Artigo
| Amides isolated from the leaves of Piper tuberculatum Jacq. (Piperaceae) with acetylcholinesterase inhibition and larvicidal activity against Aedes aegypti |
|
Brenda R. C. LeocadioI,II,* I. Programa de Pós-Graduação em Química, Departamento de Química, Universidade Federal do Amazonas, 69080-900 Manaus - AM, Brasil Received: 03/01/2025 *e-mail: ritasnunomura@gmail.com; brenda.leocadio@ufam.edu.br Piper tuberculatum Jacq. is known to be a promising source of amides, which have shown remarkable biological activities. In this study, a phytochemical investigation of the leaves of P. tuberculatum was conducted, leading to the isolation of the amides cis-piplartine (1), piplartine (2), and 4,5-dihydropiperlonguminine (3). The structures of the compounds were determined through the analysis of 1D and 2D nuclear magnetic resonance (NMR), mass spectrometry (MS), and high-resolution mass spectrometry (HRMS), as well as data comparison with the literature. The isolated amides were tested for acetylcholinesterase (AChE) inhibition assay and larvicidal activity against Aedes aegypti. The significant larvicidal activity of the isolated amides, along with the AChE inhibition results, indicate that the larvicidal activity is related to the inhibition of this enzyme. These results suggest that these amides are promising larvicidal compounds. INTRODUCTION The Piperaceae family is considered one of the most primitive angiosperms in the Piperales order, with a pantropical distribution. The genus Piper is the most abundant and comprises ca. 2,600 species.1-4 Some species have been used in traditional medicine against snake bites, insect control, and as analgesics or anti-inflammatory agents.5 The Piper species has attracted interest in the investigation of various classes of secondary metabolites, including amides, propenylphenols, lignans, neolignans, terpenes, steroids, flavones, and flavanones. Among these metabolites, amides are among the most abundant constituents in many Piper species.6-8 Several amides have been isolated from Piper, including those with isobutyl, pyrrolidine, dihydropyridone, and piperidine-type structures.9 These amides exhibit a wide range of biological activities, including anticancer,10 anxiolytic,11 and particularly promising larvicidal compounds.12 Amides isolated from Piper have shown effectiveness in mosquito control, such as Aedes aegypti, making them an interesting alternative for the development of biological and sustainable control methods against the spread of mosquito-borne diseases, such as dengue, Zika, and chikungunya.13 Some commercial insecticides, essential oils, and isolated compounds demonstrate larvicidal activity due to their effect on the cholinergic system, particularly through the inhibition of acetylcholinesterase (AChE), which has become a promising alternative for investigating the larvicidal mechanism of action of various natural products, such as essential oils and isolated compounds.10 However, it is important to highlight that insect AChE has a distinct feature compared to mammalian AChE: many insect species possess a cysteine near the active site, which is absent in mammalian AChE.14 This structural difference is crucial, because it allows the development of selective inhibitors, more effective targeting insects, while minimizing risks to non-target organisms, such as mammals.15 Therefore, to investigate the action of AChE inhibitors as insecticidal agents, it is essential to consider the specificity of insect cysteine, which enables the creation of safer and more targeted compounds that reduce negative impacts on mammals and other non-target organisms.10,16,17 Among the Piper species, P. tuberculatum Jacq., is a typical Amazonian species, popularly known as “monkey pepper", “long pepper", or “pimenta-d'Arda". This species is widely used in traditional medicine by natives, who prepare infusions from its fruits and leaves as analgesics, sedatives, as well as an antidote for snake venom and stomach issues.18 Phytochemical studies on fixed constituents of the species report a predominance of amides. Among these amides, piplartine stands out due to its significant larvicidal potential.19 In addition, this species exhibits various biological activities, such as anticancer,10 antifungal,20 and anxiolytic effects.11 Although the larvicidal activity of Piper tuberculatum against some vectors of tropical diseases is known, the mechanism of action is still poorly understood. Since AChE inhibition is often associated with larvicidal activity, this study aims to elucidate this mechanism, emphasizing the effect of the compound on AChE. In this context, the present study investigated the larvicidal activity against Aedes aegypti of the amides isolated from P. tuberculatum and determined the AChE inhibition of the isolated compounds.
