Artigo
| Synergistic system study of hydrophobic associating water-soluble polymer and viscoelastic surfactant applied in fracturing fluid for unconventional oil and gas |
|
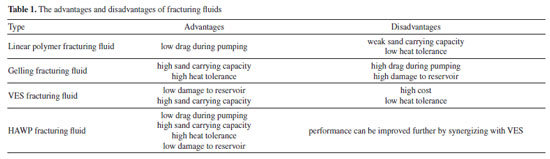
Li Yan; Baolu Yu* The 3rd Oil Production Plant, Qinghai Oilfield Branch, China National Petroleum Corporation, Haixi Mongol Tibetan Autonomous Prefecture, 816400 Qinghai, China Received: 01/09/2025 *e-mail: ybl9183@163.com Polymer-based fracturing fluids are widely used in unconventional reservoirs but often fall short in complex conditions. This study synthesizes a novel hydrophobic association water-soluble polymer (PAMNC12) and explores its synergy with viscoelastic surfactants (VESs: oleic acid amide propyl trimethylammonium chloride (OTAC), oleic acid amide propyl sulfobetaine (ODSB), oleic acid amide propyl carboxyl betaine (ODCB)) to optimize fracturing fluid performance. PAMNC12 exhibits critical association concentrations at 1029.2 and 2513.2 mg L-1. Rheological, gel-breaking, and core damage tests reveal that VES-PAMNC12 synergy significantly enhances apparent viscosity, viscoelasticity, drag reduction, heat/shear resistance, and proppant-carrying capacity while reducing residue content and core damage. Optimal dosages are 400 mg L-1 VES and 10,000 mg L-1 KCl. Among the systems, PAMNC12/OTAC shows superior thickening, heat/shear resistance (160 ºC), drag reduction (lowest settlement velocity), and minimal residue (30 mg L-1) and core damage (10.1%). PAMNC12/ODSB and PAMNC12/ODCB also demonstrate excellent heat tolerance, sand-carrying, drag reduction, and low formation damage, making them promising for hydraulic fracturing in unconventional reservoirs. This study provides a foundation for developing high-performance fracturing fluids. INTRODUCTION Hydraulic fracturing holds significant importance in the development of unconventional oil and gas.1-5 From the aspect of resource exploitation, unconventional oil and gas reservoirs (such as shale gas, tight oil, etc.) possess extremely low permeability, complex pore structures, and high resistance to the flow of oil and gas.6-10 Hydraulic fracturing can generate artificial fractures, significantly enhancing the seepage capacity of oil and gas in the reservoirs, allowing the originally hard-to-extract oil and gas to flow towards the wellbore, thereby making these unconventional resources commercially exploitable.1-3 The commonly employed water-based fracturing fluids mainly comprise the linear polymer fracturing fluid,11-13 gelling fracturing fluid,14-17 and viscoelastic surfactant (VES) clean fracturing fluid.18-20 Regarding the polymer fracturing fluid, its preparation is straightforward, and the linear polymer molecular structure confers good fluidity and low friction to the fluids,12 which is conducive to the pumping of the fracturing fluid in pipelines and helps reduce the frictional force. However, the viscosity of the linear polymer fracturing fluid is relatively low, and its capacity to carry proppants is limited. For the gelling fracturing fluid,15,16 it has a relatively high viscosity and can form a three-dimensional network structure through cross-linking reactions, enabling it to effectively carry proppants. It also possesses good shear resistance and can still maintain good stability during the fracturing process even when subjected to relatively high shear forces, allowing proppants to be evenly distributed in fractures. Additionally, the gelling fracturing fluid has a wide application range and can be utilized in formations with different temperatures and pressures. As for the clean fracturing fluid, it was introduced by Schlumberger Company in the 1990s21 by using the viscoelastic surfactant (VES) with a small molecular weight as a thickener. Stimulated by conditions22-25 such as pH, electrolyte, light, and CO2, VES molecules can assemble into various micelles, including sphere micelles, wormlike micelles, vesicles, and reverse micelles, among which wormlike micelles can endow the VES clean fracturing fluid with excellent viscoelasticity. Due to the absence of large molecule polymers, it exhibits extremely low damage to the formation matrix.22,26 Nevertheless, the cost of the VES clean fracturing fluid is relatively high because the dosage of VES is large, and its heat resistance is comparatively low. The above three water-based fracturing fluids have distinct advantages and disadvantages. The hydrophobically associating water-soluble polymer (HAWP)27-30 fracturing fluid combines the advantages of VES and polymer, having the ability to associate with each other and build a network structure in aqueous solutions. Moreover, the dosage of HAWP and its cost are relatively low, making its application widespread. However, as the depth of the exploited unconventional reservoirs increases, HAWP-based fracturing fluids have also exposed many technical problems, including insufficient heat resistance and proppant-carrying capacity.31 During the hydraulic fracturing operation, an efficient way to overcome these problems is to increase the concentration of HAWP, but this increases the friction during pumping in the pipeline and the damage rate to the formation. To improve the performance of HAWP-based fracturing fluids, some reports27,28 have proposed combining HAWP with VES to prepare fracturing fluids, thereby improving the heat resistance and proppant-carrying capacity and reducing the damage to the formation. The advantages and disadvantages of fracturing fluids were listed in Table 1. Though the HAWP fracturing fluid has advantages of multiple fracturing fluids at the same time, it still needs improvement for deeper reservoir stimulation, and the reservoir temperature reaches above 160 ºC. As reported,2,6 synergizing with VES is an excellent method to improve the performance of HAWP fracturing fluid.
