Artigo
| Antimicrobial activity of compounds isolated from mangrove-derived endophytic fungus Actinomucor elegan against Xanthomonas axonopodis pv. passiflorae |
|
Kathia Raquel Murillo PadillaI; Rayanne Priscila Vieira da ConceiçãoI; Gisele da Costa RamosI; Luana Cardoso de OliveiraII, I. Programa de Pós-Graduação em Química, Instituto de Ciências Exatas e Naturais, Universidade Federal do Pará, 66075-110 Belém - PA, Brasil Received: 02/24/2025 *e-mail: andrey@ufpa.br This study investigated bioactive compounds isolated from the mangrove endophytic fungus Actinomucor elegans AcCC18.1A for the control of Xanthomonas axonopodis pv. passiflorae (Xap), the causal agent of bacterial spot disease in passion fruit. Through classical chromatographic techniques, we isolated four phenolic compounds: tyrosol (1), 4-hydroxyphenylacetic acid (2), 4-formylphenyl-2-phenylacetate (3), and phenylacetic acid (4), with their structures elucidated through nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). Among the isolated compounds, compound 3 was characterized as a new natural product. Antimicrobial assays were performed using the broth microdilution method against four Xap strains (Xap1-Xap4), with amoxicillin (3.9 µg mL-1) and tetracycline (3.9 µg mL-1) as positive controls. All compounds exhibited inhibitory activity, with compound 2 showing the highest efficacy (7.8 µg mL-1). These results underscore the biotechnological potential of mangrove endophytic fungi as sources of bioactive molecules against agricultural phytopathogens, offering new insights for developing sustainable strategies to control bacterial spot in passion fruit cultivation. INTRODUCTION Mangroves are coastal ecosystems located between marine and terrestrial environments, exposed to tidal regimes and high salinity.1 They represent 8% of the ecosystems along the planet's coastlines, with over two-thirds of the remaining area concentrated in just eighteen countries.2 Brazil stands out as having the second-largest mangrove area globally, covering approximately 14,000 km2 along its coast, second only to Indonesia.3,4 Mangrove forests are recognized as biodiversity-rich habitats due to their unique environment, harboring a variety of plants and microorganisms, representing the second-largest ecological group of marine fungi.5,6 In the state of Pará, located in the Northern Amazon region of Brazil, mangrove vegetation dominates the entire coastal plain, extending inland along estuarine channels.7 This vegetation develops in areas influenced by saline and brackish waters, comprising characteristic species of the genera Rhizophora, Avicennia, and Laguncularia, among others.8 Rhizophora mangle L. (Rhizophoraceae), commonly known as red mangrove, is a widely distributed species in mangrove ecosystems.9 Its pharmacological potential stems from therapeutic properties such as antioxidants,10 antimicrobial,11 and cytotoxic activities, attributed to the presence of secondary metabolites, particularly polyphenols.12 Furthermore, R. mangle hosts a diversity of endophytic fungi with significant biotechnological potential.13 Endophytic fungi live in symbiosis with host plants.14 This association leads to the production of bioactive microbial metabolites that can protect plants against pathogenic microorganisms.15 In mangrove ecosystems, highly competitive environments, this chemical diversity is particularly significant,16-19 as demonstrated by Chen et al.,20 who identified 1,378 bioactive molecules from 325 fungi over three decades. These findings underscore the vast potential of microbial metabolites in the bioprospecting of novel antibacterial agents, offering new strategies to combat resistant pathogens, including the control of diseases caused by phytopathogenic agricultural bacteria.13,20 Phytopathogenic bacteria of the genus Xanthomonas pose a serious threat to the agricultural sector, leading to reduced productivity and significant economic losses.21 Bacterial spot, a disease caused by Xanthomonas axonopodis pv. passiflorae, primarily affects passionfruit crops, causing superficial lesions that can penetrate the pulp and lead to fruit rot.22 The spread of this disease has severely constrained passionfruit production in many orchards, largely due to its resistance to conventional control methods.23 Thus, research on mangrove-derived endophytic fungi demonstrates that these microorganisms represent valuable sources of bioactive compounds.20 Investigations focusing on chemical diversity and bioactivity offer a promising strategy for discovering molecules with activity against phytopathogens.19 In this study, we conducted chemical and biological analyses of four bioactive compounds isolated from the endophytic fungus A. elegans AcCC18 (obtained from mangroves) against four strains of Xanthomonasaxonopodis pv. passiflorae (Xap), aiming to control bacterial spot in passionfruit (Passiflora spp.), an economically important crop in the agricultural sector.