EXPERIMENTAL General chemical reagents High-performance liquid chromatography (HPLC)-grade solvents were purchased from Tedia Company (Fairfield, USA) and reagents from Sigma-Aldrich (St. Louis, USA). Analytical grade solvents were from Merck (Darmstadt, Germany) and Nuclear (House of Chemistry, Brazil). The ultrapure water was produced using a Milli-Q system (Millipore, Bedford, USA). Plant material The leaves of P. tuberculatum were collected at the Botanical Garden of Manaus (Museu da Amazônia) located in Manaus, Amazonas, Brazil (3º00'75" S, 59º93'97" W) and a voucher specimen (HUAM 12425) was deposited in the Herbarium of the Federal University of Amazonas. The species was identified by Profa. Dra. Micheline Carvalho Silva from the Department of Botany at the University of Brasília. The genetic access was registrated in SisGen (National System for the Management of Genetic Heritage and Associated Traditional Knowledge) under the code AE3F373. Extraction and chromatographic fractionation of the extract The P. tuberculatum leaves (62.10 g) were air-dried, powdered using a mill (SL-31, Solab, Brazil), and subjected to maceration with methanol. The obtained methanolic extract (12.17 g) was fractionated by column filtration with the following mobile phases: hexane, hexane/ethyl acetate (1:1), ethyl acetate, ethyl acetate/methanol (1:1), and methanol, using 600 mL for the first eluent system and 500 mL for the other systems. We collected five fractions which were coded and their respective yields for each mobile phase were: EMFPT-1 (0.2%), EMFPT-2 (7.9%), EMFPT-3 (4.0%), EMFPT-4 (20.3%), and EMFPT-5 (14.1%). The methanolic extract, along with the five fractions obtained, were submitted to screening for toxicity against Ae. aegypti, following World Health Organization (WHO) guidelines.21 The fraction EMFPT-3 exhibited the highest mortality rate (100%). Consequently, this fraction was subjected to further purification. Semipreparative HPLC analysis The final purification of the amides was carried out on a Shimadzu UFLC high-performance liquid chromatography system with an LC-6 AD pump; DGU-20A5 degasser; SPD-20AV UV detector; Rheodyne injector; CBM-20A communication module (Columbia, USA), equipped with a Luna C18 column (150 × 460 mm, 5 μm) (Phenomenex, Torrance, USA) at a flow rate of 3.5 mL min-1. The mobile phase consisted of Milli-Q water (A) and methanol (B) with the following elution gradient: 0-15 min, 50-100% B; 15-20 min, 100% B. The separation was monitored at 280 and 300 nm. Fractions PTF-03 (1.5 mg, 1), PTF-06 (1.2 mg, 2), and PTF-07 (1.5 mg, 3) were analyzed by high-resolution mass spectrometry (HRMS), ion-trap mass spectrometry (DI-APCI-MS), and one-dimensional (1D) and two-dimensional (2D) nuclear magnetic resonance (NMR) spectroscopy. Mass spectrometry analysis For DI-APCI-MS analysis, a stock solution (1 mg mL-1) of compounds 1-3 was prepared in MeOH (HPLC-grade). An aliquot (10 µL) of the stock solution was subsequently transferred to vials containing 1 mL of MeOH. Finally, the diluted solution (10 ppm) was analyzed by direct injection (DI) into an ion trap mass spectrometer from Thermo Scientific (San Jose, USA), LCQ-Fleet model, equipped with an APCI source operating in positive polarity. The analytical parameters used were as follows: discharge current 5 µA; vaporizer temperature 320 ºC; capillary temperature 220 ºC; sheath gas 30 psi; auxiliary gas 10 arbitrary; mass range, m/z 100-1000 Da. The MS/MS spectra were obtained through collision-induced dissociation (CID) using helium as the collision gas and collision energies from 25%. The HRMS analysis was performed in a mass spectrometer from Bruker Daltonics (Billerica, USA), model microTOF-QII, equipped with an electrospray ionization source (ESI), operating in positive mode. The analytical parameters were as follows: capillary voltage 4,500 V; sheath gas (N2) 0.4 bar; drying gas (N2) 4 L min-1; capillary temperature 200 ºC; mass range, m/z 100-400 Da. 1D and 2D NMR spectroscopy The 1D and 2D NMR spectra were acquired using an Avance III HD 500 spectrometer (Bruker, Billerica, USA) operating at 11.7 T (500.13 and 125.76 MHz for 1H and 13C, respectively). Chemical shifts (d) are reported in ppm relative to the tetramethylsilane (TMS) signal at 0.00 ppm as an internal reference and coupling constants (J) were expressed in Hertz. Deuterated