Educação
| Popping balloons: a fun activity to engage students in review of chemical nomenclature and stereochemistry by using educational games and low-cost materials |
|
José Nunes da Silva JúniorI; Antonio José Melo Leite JuniorII; Renner César Silveira JucáI,* I. Departamento de Química Orgânica e Inorgânica, Universidade Federal do Ceará, 60451-970 Fortaleza - CE, Brasil Received: 10/23/2024 *e-mail: rennerjuca@alu.ufc.br, rennerjuca@yahoo.com.br In recent years, non-traditional educational activities in the classroom have become increasingly common to motivate and engage students to study subjects considered difficult to learn. This experience report describes one of these hybrid activities, integrating digital games and low-cost materials like party balloons, rubber bands, and pins in a fun environment in the classroom as a strategy to promote learning and interaction between Brazilian undergraduate and 12th-grade students who evaluated it positively. Specifically, the activity described used two digital games on the stereochemistry and nomenclature of organic compounds. Still, it can also be applied by using any games that cover other subjects. INTRODUCTION Teaching and learning chemistry at all levels of education have been marked with several challenges. For instance, students' lack of problem-solving skills, limited spatial visualization, difficulties in understanding chemistry terminology, and poor communication between students and teachers have been previously studied. This background has prompted researchers from different settings to continuously advocate for a learning environment that appropriately addresses such difficulties.1,2 With time, educators have significantly shifted from a teacher-centered to a learner-centered approach in chemistry education research to overcome these difficulties. Particularly in the past decade, it has been proven that students learn better when they can interact with their peers instead of sitting passively in class and just listening to the teacher.3 This approach from the social cognition theory and constructivism is premised on the notion that meaningful learning can only occur if learners are provided with opportunities to interact as they attach meaning to the learned content.4 Particularly, constructivism engages students in meaningful activities that facilitate the acquisition of knowledge from their own experiences.5 In this context, game-based learning has emerged as one of the most beneficial educational approaches because it can emphasize "hands-on" and "minds-on" activities in chemistry classrooms.6,7 In parallel, the growing acceptance of digital games as mainstream entertainment has prompted consideration of how to take advantage of their potential for educational purposes. It can also be highlighted that many essential game concepts, such as motivation, relate to different theoretical foundations, such as cognitive, affective, motivational, and sociocultural.8 Hence, in the last decade, we have designed free-of-charge educational games9-15 and implemented them in the classroom as an alternative method for reviewing several chemistry topics in a fun way. In short, these games are systems where players interact in an artificial engagement governed by rules, leading to measurable outcomes.16 In light of this, player engagement is related to motivation, and it is one of the most frequently cited reasons to consider digital educational games as a learning tool because they can provide several paths to captivate and involve students.17 As a result, motivational game features can help learners stay engaged over long periods. Adaptivity is the game's capability to engage each learner by tailoring the experience to their specific situation and various game adaptation methods that can contribute significantly to learner engagement.18-21 For example, a game can engage the learner behaviorally by using gestures as input or inviting players to perform specific physical actions as part of the play. Game characters engage the learner emotionally, promoting social features such as collaborative play and team engagement.17 Besides, low-cost materials have also been used in chemical education. For example, Rwandan educators assessed the impact of using low-cost materials for effective teaching and learning chemistry in lower secondary schools. According to their results, there was an impact, improving students' achievement.22 Party balloons are a successful example of a low-cost material that chemical educators have used because they are cheap, safe, and easy-to-use alternatives to more complex and costly equipment but can also be used as effective teaching aids to demonstrate theoretical and laboratory principles of chemistry.23-27 We have observed in our previous experiences that many students do not have smartphones with adequate screens (size, resolution, etc.) or quality internet access to play digital games in classrooms, an additional difficulty for some people in less economically advantaged contexts in Brazil. This context motivated us to develop an activity where the game would still be used, but the students would not need smartphones or internet access, as the game would be projected on a screen so that everyone could interact and participate simultaneously. This change aimed to increase students' social engagement in the classroom through a fun environment that continues using digital games and now also adds a "physical layer" based on the usage of low-cost materials. Thus, this experience report presents a classroom hybrid activity that integrates digital games10-15 and low-cost materials like party balloons, rubber bands, and pins to promote learning and interaction between students.