EXPERIMENTAL Microorganism For this study, we used mangrove-derived endophytic fungus A. elegans AcCC18.1A obtained from the Bioassay and Microorganism Chemistry Laboratory collection at the Federal University of Pará, for extract preparation and compounds isolation. The strains of X. axonopodis pv. passiflorae used in the tests were obtained from passion fruit plants infected by bacterial spot in crops from four different cities in Pará State, Brazil, which were: Xap1 - X. axonopodis pv. passiflorae PA1 (Igarapé-Açu), Xap2 - X. axonopodis pv. passiflorae PA5.2 (Maracanã), Xap3 - X. axonopodis pv. passiflorae PA18 (São Francisco do Pará), Xap4 - X. axonopodis pv. passiflorae PA20 (Tomé-Açu).24 The access to genetic material was registered in SISGEN (National System for the Management of Genetic Heritage) under accession number AE88252A. Culture of Actinomucor elegans on rice and compound isolation Mangrove-derived endophytic fungus A. elegans AcCC18.1A was reactivated in Petri dishes containing potato dextrose agar (PDA) medium culture for 14 days. Afterward, ten Erlenmeyer flasks (1000 mL) containing 200 g of rice and 100 mL of distilled water per flask were autoclaved for 45 min at 121 ºC. Small cubes of PDA medium containing mycelium of A. elegans AcCC18.1A were added in 7 Erlenmeyer flasks under sterile conditions. Three flasks were used as controls. After 25 days of incubation at 25 ºC, the biomass obtained was macerated with ethanol. After filtration, the solution obtained was concentrated in a rotary evaporator to obtain the biomass extracts (10.3 g). Part of the ethanol extract (6.1 g) was partitioned with ethyl acetate and hexane, yielding acetate phase (3 g) and phase hexanic (1 g) extracts after the concentration of the solutions. The ethyl acetate phase extract was fractionated on silica gel chromatographic column eluted with hexane, ethyl acetate and methanol in a polarity gradient, yielding the compounds 1-4. Antimicrobial assays The isolated compounds were tested at concentrations from 250 to 3.125 µg mL-1 using the broth microdilution method.25 In 96-well plates, 100 μL of the liquid medium BHI (brain heart infusion) was added to each well. Then, each sample was added to the first well of each column, obtaining a concentration of 250 µg mL-1. Then successive dilution was performed to obtain 3.125 µg mL-1 in the penultimate well. After, 5 μL of the bacterial suspension was added to each well and the plates were incubated for 24 h at 35 ºC. A randomized experimental design was used with three replicates. The amoxicillin and tetracyclin were used as positive controls. The activity was evaluated by adding 10 μL of 2% TTC (2,3,5-triphenyltetrazolium chloride).26 Then, to determine the type of activity, the sample was re-inoculated in Petri dishes containing solid medium BHI and incubated for 48 h at 35 ºC. When bacterial growth was observed, it was indicated that the extract and/or compound presented bacteriostatic effects at that concentration. When there was no bacterial growth, the extract and/or compound was indicated as presenting a bactericidal effect. NMR and MS data ESIMS (electrospray ionization mass spectrometry) data were acquired in positive and negative ion mode using a Thermo-Fisher Orbitrap HRLC-MS instrument. 1D and 2D nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Ascend 400, using solvent (deuterated chloroform) signal as reference. Its 1H NMR frequency was 400 MHz, while for 13C NMR the frequency was 100 MHz. The chemical shifts are given in delta (d) values and the coupling constants (J) in hertz (Hz).