methanol (CΔ3OD) was used as solvent. cis-Piplartine (1) cis-Piplartine (1) was isolated as a white crystalline solid (1.5 mg); 1H NMR (500 MHz, methanol-d4) d 2.31 (2H, ddt, J 6.3, 4.2, 1.7 Hz, H-5), 3.80 (6H, s, OCH3), 3.87 (3H, s, OCH3), 3.95 (2H, t, J 6.4 Hz, H-6), 5.92 (1H, dt, J 9.8, 1.8 Hz, H-3), 6.41 (1H, d, J 12.8 Hz, H-8), 6.68 (1H, d, J 12.8 Hz, H-9), 6.75 (s, 2H) 6.91 (1H, dt, J 9.8, 4.4 Hz, H-4) (see Table 1S in the Supplementary Material); HRMS m/z 318.1345 (calcd. for C17H20NO5 m/z 318.1336, Δm/z theoretical -2.7 ppm) (see Figure 1S in the Supplementary Material). Piplartine (2) Piplartine (2) was isolated as a white crystalline solid (1.2 mg); 1H NMR (500 MHz, methanol-d4) d 2.59 (2H, ddt, J 6.4, 4.3, 1.8 Hz, H-5), 3.81 (3H, s, OCH3), 3.89 (6H, s, OCH3), 3.99 (2H, t, J 6.5 Hz, H-6), 6.03 (1H, dt, J 9.7, 1.8 Hz, H-3), 6.93 (2H, s, H-11/15), 7.09 (1H, dt, J 9.7, 4.4 Hz, H-4), 7.39 (1H, d, J 15.6 Hz, H-8), 7.63 (1H, d, J 15.6 Hz, H-9) (see Table 2S in the Supplementary Material); HRMS m/z 318.1331 (calcd. for C17H20NO5 m/z 318.1336, Δm/z theoretical 1.6 ppm) (see Figure 5S in the Supplementary Material). 4,5-Dihydropiperlonguminine (3) 4,5-Dihydropiperlonguminine (3) was isolated as a yellow crystalline solid (1.5 mg); 1H NMR (500 MHz, methanol-d4) d 0.90 (6H, d, J 6.6 Hz, H-3"/4"), 1.76 (1H, non, J 6.6 Hz, H-2"), 2.46 (2H, dq, J 7.3, 1.5 Hz, H-4), 2.68 (2H, t, J 7.3 Hz, H-5), 3.03 (2H, d, J 7.3 Hz, H-1"), 5.88 (2H, s, OCH2O), 5.89 (1H, dt, J 15.3, 1,5 Hz, H-2), 6.64 (1H, dd, J 7.9, 1.4 Hz, H-6'), 6.69-6.71 (1H, m, H-2'), 6.79 (1H, dt, J 15.3, 6.9 Hz, H-3) (see Table 3S in the Supplementary Material).; HRMS m/z 276.1594 (calcd. for C16H22NO3 m/z 276.15946, Δm/z theoretical 0.2 ppm) (see Figure 9S in the Supplementary Material). Larvicidal test The larvicidal bioassay against Aedes aegypti was conducted following the standard WHO larvicidal activity protocol.21 All experiments were performed at 28 ± 2 ºC and 70-85% relative humidity. Compounds 1-3 were individually dissolved in 1 mL of dimethyl sulfoxide (DMSO) and subsequently diluted with distilled water to obtain concentrations ranging from 10 to 50 µg mL-1. Third-instar larvae (n = 100) were distributed into five containers (200 mL), each containing 99 mL of distilled water and five different concentrations of the fractions. A negative control (DMSO) was tested at the same concentrations. The positive control, α-cypermethrin, was evaluated at concentrations ranging from 0.13 to 0.65 µg mL-1. All assays were performed in quintuplicate. The percentage of larvicidal activity at each concentration was calculated after 24 h using the Equation 1: Larvicidal activity (%) = (number of dead larvae / total number of larvae) × 100 (1)
Acetylcholinesterase (AChE) inhibition assay The mechanism of action was investigated by measuring AChE inhibition using a modified spectrophotometric method.22,23 Neostigmine (1 mg), used as a positive control, and AChE from Sigma-Aldrich (10 μL) were prepared in phosphate buffer (1 mL) at 0.1 M, pH 8. The isolated compounds (1 mg) were dissolved in MeOH (1 mL) and evaluated at concentrations ranging from 0.07 to 10 ppm. The assay was performed in triplicate using a 96-well microplate incubated in a light-protected environment for 15 min. Negative and positive controls were evaluated under the same conditions. The absorbance at 405 nm was read every 5 min for 30 min using a microplate reader (ELx800, Biotek, USA). The percentage of inhibition at each concentration was calculated using the Equation 2: Inhibition (%) = A2 - (A1 - A3) × 100 / A2 (2)
where A1 is the absorbance of the sample with the enzyme, A2 is the absorbance of the enzyme without the sample, and A3 is the absorbance of the sample without the enzyme. Statistical analysis The values of lethal concentration 50% (LC50) and lethal concentration 90% (LC90), chi-square (χ2), and slope ± standard deviation (SD) were determined using Probit analysis in IBM SPSS statistics for Windows, version 29.0 (IBM Corp., Armonk, USA). For the AChE assay, absorbance values were log-transformed, normalized, and analyzed using a nonlinear regression method to determine IC50 with GraphPad Prism, version 9.5.1 (GraphPad software, San Diego, USA). Significant differences were identified using one-way analysis of variance (ANOVA) followed by Tukey's test (p ≤ 0.05).