In the paper, a zwitterionic HAWP (PAMNC12) was designed and synthesized successfully, and it was used to study the synergy with some common VESs, including oleic acid amide propyl trimethylammonium chloride (OTAC), oleic acid amide propyl sulfobetaine (ODSB), and oleic acid amide propyl carboxyl betaine (ODCB). Through rheological tests, proppant-carrying tests, gel breaking tests, and core damage tests, the performance of the synergistic systems of HAWP and VESs was studied, and the best system formula was determined, which can withstand the high temperature of 160 ºC and cause low damage to the formation rocks.
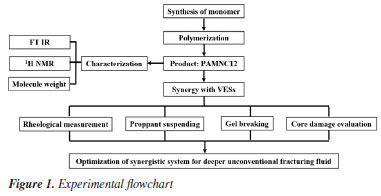
EXPERIMENTAL Materials Acrylamide (AM), maleic anhydride (MA), 1-bromododecane, N,N-dimethylaminopropyl acrylamide, ethanol (ETOH), isopropanol (IPA) and ethyl acetate, all of analytical grade, were obtained from Shanghai McLean Biochemical Technology Co., Ltd. (3-Acrylamidopropyl)trimethylammonium chloride (APTAC), 75 wt.% aqueous solution, was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Ammonium persulfate (APS) and sodium bisulfite, both of analytical grade, were obtained from Shanghai Xianding Biotechnology Co., Ltd. OTAC, ODSB, and ODCB, purity > 98%, were obtained from Shanghai Winsono New Material Technology Co., Ltd. 40 mesh ceramic proppant used for hydraulic fracturing was purchased from Shanxi Anyicheng New Material Manufacturing Co., Ltd. Artificial low-permeability rock cores, 25 mm × 50 mm, were obtained from Hai'an Huachuang Experimental Equipment Business Co., Ltd. The ceramic proppant was purchased from Zhengzhou Desair Ceramics Co., Ltd. The capsule gel breaking agent was purchased from Sichuan Energy Technology Co., Ltd. Methods The experimental flowchart is shown in Figure 1, and the experiments in the paper include polymerization, characterization, rheological measurement, proppant suspending, gel breaking and core damage evaluation to optimization of synergistic system for deeper unconventional fracturing fluid. The details of each experiment are described below.
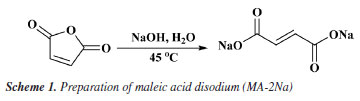
Synthesis of PAMNC12 Preparation of disodium maleate (MA-2Na) 25 g maleic anhydride was added to a flask. Then, 100 g of 20.4% NaOH solution was mixed with it. The flask was placed in a 45 ºC oil bath and stirred with a magnetic stirrer for 4 h to obtain an aqueous solution of disodium maleic acid monomer with a mass concentration of 20%. The reaction process is shown in Scheme 1.
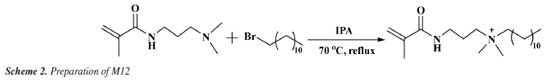
Preparation of cationic hydrophobic monomer M12 150 mL isopropanol was added to the flask. Then, 100 mmol (15.623 g) of N, N-dimethylaminopropyl acrylamide and 101 mmol (25.17 g) of bromododecane were added to the flask. The flask was placed in an oil bath at 70 ºC, stirred with a magnetic stirrer and refluxed for 8 h. The reaction process is shown in Scheme. 2. After the reaction, the solvent was removed at 50 ºC using a rotary evaporator to obtain the crude product of M12. Subsequently, the crude product was dissolved in ethyl acetate and subjected to recrystallization at -3 ºC to remove unreacted bromododecane. After three repetitions of recrystallization, the ethyl acetate was removed using a rotary evaporator at 50 ºC to obtain pure M12.
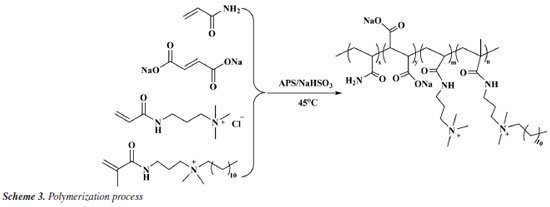
Preparation of PAMNC12 product AM, MA-2Na, and APTAC were prepared in a molar ratio of 7:1:2 to form an aqueous solution with a mass concentration of 30%. Subsequently, SDS and M12 with a mass ratio of 1% were added to obtain a monomer solution. An oxidation-reduction initiator was added to the monomer solution, with APS and NaHSO3 each accounting for 0.5% of the system. After sufficient dissolution, it was allowed to react at 45 ºC for 8 h to obtain a polymer gel block. The reaction process is shown in Scheme 3. The gel block was cut into pieces, soaked in ethanol for 2 h, taken out and dried, crushed with a grinder, and passed through an 80-mesh sieve to obtain powdered PAMNC12 product.