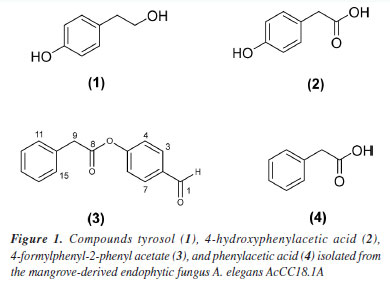
RESULTS AND DISCUSSION Isolated compounds Four compounds were isolated via silica gel column chromatography. Compounds were identified by NMR and mass spectrometry and compared with literature data for tyrosol (1), 4-hydroxyphenylacetic acid (2), 4-formylphenyl-2-phenyl acetate (3) and phenylacetic acid (4) (Figure 1).27-29
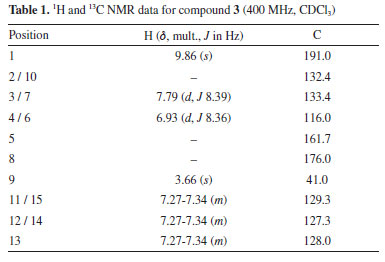
The molecular formula of compound 3 was determined as C15H12O3 using the HRESI (+) m/z 241.0860 [M + H]+. Mass spectrum (Figure 1S) and NMR spectra (Figures 2S-6S) are provided in the Supplementary Material. The 1H NMR spectrum showed signals typical for aromatic compounds as two doublets at δH 7.79 (2H, dd, J 8.39 Hz, H-3/H-7) and δH 6.95 (2H, δ, J 8.36, 4.28 Hz, H-4/H-6) compatible for a p-substituted aromatic ring; a multiplet signal was also observed at δH 7.30 (5H, m) for a mono-substituted aromatic ring. Singlet signal observed at δH 3.66 (2H, s, H-9) was consistent with benzyl CH2 protons and a singlet at δH 9.85 (1H, s, H-1) typical of the hydrogen of the aldehyde group. 13C NMR spectrum of 3 show signals corresponding to 15 carbons, of which 12 are aromatic carbons and 3 are aliphatic carbons. Analysis of the HSQC (heteronuclear single quantum coherence) spectrum reveals correlations of the signals at δH 7.29 with the carbons δC 129.4, 127.3, 128.6; of the doublets signals δH 6.95 (H-4/H-6) with δC 116.0 and δH 7.79 (H-3/H-7) with δC 133.4; of the singlet at δH 3.66 (H-9) with δC 41.0. The correlation of the H 9.85 (H-1) singlet with the carbon signal at δC 191.0 confirms the aldehyde carbonyl group. The signal at δC 176.0 shows no HSQC correlation and suggests the presence of an acetate group. These analyses evidence the presence of two aromatic systems in the compound, which are linked by an acetate group. To determine the order of connectivity to obtain the structure of compound 3, COSY (correlation spectroscopy) and HMBC (heteronuclear multiple bond correaltion) spectra were analyzed. Following the HMBC spectrum, a correlation of δH 7.79 (H-3/H-7) with the carbon of the aldehyde carbonyl δC 191.0 was observed. The COSY experiment revealed correction H-3/H-7 with H-4/H-6 with a coupling of 8.2 Hz, suggesting that the hydrogens are ortho-correlated. These correlations show that the aromatic ring A is p-substituted and the aldehyde group is attached to this ring. The methylene hydrogen at δH 3.66 (2H, s, H-9) correlates in COSY with the aromatic hydrogens of the mono-substituted ring at δH 7.30. Also, in HMBC, H-9 correlates with the carbons of the mono-substituted ring and with the carbon of the acetate carbonyl (dC 176.0) completing ring B. COSY and HMBC correlations observed are shown in Figure 2.