RESULTS AND DISCUSSION Structural elucidation of the amides isolated from P. tuberculatum The isolated compound 1 was a white crystal. Its molecular formula was determined by HRMS ([M + H]+ m/z 318.1345) as being C17H20NO5 (Figure 1S, Supplementary Material). In the DI-APCI-MS analysis, the ion at m/z 318 [M + H]+ was observed as the base peak. Upon fragmentation, an initial loss of 97 Da (m/z 221) was detected (Figure 3S, Supplementary Material). The 1H NMR spectrum (Figure 4S, Supplementary Material) exhibited signals corresponding to a tetrasubstituted and symmetrical aromatic system at dH 6.75 (s, H-11/H-15), as well as olefinic proton, signals at dH 6.41 (d, H-8) and 6.68 (d, H-9), indicating cis coupling, and at dH 6.41 (d, H-8) and 6.68 (d, H-9), indicating trans coupling. The presence of methylene protons adjacent to an electronegative atom (nitrogen) was indicated by a signal at dH 3.95 (t, H-6). Additionally, some proton resonance signals in the aliphatic region (dH 1.45-3.20) were identified. Based on the spectral data analysis and comparison with literature data,9 compound 1 was identified as cis-piplartine (Figure 1).
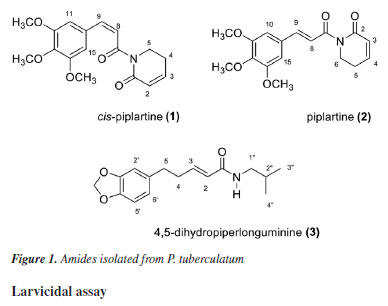
The isolated compound 2 was also a white crystal. Its molecular formula was determined by HRMS ([M + H]- m/z 318.1331) as being C17H20NO5) (Figure 6S, Supplementary Material). In the DI-APCI-MS analysis, the ion at m/z 318 [M + H]+ was observed as the base peak, and upon fragmentation, an initial loss of 97 Da (m/z 221) was detected (Figure 7S, Supplementary Material). Comparative MS and NMR analyses revealed structural similarity to compound 1. However, the 1H NMR spectrum (Figure 8S, Supplementary Material) showed a difference in the coupling constants between the olefinic protons H-8 (1H, d) and H-9 (1H, d), both with J 15.6 Hz, characteristic of vicinal trans protons. Based on spectral data analysis and literature comparison,24 compound 2 was identified as piplartine (Figure 1). The compound 3 was isolated as a yellow crystal. Its molecular formula was determined by HRMS in positive mode as C16H22NO3 ([M + H]+ m/z 276.1594). In the DI-APCI-MS analysis (Figure 10S, Supplementary Material), the ion at m/z 276 [M + H]+ was observed as the base peak. Upon fragmentation, it exhibited losses of 73 Da (m/z 203), 141 Da (m/z 135), and 115 Da (m/z 161) (Figure 11S, Supplementary Material). The 1H NMR spectrum (Figure 12S, Supplementary Material) displayed characteristic signals for aromatic protons at dH 6.64 (dd, H-6') and 6.69-6.71 (m, H-2'/H-5'), along with a singlet at dH 5.88, corresponding to protons from the methylenedioxy group. Additionally, signals in the region of olefinic proton resonances were observed at dH 6.76 (dt, H-3) and 5.89 (dt, H-2), indicating trans coupling. Proton resonance signals in the aliphatic region (dH 1.45-3.20) were also identified. The methylenedioxy group was confirmed through 3J couplings for the proton at dH 5.88 (s) with the carbons at dC 145.5 and 147.5, as observed in the heteronuclear single-quantum correlation (HSQC) experiment (Figure 13S, Supplementary Material). Therefore, after analysis of spectral data and comparison with previously reported data in the literature,24,25 the compound was identified as 4,5-dihydropiperlonguminine amide (Figure 1). Larvicidal assay The results of the larvicidal activity of the isolated amides against Ae. aegypti larvae are shown in Table 1. The data obtained on the number of dead larvae were analyzed based on the lethal concentration 50% (LC50) and lethal concentration 90% (LC90).