Characterization Infrared spectrum Potassium bromide compression method was used to prepare infrared test sample, and the IS50 Infrared Spectrometer (Thermo Fisher, US) was used to obtain infrared spectrum to characterize molecular structure. 1H NMR 1H nuclear magnetic resonance (1H NMR) spectroscopy was conducted by using 400M digital NMR spectrometer (Brooke, Germany). The sample was prepared by using deuterium water. Viscosity average molecular weight The characteristic viscosity measurement was conducted to measure the viscosity average molecular weight by using an Ubbelohde viscometer. Referring to the testing method for the intrinsic viscosity of polyacrylamide, a 1 mol L-1 sodium chloride solution and a 1000 mg L-1 polymer solution were prepared. According to the five-point dilution method, the flow time of different polymer concentration solutions was measured using an Ubbelohde viscometer at a test temperature of 25 ± 0.05 ºC. The calculation formulas for relative viscosity ηr, increased specific viscosity ηsp, and intrinsic viscosity [η] are shown in Equations 1-4.32 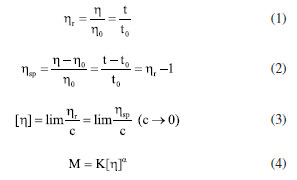 where η0 is the solvent viscosity (mPa s); T and t0 are the outflow times of the solution and solvent in the capillary (s); C is the concentration of the polymer solution (mg L-1). Critical association concentration (CAC) The HAAKE MARSIII Rheometer (Thermo Scientific, Germany) was used to measure the apparent viscosity of the solutions of PAMNC12 and VESs. With the concentration of PAMNC12 (CPAMNC12) increased, the apparent viscosity of the solutions was measured at 30 ºC and 10 s-1 to obtain the curves of CPAMNC12 vs. apparent viscosity, and the CAC values can be obtained from the curves. To compare the effect of VES type on the CAC values, the dosage of each VES in the solution was set at 200 mg L-1. Rheological test Apparent viscosity measurement By using the rotary viscometer (NDJ-95A, Shanghai, China), the apparent viscosity of the PAMNC12 solutions with VES added was measured. The rotation speed was set at 100 r min-1, and the apparent viscosity was calculated by using Equation 5.33 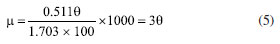 where μ represents apparent viscosity (mPa s); θ refers to the dial reading on the six-speed rotary viscometer. Viscoelastic properties test When a material is subjected to external force, some energy is stored, and some another energy is consumed by viscous deformation. This characteristic was represented by viscoelastic properties consisting of the elastic moduli (G') and viscous moduli (G"), which can be described by the Maxwell model as Equations 6 and 7.19,33 To optimize the dosage of VES, G' and G" of the PAMNC12 solutions with various VES concentrations were measured at the frequency of 1 Hz, by using the rotational rheometer (MCR 301, Anton Paar, Austria). Frequency sweep measurement was conducted, with frequency ranged from 1 to 10 Hz, to evaluate the viscoelastic properties of the solutions prepared by PAMNC12 and VESs, comprehensively. 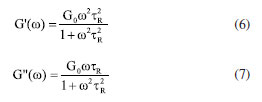 where τR is the relaxation time (s), ω is the frequency (Hz), and G0 is the plateau of the storage moduli. Drag reduction evaluation The SLSY-1849 pipeline friction test instrument (Hai'an Petroleum Research Instrument Co., Ltd, China) was used to measure drag reduction rate (DR). By measuring the pressure drop of clear water and the solutions of PAMNC12 and VESs, DR can be calculated using Equation 8.34 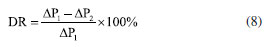 where ΔP1 refers to pressure drop of clear water (Pa); ΔP2 refers to pressure drop of slickwater (Pa). Heat and shear resistant test Heat and shear resistant tests were conducted using a HAAKE MAR III RS 600 rheometer equipped with a high pressure sealed cell (Thermo Scientific, Germany). The temperature and shear rate were set at 160 ºC and 170 s-1, respectively, and the heating and shearing process lasted for 120 min to evaluate heat and shear tolerance. Proppant suspending test The test solutions were placed in the 100 mL graduated cylinders, and then the single particle of proppant was set into the cylinder. The sediment height of proppant vs. time was recorded to measure the sediment velocity. Gel breaking and core damage test The capsule gel breaker was used to conduct gel breaking measurement at 160 ºC in the thermal aging tanks. The apparent viscosity, surface tension and residue content were measured, and the aging time was recorded. A 2 wt.% KCl solution was used to displace the cores forward with a rate of 2 mL min-1 until the pressure was balanced, and the balance pressure was maintained for 60 min, then the permeability before damage (K1) can be calculated through Darcy law. The gel breaking fluids were used to displace the cores reversely to damage the cores until the pressure reached balance, and the balance pressure was kept for 60 min. Then the forward core-flood was repeated by using 2 wt.% KCl solution to obtain the permeability after damage (K2), and the damage rates (η) were calculated by Equation 9.33 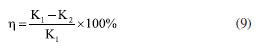
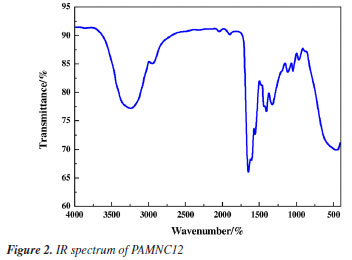
RESULTS AND DISCUSSION Characterization Infrared spectrum The molecular structure of PAMNC12 was characterized by infrared spectrum. As shown in Figure 2, there is a stretching vibration peak of N-H at 3221.5 cm-1, a stretching vibration peak of -CH2- at 2931.38 cm-1, and a stretching vibration peak of C=O at 16543.21 cm-1.