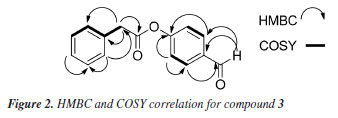
Table 1 shows 1H and 13C NMR data for 3. Based on the NMR and MS spectrometric analysis, it was possible to propose the structure of 3. Compound 3 was identified as 4-formylphenyl-2-phenyl acetate and it is the first time that 3 has been isolated as a natural product.
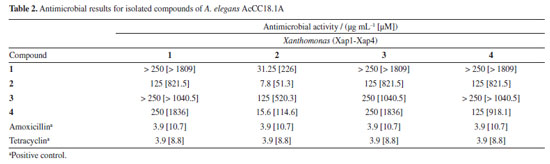
Antimicrobial assays The compounds were tested against four strains of X. axonopodis pv. passiflorae, isolated from plants infected with bacterial spot in different locations of Pará State, Brazil.24 Compound 2 showed significant activity at the lowest tested concentration among the compounds. The results are summarized in Table 2.
There are few reports on chemical studies of A. elegans AcCC18.1A, so this work contributes to the understanding of the secondary metabolites produced by A. elegans. In a study by Yin et al.,30 a significant increase in total and soluble phenolic along with a decrease in insoluble phenolic content in tofu were observed after fermentation with A. elegans. In our studies, phenolic compounds derived from tyrosol were obtained. Phenolics are ubiquitous secondary metabolites. They are aromatic compounds synthesized by phenylpropanoid pathway. Phenolics have been the focus of many findings on plant-defenses mechanisms to pathogens, including bacteria, fungi, and viruses, and major abiotic stresses like drought, UV and salinity.31 These findings align with our study, as the fungus A. elegans AcCC18.1A is a mangrove-derived fungus that lives in high levels of salinity and produces phenolic compounds as its main secondary metabolites. The main beneficial properties of phenolic compounds are their antioxidant effects, which contribute to their potential health benefits. These include the prevention of cancer, cardiovascular diseases, and neurodegenerative disorders such as Alzheimer's.32 In addition to their antioxidant activity, phenolic compounds have demonstrated antimicrobial properties.33 In this study, we isolated the phenolics compounds tyrosol (1), 4-hydroxyphenylacetic acid (2), 4-formylphenyl-2-phenyl acetate (3), and phenylacetic acid (4), with compound 4-formylphenyl-2-phenyl acetate first reported as a natural product. Tyrosol is a simple phenolic compound of natural origin that plays a defensive role in plants against pathogens and herbivores.34 Numerous studies35,36 highlight its antioxidant, anti-inflammatory, anticarcinogenic, antiplatelet, antimicrobial and antifungal activities. 4-Hydroxyphenylacetic acid (4-HPCA) is another phenolic compound with demonstrated antioxidant and antimicrobial effects. Notably, 4-HPCA exhibits strong antimicrobial activity against Listeria monocytogenes, inhibiting its growth in a dose-dependent manner.37 Phenylacetic acid showed antimicrobial activity against various bacteria and yeasts, including Staphylococcus aureus, Escherichia coli, and Candida albicans.38 Several studies have reported the antimicrobial activity of phenolic compounds and their derivatives against animal pathogens. However, a few investigations have explored their potential use of phenolic compounds against bacteria that affect crops, such as bacteria of the genus Xanthomonas, which cause approximately 350 plant diseases worldwide and significantly reduce crop yields.21 The heat-treated extract of palm dates showed phenolic compounds like tyrosol as the main constituents, which showed activity against X. campestris.39 Abo-Elyousr et al.40 have shown the induction of phenolic compounds by salicylic and benzoic acids for controlling the common blight of beans caused by X. axonopodis pv. phaseoli. Fan et al.41 reported that phenolic compounds can suppress the disease symptoms of X. oryzae pv. oryzae and X. oryzae pv. oryzicola on rice. These studies show that the use of phenolic compounds can be a good strategy for combating diseases caused by bacteria of the Xanthomonas genus. However, there are no reports of the activity of phenolic compounds derived from tyrosol against X. axonopodis pv. passiflorae, which causes bacterial spot on passion fruit. The passion fruit crop is one of the most affected by this bacterium. Due to its rapid propagation, management difficulties, problems with chemical control, and the severity of crop losses, the control of Xanthomonas is a difficult obstacle to be surpassed for agriculture worldwide.22 In a previous work of our group,24 the ACN extract of the fungus G. mangiferae showed a good inhibition of X. axonopodis pv. passiflorae and, also active compounds sydowinol and sydowinin A were isolated. In this study, all the compounds were able to inhibit the growth of the bacterium X. axonopodis pv. passifloraein vitro, especially compound 2, which was active up to 7.8 µg mL-1. The results obtained for the compounds isolated from mangrove-derived endophytic fungus A. elegans AcCC18.1A are promising and demonstrate the importance of studies aimed at finding bioactive compounds from mangrove-derived endophytic fungus, that can be used as prototypes for the development of natural pesticides.