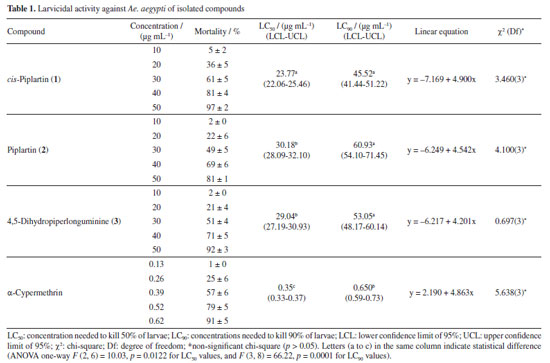
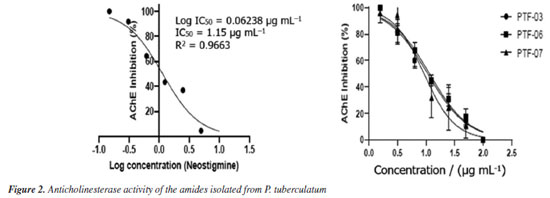
The amide cis-piplartine, at the three highest tested concentrations (30, 40, and 50 µg mL-1), demonstrated larvicidal potential with mortality rates of 61 ± 5, 81 ± 4, and 97 ± 2%, respectively, after 24 h of exposure, and presented an LC50 of 23.77 µg mL-1. This is the first study to report the larvicidal activity of this amide against Ae. aegypti, with promising activity. For the amide piplartine, its two highest concentrations (40 and 50 µg mL-1) showed high mortality rates of 71 ± 5 and 81 ± 1%, respectively, with an LC50 of 30.18 µg mL-1. According to the literature,26 piplartine exhibited larvicidal activity in a study conducted at seven concentrations (1, 10, 30, 50, 70, 100, and 300 µg mL-1) in third-instar Ae. aegypti larvae. However, in that study, high mortality rate of 100% was only observed at the highest concentration (300 µg mL-1). In a comparative analysis between the results presented in this study and those reported in the literature, this study demonstrated that at a concentration of 50 µg mL-1, the activity was more significant than previously reported. The amide 4,5-dihydropiperlonguminine exhibited high larvicidal activity, with mortality rates of 71 ± 5 and 92 ± 3% at concentrations of 40 and 50 µg mL-1, respectively, and an LC50 of 29.04 µg mL-1. Based on this, compared to the literature,13 amides not only belong to one of the two groups with the highest number of larvicidal components but also include the most active substances. This is the first study to report the larvicidal activity of 4,5-dihydropiperlonguminine against Ae. aegypti. The observed LC50 values of the isolated amides piplartine and cis-piplartine further support this class as promising chemical agents for the control of tropical diseases such as dengue. The use of amides as larvicidal agents offers a promising alternative in integrated pest management. These compounds can potentially control vector populations effectively and sustainably, while minimizing environmental impact.24 The results of this study demonstrate the significant larvicidal activity of these compounds, suggesting their potential as valuable tools for controlling tropical diseases like dengue, Zika, and chikungunya. A more detailed analysis is needed to evaluate the efficacy of amides compared to other insecticides, particularly regarding their potency and selective toxicity. Non-target aquatic insects, including families like Notonectidae, Gerridae, and others, were exposed to piplartine and DMSO at 264.4 µg mL-1 for 30 days. Both treatments resulted in 100% survival, showing no toxicity. In contrast, exposure to α-cypermethrin at a much lower concentration of 0.025 µg mL-1 (10.5 times lower than piplartine and DMSO) caused rapid toxicity, with only 9.1% survival within three days. This significant difference highlights the relative safety of piplartine and DMSO compared to the pronounced toxicity of α-cypermethrin. Comparing natural amides to common chemical insecticides, it is crucial to consider not only their efficacy in vector control but also their potential impact on non-target organisms and the environment.14 The selective toxicity of amides, especially toward insects, is a key factor that distinguishes them from other insecticides, which tend to affect a broader range of species.27 Insects like Ae. aegypti possess unique biochemical features, such as cysteine near the active site of AChE, which allows the development of selective inhibitors that mainly target insects without significantly harming non-target organisms.11 The environmental impact of amides, particularly on non-target species, must be carefully assessed to ensure that their use does not negatively affect biodiversity or other species.9 A thorough evaluation of these factors will help determine the safety and feasibility of using amides as insecticides on a larger scale.8 While amides may provide a less toxic and more sustainable alternative to traditional chemical insecticides, it