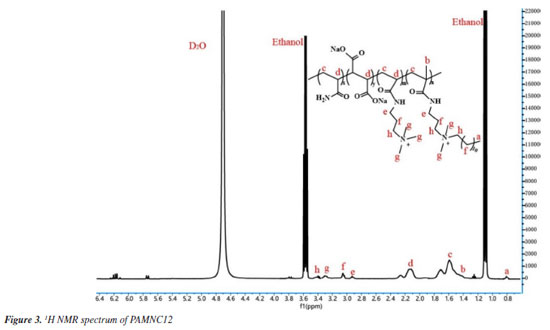
The molecular structure of PAMNC12 was also characterized by 1H NMR spectrum as shown in Figure 3. The chemical shifts corresponding to resonances of protons are as follows: d 0.81 ppm (a) for the three protons resonances of methyl (-CH2-CH2-CH3); d 1.43 ppm (b) for the three protons resonances of methyl (-C-CH3); d 1.61 ppm (c) for the two protons resonances of methylene (-CH-CH2- or -C-CH2-); d 2.13 ppm (d) for the proton resonance of methine (-CH-CH2-); d 2.95 ppm (e) for the two protons resonances of methylene (NH-CH2-); d 3.08 ppm (f) for the two protons resonances of methylene (-CH2-CH2-CH2-); d 3.31 ppm (g) for the three protons resonances of methyl (-N+-CH3); d 3.41 ppm (h) for the two protons resonances of methylene (-N+-CH2-).
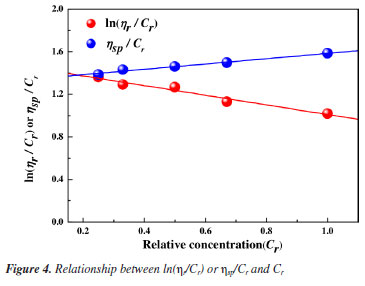
Viscosity-average molecular weight Based on the outflow time of the PAMNC12 solutions with various concentrations, the values of ln(ηr/Cr) and ηsp/Cr were calculated. The straight lines fitted by ln(ηr/Cr) and ηsp/Cr with Cr are shown in Figure 4, and the average intercept of two straight lines is 1.398. By converting the relative concentration, the value of [η] is 1398 mg L-1. Using the K value of 802 and the α value of 1.25, given in the national standard, the viscosity average molecular weight can be calculated as 4.27 × 106 g mol-1.
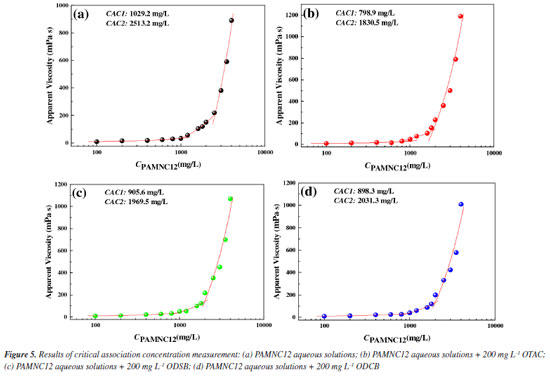
Critical association concentration (CAC) CAC refers to the lowest concentration at which hydrophobic associating water-soluble polymers can associate with themselves or with each other under specific conditions. In the paper, the CAC of PAMNC12 was tested and the effect of VES types on CAC was studied as shown in Figure 5. Without any VES added, the CAC1 and CAC2 of PAMNC12 are 1029.2 and 2513.2 mg L-1, respectively, as shown in Figure 5a. CAC1 mainly marks the transition from intramolecular association to intermolecular association. CAC2 means that a relatively complete three-dimensional network structure is formed in the system when the concentration is higher than CAC2.
As shown in Figures 5b-5d, the addition of VES can effectively lower CAC1 and CAC2 of PAMNC12, and the lower CAC values of hydrophobic associating water-soluble polymers indicate that the molecules are highly prone to association and are more likely to form 3D network structures in water to achieve thickening. To some degree, the lower CAC values of polymers can reduce usage and minimize costs when the polymers are used in fracturing fluids or EOR. Among these VESs, OTAC has the best performance in lowering CAC. This is attributed to the fact that OTAC is extremely sensitive to Cl-. A small amount of Cl- can compress the equilibrium area of OTAC at the aggregate surface and stimulate the formation of wormlike micelles. Compared with OTAC, ODSB and ODCB are insensitive to salinity since they are both betaine VESs. The length of wormlike micelles formed by ODSB and ODCB in the solutions is shorter. Thus, the degree of association in the synergistic system of PAMNC12 and ODSB or ODCB is relatively weak. The capability order of reducing CAC: OTAC > ODSB > ODCB. Rheological measurement Effect of VES concentration on apparent viscosity The effect of VES concentration on apparent viscosity of PAMNC12 aqueous solutions were studied by apparent viscosity measurement. The dosage of PAMNC12 was 3000 mg L-1 and the shear rate was set at 170 s-1. As shown in Figure 6, generally, OTAC exhibits superior thickening-assist capacity to the others, which is consistent with the CAC test results. The curves of viscosity vs. VES concentration exhibit the trends of rising first and then falling, and the peak viscosity values of the three system were all reached at the VES concentration of 400 mg L-1.