CONCLUSIONS Fractionation of the biomass extract from the mangrove-derived endophytic fungus A. elegans AcCC18.1A yielded four compounds: tyrosol (1), 4-hydroxyphenylacetic acid (2), 4-formylphenyl-2-phenyl acetate (3), and phenylacetic acid (4). Compound 3 is reported here for the first time as a natural product. All tested compounds demonstrated in vitro inhibitory effects against X.axonopodis pv. passiflorae, with compound 2 showing the highest efficacy. These findings highlight the potential of mangrove-derived fungal metabolites as sustainable alternatives to conventional synthetic pesticides, offering eco-friendly strategies to combat bacterial spot, while reducing environmental and public health risks.
SUPPLEMENTARY MATERIAL Spectrometric information (mass and NMR) for compounds 3 are provided in Figures 1S-6S, available at http://quimicanova.sbq.org.br/, as a PDF file, with free access.
DATA AVAILABILITY STATEMENT All data are available in the text.
ACKNOWLEDGMENTS This research was funded by the Fundação Amazônia de Amparo a Estudos e Pesquisa do Pará (FAPESPA), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant 308631/2021-8), Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES). P. S. B. Marinho is grateful to CNPq (grant: 406269/2022-0). A. M. R. Marinho is grateful to CNPq (grant: 310540/2022-4).
REFERENCES 1. Friess, D. A.; Rogers, K.; Lovelock, C. E.; Krauss, K. W.; Hamilton, S. E.; Lee, S. Y.; Lucas, R.; Primavera, J.; Rajkaran, A.; Shi, S.; Annual Review of Environment and Resources 2019, 44, 89. [Crossref] 2. Barbier, E. B.; Mar. Pollut. Bull. 2016, 109, 676. [Crossref] 3. de Lacerda, L. D.; Ward, R. D.; Godoy, M. D. P.; Meireles, A. J. A.; Borges, R.; Ferreira, A. C.; Frontiers in Forests and Global Change 2021, 4, 653096. [Crossref] 4. Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio); Atlas dos Manguezais do Brasil; ICMBio: Brasília, 2018, p. 176. 5. Nagelkerken, I.; Blaber, S. J. M.; Bouillon, S.; Green, P.; Haywood, M.; Kirton, L. G.; Meynecke, J.-O.; Pawlik, J.; Penrose, H. M.; Sasekumar, A.; Somerfield, P. J.; Aquat. Bot. 2008, 89, 155. [Crossref] 6. Liu, Y.; Xue, X.; Zhou, L.; Yang, W.; She, Z.; Liao, Q.; Feng, Y.; Chen, X.; Zhang, Y.; Chem. Biodiversity 2023, 20, e202300735. [Crossref] 7. Barbosa, I. C. C.; Müller, R. C. S.; Alves, C. N.; Berrêdo, J. F.; Souza Filho, P. W. M.; Rev. Virtual Quim. 2015, 7, 1087. [Crossref] 8. Da