EXPERIMENTAL Tutor guide of the activity - Chemical Nomenclature The chapter Nomenclature of organic compounds is taught in five or six 100-min classes at the Federal University of Ceará in Brazil. Despite its significance, students' perceptions of chemistry as a set of complicated rules applied to strange concepts make learning challenging. Educators have also expressed annoyance with studying chemical nomenclature, and students have often felt that it is a pointless exercise.28 To overcome this scenario, in 2018, we published the design of the Nomenclature Bets game10 and its use in a tournament. This tournament was an academic competition between groups of students who used their knowledge of the nomenclature of organic compounds as a didactic strategy to promote students' interaction and learning. We continually used the Nomenclature Bets in tournaments. However, in March 2020, the COVID-19 pandemic disrupted face-to-face activities. Confronting this scenario, we upgraded the game to an online version (Nomenclature Bets 2.0) to be used remotely by students from their homes.15 After the COVID-19 pandemic, we continued using the Nomenclature Bets 2.0 in a fun and collaborative environment in presential classes, in which groups of four students played the game. In May 2023, we planned an activity (Popping Balloons) using the same online version of the Nomenclature Bets10 in a challenging competition involving sixteen Pharmacy students from the Federal University of Ceará. In September 2023, we applied the same activity using the same game in another class of twelve Pharmacy undergraduate students as an alternative activity to the traditional solving-problem class. In both cases, the students were enrolled in a class of Introductory Organic Chemistry. We implemented the activity immediately after the end of the unit on the nomenclature of organic compounds, which had six classes with 100 min duration each. Before the activity day Initially, we sorted the 16 students in descending order of performances in previous exams in the course. Then, we separated the students into quartets according to their position in the order, setting up four competition groups with one student from each quartet, ensuring that the sub-groups were internally heterogeneous and homogeneous among themselves, making the competitions more balanced. Prior grades served exclusively as a technical criterion for group formation, with no intention of ranking students or judging their abilities. The group formation followed Grumbler's Algorithm,29 which is often used in research involving tournament dynamics and playful activities, ensuring better group balance and fostering fair and healthy competition. Afterward, each competition group chose one color of balloons to represent them in the activity: blue, red, green, and yellow (Figure 1).
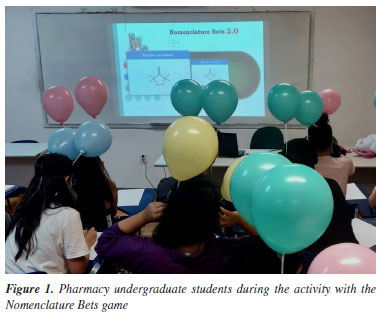
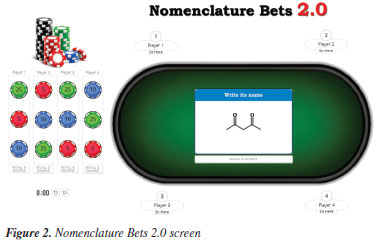
Later, we encouraged the students to study together before the activity day, when they would not have any direct support from the members of their group. Although the activity had an individual character, that is, only one student would win the activity and be rewarded with one extra point in their grade, all students from the winner's group would also be rewarded with a half-point in their grades to foster partnership. Activity day Initially, each group member attached two balloons with their group colors to their head with the help of an elastic band and two plastic rods (Figure 1 and Figure 1S, see Supplementary Material). The time spent for this preliminary preparation was 15 min on average. Next, the Professor projected the Nomenclature Bets 2.0 game10 screen on a whiteboard (Figure 1 and Figure 2S, see Supplementary Material). It is essential to highlight that the students did not sit near each other members of their groups because no exchange of answers was permitted among them during the activity. The steps of Popping Balloons are listed below:
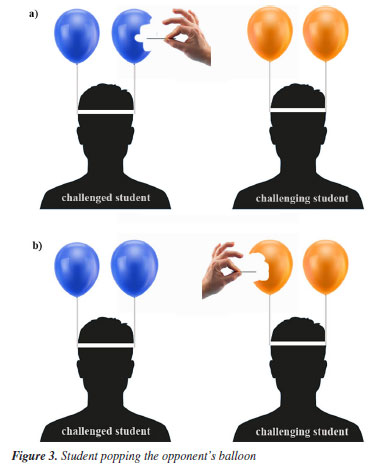
The activity repeats steps 2-5 until only one student remains with balloons and becomes the winner, getting rewarded with one extra point in their grade. The members of the winner's group also win half a point in their grades. The activity motivated all students untill the end of the competition. Even those students who had already had their balloons popped continued to cheer on the other members of their groups. The activity time involving the 16 students was 75 min. Instructors can adjust the activity time by varying the number of students and the number of balloons for each student. They can also develop this activity by changing the number of competition groups or even the number of students in each group since they cannot discuss questions and answers with other students during the activity. Direct interaction is only recommended in the preparation stage before the activity day to promote the engagement and sharing of knowledge between the students of the competition groups. Finally, to maintain the competition balance, the professor must remember that each group must have the same number of balloons attached to the members' heads. Tutor guide of the activity - Stereochemistry Many students find stereochemistry challenging to grasp because of the struggle to visualize three-dimensional structures and comprehend concepts like asymmetric carbon and chirality. As a consequence, educators have noted challenges in teaching stereochemistry to high school and college students dating back to the 1940s.28,30-33 So, given the good results obtained in the previous activity, we evaluated the students' engagement in another context. Therefore, in October 2023, we implemented a similar activity, but now addressing stereochemistry in high school. For that, we worked with three high school classes (thirty-nine 12th-grade students in total) from three different schools, Colégio Maximus (10), Escola de Ensino Fundamental e Médio Prof. Hermenegildo Firmeza (11), and Escola de Ensino Fundamental e Médio Prof. Adalgisa Bonfim Soares (18) in Fortaleza, Brazil. Below, we describe how we applied the Popping Balloons activity in high school. The process was the same in all classes. However, there was only one difference. While we used the Nomenclature Bets 2.010 in the University, we used the Stereochemistry Game12 (Figure 4 and Figure 4S, see Supplementary Material) in high school.
Before and during the activity day We separated the 39 students into nine competition groups, with four students and another with three. To maintain the competition balance, two members of this last group attached three balloons to their heads, while the third student attached only two balloons, totaling eight as the other groups had (4 members with two balloons each). We followed the same procedures described previously and projected the Stereochemistry Game12 screen on a whiteboard (Figure 5). The activity time was 38 min on average.

It is worth noting that during the activity, whether using the cards from the Nomenclature Bets game or the Stereochemistry game, the professor played an active role by discussing correct and incorrect answers to clarify doubts and enhance students' understanding of the topics covered. Thus, even though this was not the primary focus of the experiment, the activity indirectly contributed to reinforcing learning and addressing potential conceptual errors during the interaction. Activity day Initially, each group member attached two balloons with their group colors to their head with the help of an elastic band and two plastic rods (Figure 5). The time spent for this preliminary preparation was 10 min on average. Next, the Professor projected the Stereochemistry Game12 screen on a whiteboard (Figure 5). The activity time was 38 min on average and proved enjoyable for undergraduate and high school students, eliciting enthusiastic participation from all. We evaluated the learners' perceptions, and the findings are presented below.