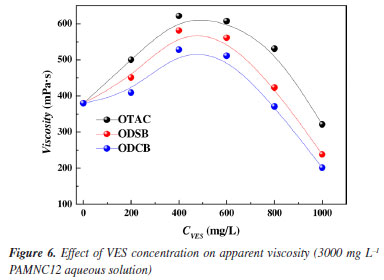
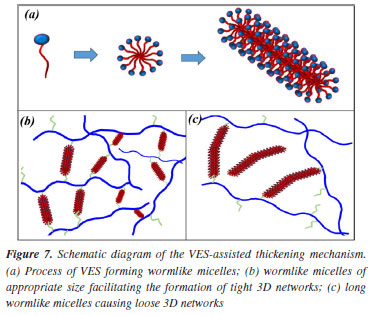
The phenomenon described above can be attributed to the change of net structures in aqueous solutions, and Figure 7 explains the VES-assisted thickening mechanism by the schematic diagram. As shown in Figure 7a, the VES molecules assemble into sphere micelles and then wormlike micelles with VES concentration increased; The wormlike micelles with suitable size assist the hydrophobic associating water-soluble polymers to construct tight net structures as shown in Figure 7b. However, with the VES concentration increased and wormlike micelle lengthening the net structure becomes loose as shown in Figure 7c.
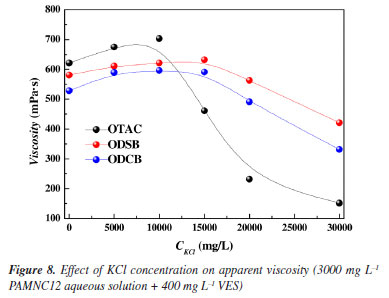
Effect of KCl concentration on apparent viscosity Figure 8 shows the effect of KCl concentration on the apparent viscosity of 3000 mg L-1 PAMNC12 aqueous solutions with 400 mg L-1 VES added. With KCl concentration increased, the apparent viscosity of solutions with OTAC added increases and then falls down sharply when beyond 10000 mg L-1. This is attributed to the extreme sensitivity of OTAC as reported34 for cationic VES, and the wormlike micelles disappear when the salinity is comparably high. As reported, betaine VES is comparable insensitive to salinity, thus the viscosity of the solutions with ODSB and ODCB added keeps stable, and starts to fall down gently when KCl concentration reaches 20000 mg L-1. The main reason for the decrease of viscosity lies in the curling of polymers caused by high salinity.
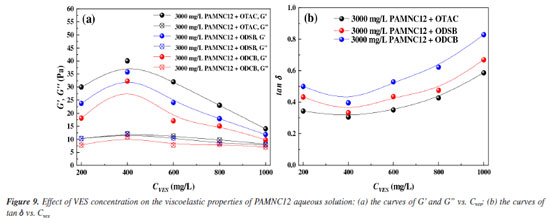
Viscoelastic properties The effect of VES concentration on viscoelastic properties was studied by measuring the G' and G" of each solution. As shown in Figure 9a, the values of G' are higher than the values of G", which indicates that the systems constructed by PAMNC12 and each VES present viscoelastic properties with elastic prominence. All solutions reach the peak values of G' at the VES concentration of 400 mg L-1, which is consistent with the effect of VES concentration on apparent viscosity. The mechanism can also be explained by Figure 7. The value of tan δ (tan δ = G"/G') represents the magnitude of the energy loss,27 and it is obvious that at the VES concentration of 400 mg L-1 each solution prepared by PAMNC12 and VES exhibits the lowest energy loss, which indicates that the VES concentration of 400 mg L-1 can result in the best net structure constructed in PAMNC12 aqueous solutions.
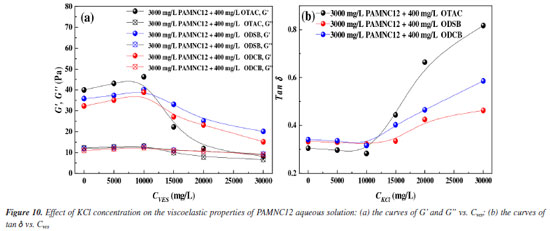
By measuring the G' and G'' of each solution, the effect of KCl concentration on viscoelastic properties was also studied as shown in Figure 10. The results are consistent with the effect of KCl concentration on apparent viscosity. The synergistic system of PAMNC12 and OTAC exhibits the best viscoelastic properties at the KCl concentration of 10000 mg L-1. With further increase of KCl concentration, the energy loss of the synergistic system of PAMNC12 and OTAC increases sharply, which means the net structure in aqueous solution is broken and lose most energy storage capacity.