Cruz, C. C.; Mendoza, U. N.; Queiroz, J. B.; Berrêdo, J. F.; Da Costa Neto, S. V.; Lara, R. J.; Plant Soil 2013, 370, 393. [Crossref] 9. DeYoe, H.; Lonard, R. I.; Judd, F. W.; Stalter, R.; Feller, I.; J. Coastal Res. 2020, 36, 857. [Crossref] 10. Damm, B. M.; Schaffel, I. F.; dos Santos, G. F. S.; Azevedo, L. E. S.; Ferreira, R. Q.; de Moura, P. R. G.; J. Chem. Educ. 2023, 100, 4449. [Crossref] 11. Pontes, A. L. S.; Leal, C. M.; Lucas, M. P.; da Silva, G. C. M.; Peixoto, J. V. B. A.; Succar, J. B.; Flores, V. R.; Direito, I. C. N.; da Silva, A. J. R.; Chaves, F. O.; Fingolo, C. E.; Chem. Biodiversity 2024, 21, e202400687. [Crossref] 12. Regalado, A. I.; Sánchez, L. M.; Mancebo, B.; J. Pharm. Pharmacogn. Res. 2016, 4, 1. [Crossref] 13. Wu, J.; Xiao, Q.; Xu, J.; Li, M.-Y.; Pan, J.-Y.; Yang, M.; Nat. Prod. Rep. 2008, 25, 955. [Crossref] 14. Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D.; Appl. Microbiol. Biotechnol. 2019, 103, 3327. [Crossref] 15. Kusari, S.; Hertweck, C.; Spiteller, M.; Chem. Biol. 2012, 19, 792. [Crossref] 16. Lu, P.; Shi, Y.; Zhang, J.; Hong, K.; Xue, Y.; Liu, L.; Int. J. Biol. Macromol. 2024, 257, 128808. [Crossref] 17. Yuan, Y.; Wang, G.; She, Z.; Chen, Y.; Kang, W.; Fitoterapia 2023, 171, 105692. [Crossref] 18. Cadamuro, R. D.; Bastos, I. M. A. S.; Silva, I. T.; da Cruz, A. C. C.; Robl, D.; Sandjo, L. P.; Alves Jr., S.; Lorenzo, J. M.; Rodríguez-Lázaro, D.; Treichel, H.; Steindel, M.; Fongaro, G.; J. Fungi 2021, 7, 455. [Crossref] 19. Li, K.; Chen, S.; Pang, X.; Cai, J.; Zhang, X.; Liu, Y.; Zhu, Y.; Zhou, X.; Eur. J. Med. Chem. 2022, 230, 114117. [Crossref] 20. Chen, S.; Cai, R.; Liu, Z.; Cui, H.; She, Z.; Nat. Prod. Rep. 2022, 39, 560. [Crossref] 21. Stefani, E.; Obradović, A.; Gašić, K.; Altin, I.; Nagy, I. K.; Kovács, T.; Microorganisms 2021, 9, 1056. [Crossref] 22. Marin, V. R.; Ferrarezi, J. H.; Vieira, G.; Sass, D. C.; World J. Microbiol. Biotechnol. 2019, 35, 72. [Crossref] 23. de Oliveira, L. C.; Nakasone, A. K.; Silva, C. S.; Carvalho, K. B. A.; Rev. Ceres 2023, 70, 124. [Crossref] 24. de Oliveira, L. C.; da Costa, W. C. L.; Vinagre, V. G.; Siqueira, J. E. S.; Silva, S. C.; Silva, S. Y. S.; Marinho, A. N. R.; Rocha, D. C. C.; Marinho, P. S. B.; Nakasone, A. K.; Marinho, A. M. R.; Agronomy 2022, 12, 1690. [Crossref] 25. Ramos, G. C.; Silva-Silva, J. V.; Watanabe, L. A.; Siqueira, J. E. S.; Almeida-Souza, F.; Calabrese, K. S.; Marinho, A. M. R.; Marinho, P. S. B.; de Oliveira, A. S.; Antibiotics 2022, 11, 1332. [Crossref] 26. Gabrielson, J.; Hart, M.; Jarelöv, A.; Kühn, I.; McKenzie, D.; Möllby, R.; J. Microbiol. Methods 2002, 50, 63. [Crossref] 27. Park, C. H.; Kim, K. H.; Lee, I. K.; Lee, S. Y.; Choi, S. U.; Lee, J. H.; Lee, K. R.; Arch. Pharm. Res. 2011, 34, 1289. [Crossref] 28. Zhang, Y.