is essential to balance their effectiveness in vector control with environmental protection and public health.9 According to studies,27,28 amides can trigger the overproduction of reactive oxygen and nitrogen species (RONS), leading to oxidative stress, cellular damage, and changes in the activity of key defense enzymes. These enzymes include catalase (CAT), glutathione S-transferase (GST), thiols, esterases, and mixed-function oxidases (MFOs). Additionally, plant-derived compounds often target acetylcholinesterase (AChE), disrupting neural function in insects, including those from the Aedes and Anopheles genera. In fact, a recent study29 identified piplartine, a compound isolated for the first time from P. purusanum (Piperaceae), as a potent larvicide against Ae. aegypti and An. darlingi, with LC50 values of 14.56 and 26.44 µg mL-1, respectively. This larvicidal activity was primarily attributed to the overproduction of reactive oxygen and nitrogen species, reaching 66.67 ± 7 and 86.33 ± 6%, likely leading to oxidative stress and cellular damage in mosquito larvae. Furthermore, piplartine was found to enhance the activity of key detoxifying enzymes, which play a crucial role in managing oxidative stress.29 These include catalase, responsible for breaking down hydrogen peroxide (87.00 ± 9 and 94.67 ± 9 µmol of H2O2 consumed per min per mg of protein), glutathione S-transferase (76.00 ± 1 and 134.00 ± 1 µmol min mg-1), mixed-function oxidase (26.67 ± 5 and 55.00 ± 1 nmol cti mg-1 protein), as well as α-esterase and β-esterase, with activity levels ranging from 27.67 ± 7 to 46.33 ± 1 nmol cti mg-1 protein. Several significant alterations were observed in the digestive tract of Ae. aegypti larvae, indicating cellular stress followed by apoptosis after exposure to amides, as described in the literature,26,30 including intense cytoplasmic vacuolization, shortened and sparse microvilli, the presence of myelin structures, and disorganized cellular architecture. Additionally, some midgut cell nuclei exhibited deoxyribonucleic acid (DNA) fragmentation, further highlighting the extent of cellular damage. Notably, the presence of myelin structures in mosquito larvae cells may serve as an indicator of cellular stress, as these formations are often associated with cell damage and degradation in various biological contexts. These findings provide strong evidence that amides, such as piplartine, exert their larvicidal effects not only by inducing oxidative stress but also by modulating the activity of detoxifying enzymes, which may contribute to the overall toxicity observed in mosquito larvae. In addition to amides, various classes of natural products found in Piper species are rich in bioactive compounds. Studies31 have shown that phenolic compounds possess anticancer, antimicrobial, and antimutagenic properties. Lignans and neolignans isolated from these species have shown promise as agents against leishmaniasis.32 Alkaloids have also demonstrated high efficacy in cancer treatment.33,34 Furthermore, the pharmacological activities of steroids, terpenes, chalcones, dihydrochalcones, and essential oils indicate the great potential of these substances as health-promoting agents with broad pharmaceutical applications.6 AChE inhibition assay The larvicidal activity of the amides investigated in the present study may be associated with their ability to inhibit AChE, an essential enzyme for cholinergic neurotransmission in insects. AChE plays a crucial role in regulating synaptic transmission by hydrolyzing acetylcholine (ACh) into choline and acetic acid, allowing for the termination of neural excitation.35,36 The inhibition of this enzyme results in the excessive accumulation of ACh in the synaptic cleft, leading to neural hyperexcitation, motor dysfunction, and eventually, paralysis and death of the affected organism.37 The enzymatic assays conducted in this study demonstrated that the three tested amides exhibited significant AChE inhibition, with IC50 values lower than that of neostigmine, a widely used standard inhibitor (Figure 2). The compound 4,5-dihydropiperlonguminine showed the highest efficiency in inhibiting AChE (IC50 of 0.96 µg mL-1), followed by cis-piplartine (IC50 of 1.02 µg mL-1) and piplartine (IC50 of 1.05 µg mL-1), suggesting a strong interaction of these molecules with the active site of the enzyme.38 The magnitude of this inhibition indicates that these compounds may directly affect the nervous system of the larvae, interfering with motor coordination and vital functions such as feeding and response to environmental stimuli.39