Therefore, through the study of VES and KCl concentration effect on the viscoelastic properties of the synergistic system solutions, the optimal VES and KCl dosage should be 400 and 10000 mg L-1, respectively. With the PAMNC12 dosage of 3000 mg L-1, the dynamic frequency sweep of each synergistic system solution was conducted, and the results are shown in Figure 11. The values of G' and G" increase with the frequency increased from 0.1 to 10 Hz generally, and the value of G' is always much higher than the value of G", which indicate the elastic properties dominate in all synergistic system solutions. Among these systems, the synergistic system of PAMNC12 with OTAC exhibits the best viscoelastic properties.

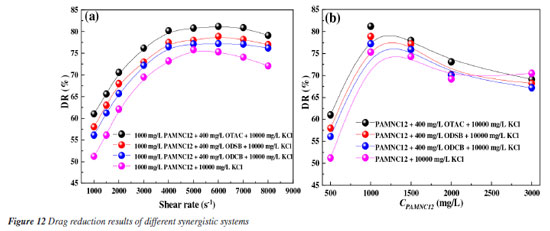
Drag reduction rate Drag reduction rate is a crucial index to evaluate the performance of fracturing fluid, especially for unconventional reservoir fracturing operation that requires great pump rate. With shear rate increased from 1000 to 8000 s-1, the drag reduction rates of the three synergistic system solutions and the blank sample (PAMNC12 solution without VES added) were measured and the results are shown in Figure 12a. It is obvious that the synergistic systems own superior drag reduction performance to the blank sample, which is attributed to the great elastic properties. As reported,2 the elasticity of fluid can assist to store turbulent energy and release it to promote fluid flow. With shear rate increased, the drag reduction rate increases because the friction resistance of clean water increases significantly. As proven above, the synergistic system of PAMNC12 and OTAC owns the best elastic properties and least energy loss among the three synergistic systems, thus it exhibits the best drag reduction rate. When the shear rate is 6000 s-1, the drag reduction of the synergistic system of PAMNC12 and OTAC reached the peak value of 81.2%.
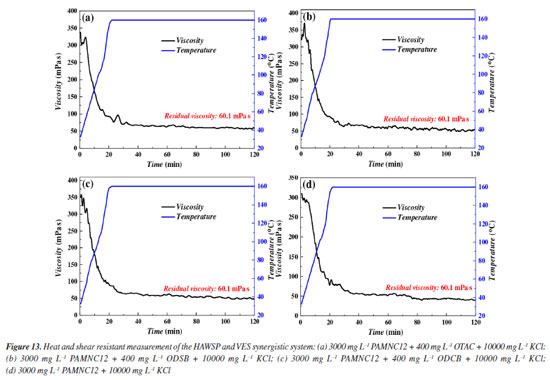
As is known, the higher apparent viscosities also cause large friction during pipe flowing, and the dosage of polymer is the key factor for the apparent viscosity. Thus, the drag reduction rates vs. PAMNC12 concentration were measured as shown in Figure 12b, and it is shown that the drag rate increases and then fall down. The peak value is at 1000 mg L-1. This is because the net structure is not tight enough to impart elastic properties to the fluid when CPAMNC12 is lower than 1000 mg L-1. When CPAMNC12 is above 1000 mg L-1, the high apparent viscosity causes large flowing friction. Heat and shear resistance test Due to the high temperature of oil and gas reservoir and high shear rate during pumping in the pipe, it is very crucial that fracturing fluids owns sufficient heat and shear resistance to ensure the proppant carrying capacity in fractures. The heat and shear resistance tests of the three synergistic systems and the blank sample (PAMNC12 solution without VES added) were conducted and the results are shown in Figure 13. After 120 min of shear at 170 s-1 and 160 ºC, the synergistic systems of PAMNC12/OTAC, PAMNC12/ODSB, and PAMNC12/ODCB have residual viscosities of 60.1, 55.1, and 50.4 mPa s, respectively, and the residual viscosity of the blank sample is only 38 mPa s, which indicates that the VESs act as excellent auxiliary agents to improve heat and shear resistance. Among these VESs, OTAC owns the best synergy with PAMNC12 which leaves a residual viscosity above 60 mPa s under 170 s-1 and 160 ºC after 120 min shearing.
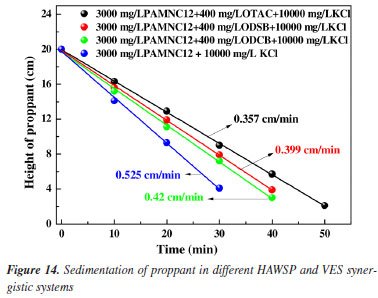
Proppant suspending The proppant suspending capacities of the three synergistic systems and the blank sample (PAMNC12 solution without VES added) were measured under 95 ºC in the cylinders. Heights of proppant in the cylinders vs. time were recorded, and the settlement velocities are calculated by linear fitting as shown in Figure 14. The settlement velocities in the synergistic systems of PAMNC12/OTAC, PAMNC12/ODSB, and PAMNC12/ODCB are 0.357, 0.399 and 0.420 cm min-1, respectively, and the settlement velocity in the blank sample is 0.525 cm min-1, which indicates that the PAMNC12/OTAC synergistic system owns the best proppant suspending capacity.