-K.; Wang, T.-T.; Huo, X.-M.; Xue, Z.; Zhang, H.-Y.; Zeng, Y.-R.; Tan, C.-J.; Chem. Nat. Compd. 2023, 59, 771. [Crossref] 29. Sajid, I.; Shaaban, K. A.; Hasnain, S.; Braz. J. Microbiol. 2011, 42, 592. [Crossref] 30. Yin, L.; Zhang, Y.; Wu, H.; Wang, Z.; Dai, Y.; Zhou, J.; Liu, X.; Dong, M.; Xia, X.; LWT 2020, 133, 110087. [Crossref] 31. Kumar, S.; Abedin, Md. M.; Singh, A. K.; Das, S.; Plant Phenolics in Sustainable Agriculture; Springer Singapore: Singapore, 2020. 32. Coronado H., M.; Vega y León, S.; Gutiérrez T., R.; Vázquez F., M.; Radilla V., C.; Revista Chilena de Nutrición 2015, 42, 206. [Crossref] 33. Daglia, M.; Curr. Opin. Biotechnol. 2012, 23, 174. [Crossref] 34. Rodríguez-Morató, J.; Boronat, A.; Kotronoulas, A.; Pujadas, M.; Pastor, A.; Olesti, E.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Farré, M.; de la Torre, R.; Drug Metab. Rev. 2016, 48, 218. [Crossref] 35. de Arruda, G. L.; de Moraes, G. K. A.; Chagas Junior, A. F.; Araujo, A. R.; Chapla, V. M.; Nat. Prod. Res. 2022, 36, 3999. [Crossref] 36. Fernández-Prior, Á.; Bermúdez-Oria, A.; Fernández-Bolaños, J.; Espejo-Calvo, J. A.; López-Maestro, F.; Rodríguez-Gutiérrez, G.; Molecules 2022, 27, 8380. [Crossref] 37. Liu, Y.; Shi, C.; Zhang, G.; Zhan, H.; Liu, B.; Li, C.; Wang, L.; Wang, H.; Wang, J.; Biochem. Biophys. Res. Commun. 2021, 572, 145. [Crossref] 38. Kim, Y.; Cho, J.-Y.; Kuk, J.-H.; Moon, J.-H.; Cho, J.-I.; Kim, Y.-C.; Park, K.-H.; Curr. Microbiol. 2004, 48, 312. [Crossref] 39. Mrabet, A.; García-Borrego, A.; Jiménez-Araujo, A.; Fernández-Bolaños, J.; Sindic, M.; Rodríguez-Gutiérrez, G.; LWT-Food Sci. Technol. 2017, 79, 416. [Crossref] 40. Abo-Elyousr, K. A. M.; Imran, M.; Almasoudi, N. M.; Ali, E. F.; Hassan, S.; Sallam, N. M. A.; Youssef, K.; Abdel-Rahim, I. R.; Bagy, H. M. M. K.; J. Plant Pathol. 2022, 104, 947. [Crossref] 41. Fan, S.; Tian, F.; Li, J.; Hutchins, W.; Chen, H.; Yang, F.; Yuan, X.; Cui, Z.; Yang, C.-H.; He, C.; Mol. Plant Pathol. 2017, 18, 555. [Crossref]
Guest Editor handled this article: Lucas S. Abreu |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access