Although we performed the AChE assay using a Sigma-Aldrich kit, which contains AChE from Electrophorus electricus (Gymnotidae), an invertebrate, a significant decrease in AChE activity was observed in Ae. aegypti and An. darlingi larvae after exposure to amides, such as piplartine. The activity levels were recorded at 43.33 ± 7 and 48.00 ± 2 µmol min mg-1 of protein, respectively, compared to the control groups: α-cypermethrin, with values of 24.00 ± 6 and 18.33 ± 3 µmol min mg-1, and DMSO, with values of 87.33 ± 1 and 146.30 ± 3 µmol min mg-1, as described.35 It is important to highlight that this assay was conducted with the supernatant produced from Ae. aegypti and An. darlingi larvae, indicating the presence of cysteine. These results suggest that amides affect both invertebrate AChE and insect AChE, indicating that their use should be carefully controlled to avoid contact with invertebrates. The correlation between larvicidal activity and AChE inhibition has been widely described for other natural compounds, such as alkaloids, terpenoids, and amides, reinforcing the hypothesis that this mechanism may be involved in the toxicity of the studied amides.35 Previous studies40,41 indicate that AChE inhibitors in Ae. aegypti larvae can impair their ability to move, making it difficult to search for food and increasing vulnerability to death from metabolic stress and starvation. Furthermore, the loss of neuromuscular control can lead to erratic behavior and convulsions, resulting in premature death of the exposed individuals. Thus, the results obtained reinforce the potential of the isolated amides as promising candidates for the development of new natural larvicides.42 The combination of high larvicidal efficacy and a possible action mechanism based on selective neurotoxicity for insects is a desirable feature for compounds used in vector control.43 Moreover, AChE inhibition may act synergistically with other toxicity mechanisms of these amides, which warrants further exploration in future studies for a deeper understanding of their biological action.44
CONCLUSIONS This is the first report of the larvicidal activity of cis-piplartine and 4,5-dihydropiperlonguminine. Additionally, this is the first report of AChE inhibitory activity for the isolated amides, except for piplartine. The observed results suggest that cis-piplartine, piplartine, and 4,5-dihydropiperlonguminine may be potential natural larvicides against Ae. aegypti, as they exhibited significant larvicidal activity. Moreover, one of their possible mechanisms of action could involve AChE enzyme inhibition, which was notably significant, showing values comparable to neostigmine, used as the positive control.
SUPPLEMENTARY MATERIAL Complementary material for this work (1H NMR and HSQC spectra, as well as the MS and HRMS spectra of the compounds isolated from P. tuberculatum) is available at http://quimicanova.sbq.org.br/, as a PDF file, with free access.
DATA AVAILABILITY STATEMENT All data are available in the text.
ACKNOWLEDGMENTS This study was conducted with financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) e Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM, notice No. 013/2022 Programa FAPEAM Produtividade em CT&I e Programa de Apoio à Pós-Graduação strictu sensu - POSGRAD edition 2022-2023). The authors also thank to Pós-Graduação em Química, Central Analítica - UFAM, Financiadora de Estudos e Projetos (FINEP) e Instituto Nacional de Pesquisas da Amazônia (INPA).