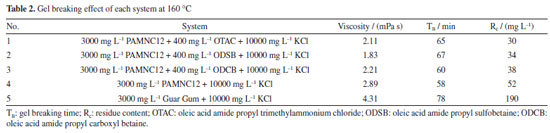
Gel breaking performance With 500 mg L-1 capsuled APS added, the gel breaking measurements were conducted under 160 ºC in the thermal aging tanks. The results are listed in Table 2, the gel breaking fluids of the three synergistic systems all exhibit low viscosity, short gel breaking time (TB) and low residue content (Rc). The gel breaking fluid of blank sample also has low viscosity and short TB, but the Rc is comparably higher because no surfactant was added, and the solubility of polymer fragments is lower. The guar gum system (3000 mg L-1 Guar Gum + 10000 mg L-1 KCl) was used as comparable sample, and the viscosity, TB and Rc are all much higher than those of the other systems. It is obvious that PAMNC12/OTAC system owns the best comprehensive gel breaking performance.
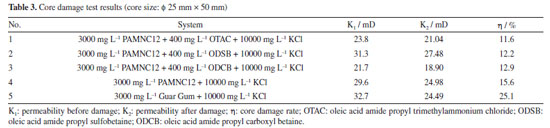
Core damage evaluation Through core-flood experiments, the core damages were evaluated, and the core damage rates (η) caused by the three synergistic systems and the blank sample (PAMNC12 solution without VES added) were all below 20% as shown in Table 3, among which the gel breaking fluid of PAMNC12/OTAC system causes only 11.6% damage rate. However, the gel breaking fluid of the traditional guar gum system caused 25.1% damage rate. It is obvious that the three synergistic systems caused lower damage rate than the PAMNC12 solution without VES added and the traditional guar gum system.
CONCLUSIONS According to the results of FTIR and 1H NMR, the zwitterionic HAWP, named PAMNC12, was successfully synthesized, with a viscosity average molecular weight of 4.27 × 106 g mol-1, and CAC1 and CAC2 of PAMNC12 were 1029.2 and 2513.2 mg L-1, respectively. The synergistic effect of PAMNC12 with VESs was investigated systematically, and the synergistic systems were optimized as fracturing fluid for deeper unconventional reservoir with temperature above 160 ºC. OTAC performs best among the three VESs, and it can lower the CAC1 and CAC2 to 798.9 and 1830.5 mg L-1, respectively. In the synergistic systems of PAMNC12 and VESs, the optimal dosage of VESs and KCl were 400 and 10000 mg L-1, respectively, to assist thickening capacity and improve elastic properties, and PAMNC12/OTAC synergistic system is the best synergistic system to construct the tight net structures and improve rheological properties. Meanwhile, as to the proppant-carrying capacity, heat and shear resistance, gel breaking and core damage, PAMNC12/OTAC synergistic system still performs best due to the toughest association force, which is the optimized synergistic system used as fracturing fluid for deeper unconventional reservoir stimulation. The drag reduction under the shear rate of 6000 s-1 can reach 81.2%, and its heat resistance reaches 160 ºC, which can meet the requirement of large pump rate during massive hydraulic fracturing for deeper unconventional reservoir. The core damage rates caused by the synergistic system was just 11.6%, much lower than the single polymer systems, especially the guar gum system. The experimental results proved that the optimized PAMNC12/OTAC synergistic system provides an excellent access to develop the deeper unconventional reservoir with high temperature.