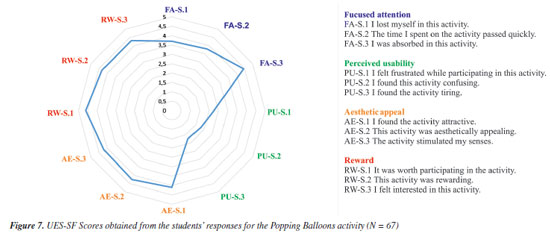
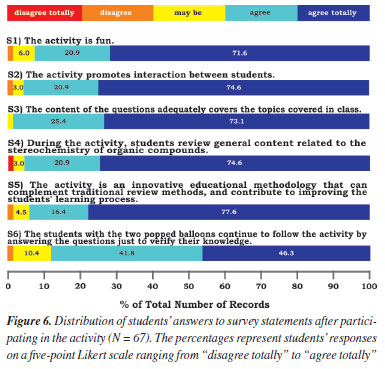
RESULTS AND DISCUSSION Evaluation of the activity - students' opinions We invited the 67 students (28 undergraduate and 39 high school) who participated in the Popping Balloons activity to respond to a survey of twenty-one statements using a Likert-type scale34 (see Supplementary Material). All Pharmacy students agreed to participate and signed the Informed Consent Form we provided (see Supplementary Material). Also, the high school students agreed, and their parents signed the Assent Form we provided (see Supplementary Material). The first six statements (S1-S6) refer to the activity as a tool to review the nomenclature of organic compounds (for undergraduate students) or Stereochemistry (for high school students). The following twelve statements (S7-S18) refer to the User Engagement Scale short form (UES-SF),35 one of the best-known and most straightforward methods of ascertaining participant engagement levels in an activity. The last three statements request a rate for the activity and its positive and negative aspects (S19-S21). Based on student responses (Figure 6), we can confidently affirm that they enjoyed the Popping Balloons activity, with agreement rates to the statements ranging from 88.1 to 98.5%. In addition, the findings showed that 92.5% of students considered the activity fun (S1). 95.5% agreed that the activity promotes interaction between students (S2) while reviewing content related to the nomenclature or stereochemistry of organic compounds (S3, 98.5%) because the games adequately cover the subjects seen in class (S4, 95.5%). Also, 94.0% agreed that the activity is an innovative educational methodology that can complement traditional review methods and improve the student learning process (S5). Finally, 88.1% of the students agreed that those who had their two balloons popped during the activity continued to follow its dynamics by answering the questions to test their knowledge (S6).
User engagement Measuring user engagement has become essential in different activities, particularly educational ones.36 To assess the level of student involvement in the Popping Balloons activity, we employed the UES-SF.35 It is a tool composed of 12 questions (S7-S18, see Supplementary Material) divided into four domains:
Figure 7 presents the rates in the four domains of the UES-SF obtained from all 67 undergraduate and high school students who participated in the Popping Balloons. Since we detected no significant differences between the undergraduate and high school students, the overall engagement UES-SF Score was 4.16 - the closer to 5, the greater the engagement.
SWOT analysis - Professors' and students' perceptions The survey required students to rate the Popping Balloons activity (S19, Supplementary Material) by assigning a score on a scale of 0 to 10. The average scores of college and high school students were 8.6 ± 1.9 and 9.8 ± 0.4, respectively. Despite these reasonable rates, we adopted the SWOT (Strengths, Weaknesses, Opportunities, and Threats) analysis37 to represent the activity's overall instructor and student perceptions. We opted for the SWOT analysis as it provides a structured framework for organizing the variables that could improve our comprehension of the advantages and disadvantages associated with the implementation of the Popping Ballons activity in the classroom. In order to advance with the SWOT analysis, the professors involved in designing and implementing the activity participated in a brainstorming session to reflect on their perceptions throughout the entire process. They also considered the positive and negative points indicated by students in the survey (S20-S21, see Supplementary Material). This brainstorming session tried to address the following questions (directly involved in the SWOT analysis):
The results from the brainstorming were: Strengths
Weaknesses
Opportunities
Threats
As we found no significant differences between undergraduate and high school students' opinions, the results above can assist us in developing new activities to be used in the classroom.
