, M. M.; Energy Fuels 2010, 24, 2109. [Crossref] 21. de Jesus, A.; Zmozinski, A. V.; Laroque, D. O.; da Silva, M. M.; Quim. Nova 2021, 44, 205. [Crossref] 22. Lima, A. S.; Silva, D. G.; Teixeira, L. S. G.; Environ. Monit. Assess. 2015, 187, 4122. [Link] accessed in January 2024 23. Almeida, E. S.; Richter, E. M.; Munoz, R. A. A.; Electroanalysis 2016, 28, 633. [Crossref] 24. Welz, B.; Becker-Ross, H.; Florek, S.; Heitmann, U.; High-Resolution Continuum Source AAS: The Better Way to Do Atomic Absorption Spectrometry, 1st ed.; Wiley-VCH: Weinheim, 2005. 25. de Oliveira, L. C. C.; Vieira, M. A.; Ribeiro, A. S.; Baptista, P. M.; de Campos, R. C.; J. Braz. Chem. Soc. 2012, 23, 1400. [Crossref] 26. Guzatto, R.; de Martini, T. L.; Samios, D.; Fuel Process. Technol. 2011, 92, 2083. [Crossref] 27. Santos, S. J.; Braun, J. V.; Espíndola, G. C.; da Silva, J. A.; Renner, R. E.; Fontoura, L. A. M.; J. Braz. Chem. Soc. 2024, 35, 1. [Crossref] 28. Schaumlöffel, L. S.; Fontoura, L. A. M.; Santos, S. J.; Pontes, L. F.; Gutterres, M.; Fuel 2021, 292, 120198. [Crossref] 29. Santos, S. J.; Dutra, C. E. M.; Fontoura, L. A. M.; Quim. Nova 2023, 46, 818. [Crossref] 30. Kumar, R.; Bansal, V.; Patel, M. B.; Sarpal, A. S.; Energy Fuels 2012, 26, 7005. [Crossref] 31. Ivanova, M.; Hanganu, A.; Dumitriu, R.; Tociu, M.; Ivanov, G.; Stavarache, C.; Popescu, L.; Ghendov-Mosanu, A.; Sturza, R.; Deleanu, C.; Chira, N.; Foods 2022, 11, 1466. [Crossref] 32. ASTM D1298-99: Standard Test Method for Relative Density (Specific Gravity), or API Gravity of Crude Petroleum and Liquid Petroleum Roducts by Hydrometer Method, Philadelphia, 2017. 33. ASTM D445-06: Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity), Philadelphia, 2012. 34. ASTM D97-17b: Standard Test Method for Pour Point of Petroleum Products, Philadelphia, 2022. 35. Sarpal, A. S.; Silva, S. R.; Silva, P. R. M.; Monteiro, T. V.; Itacolomy, J.; Cunha, V. S.; Daroda, R. J.; Energy Fuels 2015, 29, 7956. [Crossref] 36. Chaves, E. S.; Saint'Pierre, T. D.; dos Santos, E. J.; Tormen, L.; Bascuñan, V. L. A. F.; Curtius, A. J.; J. Braz. Chem. Soc. 2008, 19, 856. [Crossref] 37. Vieira, M. A.; de Oliveira, L. C. C.; Goncalves, R. A.; de Souza, V.; de Campos, R. C.; Energy Fuels 2009, 23, 5942. [Crossref] 38. Antunes, G. A.; dos Santos, H. S.; da Silva, Y. P.; Silva, M. M.; Piatnicki, C. M. S.; Samios, D.; Energy Fuels 2017, 31, 2944. [Crossref] 39. Welz, B.; Sperling, M.; Atomic Absorption Spectrometry, 3rd ed.; Wiley-VCH: Weinheim, 1999. 40. Andrade, J. M.; Estévez-Pérez, M. G.; Anal. Chim. Acta 2014, 838, 1. [Crossref] 41. Instituto Nacional de Metrologia, Normalização e Qualidade Industrial (INMETRO); DOQ-CGCRE-008, Orientação sobre Validação de Métodos Analíticos, 2020. [Link] accessed in January 2024 42. Agência Nacional do Petróleo, Gás Natural e Biocombustíveis (ANP); Resolução ANP No. 856, de 22 de outubro de 2021, Estabelece as Especificações do Querosene de Aviação JET A e JET A-1, dos Querosenes de Aviação Alternativos e do Querosene de Aviação C (JET C), bem como as Obrigações Quanto ao Controle da Qualidade a Serem Atendidas pelos Agentes Econômicos que Comercializam esses Produtos em Território Nacional; Diário Oficial da União (DOU), Brasília, No. 201, de 25/10/2021, p. 89. [Link] accessed in January 2024 43. Agência Nacional do Petróleo, Gás Natural e Biocombustíveis (ANP); Resolução No. 920, de 04 de abril de 2023, Estabelece a Especificação do Biodiesel e as Obrigações quanto ao Controle da Qualidade a serem Atendidas pelos Agentes Econômicos que Comercializem o Produto em Território Nacional; Diário Oficial da União (DOU), Brasília, No. 66, de 05/04/2023, p. 51. [Link] accessed in January 2024 44. Real Statistics using Excel, https://real-statistics.com/students-t-distribution/problems-data-t-tests/yuen-welchs-test/, accessed in January 2024. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access