REFERENCES 1. Al-Hajri, S.; Negash, B. M.; Rahman, M. M.; Haroun, M.; Al-Shami, T. M.; ACS Omega 2022, 7, 7431. [Crossref] 2. Yang, B.; Zhao, J.; Mao, J.; Tan, H.; Zhang, Y.; Song, Z.; J. Nat. Gas Sci. Eng. 2019, 62, 302. [Crossref] 3. Bahamdan, A.; Daly, W. H.; Polym. Adv. Technol. 2007, 18, 652. [Crossref] 4. Banerjee, C.; Ghosh, S.; Sen, G.; Mishra, S.; Shukla, P.; Bandopadhyay, R.; Carbohydr. Polym. 2013, 92, 675. [Crossref] 5. Jiang, Y.; Liang, W.; Lian, H.; He, W.; International Journal of Rock Mechanics and Mining Sciences 2025, 186, 106013. [Crossref] 6. Yang, Y.; Li, X.; Yang, X.; Li, X.; J. Pet. Sci. Eng. 2022, 219, 111081. [Crossref] 7. Liu, Y.; Wang, F.; Wang, Y.; Li, B.; Zhang, D.; Yang, G.; Zhi, J.; Sun, S.; Wang, X.; Deng, Q.; Xu, H.; Petroleum Exploration and Development 2022, 49, 864. [Crossref] 8. Bian, X.; Zhang, S.; Zhang, J.; Wang, D.; Petroleum Exploration and Development 2015, 42, 705. [Crossref] 9. Liang, T.; Achour, S. H.; Longoria, R. A.; DiCarlo, R. A.; Nguyen, Q. P.; J. Pet. Sci. Eng. 2017, 157, 631. [Crossref] 10. Zhang, W.; Wan, L.; Fan, Y.; Zhang, X.; Zhou, J.; Chen, Y.; Li, H.; Liu, X.; Zhang, Y.; Wang, L.; J. Mol. Liq. 2023, 390, 123168. [Crossref] 11. Al-Taq, A.; Aljawad, M.; Alade, O.; Al-Ajwad, H.; Murtaza, M.; Al-Abdrabalnabi, R.; Alrustum, A.; Mahmoud, M.; Geoenergy Sci. Eng. 2024, 242, 213260. [Crossref] 12. Makki, S.; Maalouf, E.; Yehya, A.; Heliyon 2025, 11, e40883. [Crossref] 13. Yang, D.; Yang, B.; Chen, P.; Tang, Q.; Yang, B.; Li, W.; Zhong, Y.; Wang, Y.; Zhang, H.; Chem. Eng. J. 2024, 499, 156456. [Crossref] 14. Xu, Z.; Zhao, M.; Sun, N.; Meng, X.; Yang, Z.; Xie, Y.; Ding, F.; Dong, Y.; Gao, M.; Wu, Y.; Li, L.; Dai, C.; Colloids Surf., A 2025, 708, 135967. [Crossref] 15. Wu, Y.; Yan, X.; Huang, Y.; Zhao, M.; Zhang, L.; Dai, C.; Energy 2024, 293, 130632. [Crossref] 16. Lin, X.; Zhang, S.; Wang, Q.; Feng, Y.; Shuai, Y.; J. Nat. Gas Sci. Eng. 2015, 25, 367. [Crossref] 17. Zhang, C.; Wang, Y.; Yin, Z.; Yan, Y.; Wang, Z.; Wang, H.; Int. J. Biol. Macromol. 2024, 277, 134445. [Crossref] 18. Zhang, W.; Wang, L.; Li, H.; Xu, P.; Yi, F.; Chen, Y.; Liu, X.; Wang, L.; J. Mol. Liq. 2023, 385, 122275. [Crossref] 19. Yang, C.; Hu, Z.; Song, Z.; Bai, J.; Zhang, Y.; Luo, J.; Du, Y.; Jiang, Q.; J. Appl. Polym. Sci. 2017, 134, 44602. [Crossref] 20. Mao, J.; Yang, X.; Wang, D.; Li, D. Y.; Zhao, J.; RSC Adv. 2016, 6, 88426. [Crossref] 21. Samuel, M.; Card, R. J.; Nelson, E. B.; Brown, J. E.; Vinod, P. S.; Temple, H. L.; Fu, D. K.; SPE Drill. Completion 1999, 14, 240. [Crossref] 22. Sing, M. K.; Wang, Z.-G.; McKinley, G. H.; Olsen, B. D.; Soft Matter 2015, 11, 2085. [Crossref] 23. Chu, Z.; Feng, Y.; Chem. Commun. 2010, 46, 9028. [Crossref] 24. Pucci, A.; Bizzarri, R.; Ruggeri, G.; Soft Matter 2011, 7, 3689. [Crossref] 25. Moon, J. R.; Jeon, Y. S.; Chung, D. J.; Kim, D.; Kim, J.-H.; Macromol. Res. 2011, 19, 515. [Crossref] 26. Zhang, W.; Li, H.; Fan, Y.; Lv, M.; Zhou, Y.; Wang, L.; Wang, L.; Yang, X.; J. Mol. Liq. 2024, 415, 126408. [Crossref] 27. Yang, D.; Yang, B.; Ren, M.; Liu, Y.; Cao, H.; Jiang, Z.; Zhang, H.; J. Mol. Liq. 2023, 377, 121546. [Crossref] 28. Pu, W.; Du, D.; Liu, R.; J. Pet. Sci. Eng. 2018, 167, 568. [Crossref] 29. Zhao, J.; Yang, B.; Mao, J.; Zhang, Y.; Yang, X.; Zhang, Z.; Shao, Y.; Energy Fuels 2018, 32, 3039. [Crossref] 30. Volpert, E.; Selb, J.; Candau, F.; Polymer 1998, 39, 1025. [Crossref] 31. Cao, W.; Xie, K.; Lu, X.; Chen, Q.; Tian, Z.; Lin, W.; J. Pet. Sci. Eng. 2020, 192, 107251. [Crossref] 32. Lodge, T. P.; Hiemenz, P. C.; Polymer Chemistry, 3rd ed.; CRC Press: Boca Raton, 2020. [Crossref] 33. Zhang, W.; Mao, J.; Yang, Y.; Chai, H.; Shuang, Z.; Wang, L.; Yang, X.; Wang, L.; ChemistrySelect 2024, 9, e202400399. [Crossref] 34. Tan, H.; Mao, J.; Zhang, W.; Yang, B.; Yang, X.; Zhang, Y.; Lin, C.; Feng, J.; Zhang, H.; Polymers 2020, 12, 955. [Crossref]
Associate Editor handled this article: Boniek G. Vaz |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access