CONCLUSIONS The Popping Balloons activity requires minimal preparation time and uses low-cost materials. Educators can use any educational games or even online quizzes available on the Internet (Kahoot, Mentimeter, Quizizz, etc.) or still prepare a set of specific questions to facilitate the review of topics covered in the classroom. Furthermore, the activity transformed a traditional classroom with chairs and a whiteboard into a place of fun, in which students had their knowledge tested and could review the content seen previously in an environment that promoted integration between them. Both high school and undergraduate students evaluated the activity positively and expressed their desire to participate again during the course. In this scenario, students would be motivated to proactively engage in pre-activity studies, aiming for improved performance in subsequent activities. Therefore, the Popping Balloons activity can be applied in specific classes, as described in this experience report, or periodically, by using other games in all units.
SUPPLEMENTARY MATERIAL Complementary material for this work is available at http://quimicanova.sbq.org.br/, as a PDF file, with free access.
ACKNOWLEDGMENTS The authors acknowledge Mr. Rob Middleton from PlayingCards.io LLC for all the support in hosting the Nomenclature Bets and Stereochemistry Game.
REFERENCES 1. Childs, P. E.; Sheehan, M.; Chem. Educ. Res. Pract. 2009, 10, 204. [Crossref] 2. Erman, E.; J. Res. Sci. Teach. 2017, 54, 520. [Crossref] 3. Byusa, E.; Kampire, E.; Mwesigye, A. R.; Heliyon 2022, 8, e09541. [Crossref] 4. Amineh, R. J.; Asl, H. D.; Journal of Social Sciences, Literature and Languages 2015, 1, 9. [Link] accessed in March 2025 5. Shah, R. K.; Shanlax International Journal of Education 2019, 7, 1. [Crossref] 6. North, B.; Diab, M.; Lameras, P.; Zaraik, J.; Philippe, S.; Müller, J.; Fischer, H.; IEEE Global Engineering Education Conference (EDUCON); Vienna, Austria, 2021. [Crossref] 7. Yilmaz, K.; The Clearing House: A Journal of Educational Strategies, Issues and Ideas 2011, 84, 204. [Crossref] 8. Shaffer, D. W.; Halverson, R.; Squire, K. R.; Gee, J. P.; WCER Working Paper No. 2005-4; Wisconsin Center for Education Research (NJ1), 2005. [Link] accessed in March 2025 9. da Silva Júnior, J. N.; Nobre, D. J.; do Nascimento, R. S.; Torres, G. S.; Leite, A. J. M.; Monteiro, A. J.; Alexandre, F. S. O.; Rodríguez, M. T.; Rojo, M. J.; J. Chem. Educ. 2018, 95, 899. [Crossref] 10. da Silva Junior, J. N.; Lima, M. A. S.; Nunes, F. M.; Leite Junior, A. J. M.; Alexandre, F. S. O.; Assis, D. C. O.; Nobre, D. J.; Winum, J.-Y.; Monteiro, A. J.; de Lima, D. T. F.; J. Chem. Educ. 2022, 99, 2208. [Crossref] 11. Lima, M. A. S.; Monteiro, Á. C.; Leite Junior, A. J. M.; Matos, I. S. A.; Alexandre, F. S. O.; Nobre, D. J.; Monteiro, A. J.; da Silva Júnior, J. N.; J. Chem. Educ. 2019, 96, 801. [Crossref] 12. da Silva Júnior, J. N.; Uchoa, D. E. A.; Lima, M. A. S.; Monteiro, A. J.; Leite Junior, A. J. M.; Winum, J.-Y.; Basso, A.; J. Chem. Educ. 2021, 98, 3055. [Crossref] 13. da Silva Júnior, J. N.; Leite Junior, A. J. M.; Winum, J.-Y.; Basso, A.; de Sousa, U. S.; do Nascimento, D. M.; Alves, S. M.; Education for Chemical Engineers 2021, 36, 90. [Crossref] 14. da Silva Júnior, J. N.; Lima, M. A. S.; Pimenta, A. T. Á.; Nunes, F. M.; Monteiro, Á. C.; de Sousa, U. S.; Leite Júnior, A. J. M.; Zampieri, D.; Alexandre, F. S. O.; de Sousa, U. S.; Pacioni, N. L.; Winum, J.-Y.; Education for Chemical Engineers 2021, 34, 106. [Crossref] 15. da Silva Junior, J. N.; Winum, J.-Y.; Basso, A.; Gelati, L.; Moni, L.; Leite Junior, A. J. M.; Mafezoli, J.; Zampieri, S.; Alexandre, F. S. O.; Veja, K. B.; Monteiro, A. J.; J. Chem. Educ. 2022, 99, 3315. [Crossref] 16. Tekinbas, K. S.; Zimmerman, E.; Rules of Play: Game Design Fundamentals, 1st ed.; eTextbooks for Students, 2003. [Link] accessed in March 2025 17. Gee, J. P.; Computers in Entertainment (CIE) 2003, 1, 20. [Crossref] 18. Andersen, E.; Proceedings of the International Conference on the Foundations of Digital Games 2012, 279. [Crossref] 19. Leutner, D.; Learning and Instruction 1993, 3, 113. [Crossref] 20. Plass, J. L.; Chun, D. M.; Mayer, R. E.; Leutner, D.; Journal of Educational Psychology 1998, 90, 25. [Crossref] 21. Turkay, S.; The Effects of Customization on Player Experiences in an Extended Online Social Game: A Mixed Method Study; PhD Thesis, Teachers College, Columbia University ProQuest Dissertations & Theses, New York, USA, 2013. [Link] accessed in March 2025 22. Ncutinamagara, E.; Nkurunziza, J. B.; Makhosi, N.; Journal of Research Innovation and Implications in Education 2023, 7, 63. [Link] accessed in March 2025 23. Dewhurst, F.; Dewhurst, F. R.; J. Chem. Educ. 1981, 58, 44. [Crossref] 24. Arnaiz, F. J.; J. Chem. Educ. 1993, 70, 1020. [Crossref] 25. Maynard, J. H.; Wolsey, W.; J. Chem. Educ. 2008, 85, 519. [Crossref] 26. Saura-Sanmartin, A.; Lopez-Sanchez, J.; Lopez-Leonardo, C.; Pastor, A.; Berna, J.; J. Chem. Educ. 2023, 100, 3355. [Crossref] 27. Williams, K. R.; J. Chem. Educ. 2005, 82, 1448. [Crossref] 28. Mullin, J.; Courtney, P.; J. Chem. Educ. 1996, 73, A130. [Crossref] 29. The GRumbler, https://msparrow.scholars.harvard.edu//grumbler, accessed in March 2025. 30. Fromm, F.; J. Chem. Educ. 1945, 22, 43. [Crossref] 31. Shine, H. J.; J. Chem. Educ. 1957, 34, 355. [Crossref] 32. Evans, G. G.; J. Chem. Educ. 1963, 40, 438. [Crossref] 33. Beauchamp, P. S.; J. Chem. Educ. 1984, 61, 666. [Crossref] 34. Likert, R.; Archives of Psychology 1932, 22140, 55. [Link] accessed in March 2025 35. O'Brien, H. L.; Cairns, P.; Hall, M.; International Journal of Human-Computer Studies 2018, 112, 28. [Crossref] 36. Hamari, J.; Shernoff, D. J.; Rowe, E.; Coller, B.; Asbell-Clarke, J.; Edwards, T.; Computers in Human Behavior 2016, 54, 170. [Crossref] 37. Gürel, E.; Tat, M.; Journal of International Social Research 2017, 10, 994. [Link] accessed in March 2025
Associate Editor handled this article: Nyuara A. S. Mesquita |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access