REFERENCES 1. The Angiosperm Phylogeny Group; Chase, M. W.; Christenhusz, M. J. M.; Fay, M. F.; Byng, J. W.; Judd, W. S.; Soltis, D. E.; Mabberley, D. J.; Sennikov, A. N.; Soltis, P. S.; Stevens, P. F.; Bot. J. Linn. 2016, 20, 181. [Crossref] 2. Davidse, G.; Ulloa, U. C.; Hernandez, H.; Flora Mesoamericana, Parte 2: Piperaceae, volumen 2; Missouri Botanical Garden Press: New York, 2021. 3. Martinez, C.; Carvalho, M. R.; Madrinan, S.; Jaramillo, C. A.; Am. J. Bot. 2015, 102, 289. [Crossref] 4. Jaramillo, M. A.; Manos, P. S.; Am. J. Bot. 2001, 88, 716. [Crossref] 5. Rito, D. S.; Vieira, E. F. T.; de Menezes, I. C.; Lameira, O. A.; Poltronieri, M. C.; de Lemos, O. F.; Rodrigues, S. M.; Research, Society and Development 2021, 10, e33410615686. [Crossref] 6. Parmar, V.; Jain, S. C.; Bisht, K. S.; Jain, R.; Tanela, P.; Jha, A.; Tyagi, O. D.; Prasad, A. D.; Wengel, J.; Olsen, C. E.; Boll, P. M.; Phytochemistry 1997, 49, 597. [Crossref] 7. Wu, Q. L.; Wang, S. P.; Tu, G. Z.; Feng, Y.; Yang, J. S.; Phytochemistry 1997, 44, 727. [Crossref] 8. Yamaguchi, L. F.; Lago, J. H. G.; Tanikazi, T. M.; Mascio, P. D.; Kato, M. J.; Phytochemistry 2006, 67, 1838. [Crossref] 9. Navickiene, H. M. D.; Alécio, A. C.; Kato, M. J.; Bolzani, V. D. S.; Young, M. C. M.; Cavalheiro, A. J.; Furlan, M.; Phytochemistry 2000, 55, 626. [Crossref] 10. da Nóbrega, F. R.; Ozdemir, O.; Sousa, S. C. S. N.; Barboza, J. N.; Turkez, H.; de Sousa, D. P.; Molecules 2018, 23, 1382. [Crossref] 11. Zimath, P. L.; Ribeiro, T. C.; Dalmagro, A. P.; Santos, M. E. D.; Gasparetto, A.; Cruz, A. B.; Souza, M. M. D.; Rev. Fitos 2017, 11, 153. [Crossref] 12. Spletozer, A. G.; Santos, C. R. D.; Sanches, L. A.; Garlet, J.; Ciência Florestal 2021, 31, 997. [Crossref] 13. Garcez, W. S.; Garcez, F. R.; da Silva, L. M.; Sarmento, U. C.; Rev. Virtual Quim. 2013, 5, 393. [Crossref] 14. Costa, A. A.; Gonzalez, P. V.; Harburguer, L. V.; Masuh, H. M.; J. Med. Entomol. 2018, 55, 1104. [Crossref] 15. Govindarajan, M.; Rajeswary, M.; Hoti, S. L.; Benelli, G.; Res. Vet. Sci. 2016, 104, 77. [Crossref] 16. Huang, Y.; Lin, M.; Jia, M.; Hu, J.; Zhu, L.; Pest Manage. Sci. 2020, 76, 542. [Crossref] 17. de Oliveira, A. C.; Simões, R. C.; Lima, C. A.; da Silva, F. M.; Nunomura, S. M.; Roque, R. A.; Nunomura, R. C.; Environ. Sci. Pollut. Res. 2022, 29, 47253. [Crossref] 18. de Araujo-Junior, J. X.; da Cunha, E. V.; Chaves, M. C. D. O.; Gray, A. I.; Phytochemistry 1997, 44, 561. [Crossref] 19. Pohlit, A. M.; Quinard, E. L. J.; Nunomura, S. M.; Tadei, W. P.; Hidalgo, A. D. F.; Pinto, A. C. D. S.; Graça, Y. R.; Acta Amazonica 2004, 34, 105. [Crossref] 20. Palacios, Z. G.; Delgado, G. E.; Moreno, M. C.; Kato, M. J.; Rojas, C.; Revista Peruana de Biologia 2009, 16, 209. [Crossref] 21. World Health Organization (WHO), https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue, accessed in January 2025. 22. Ellman, G. L.; Courtney, K. D.; Andres Junior, V.; Featherstone, R. M.; Biochem. Pharmacol. 1961, 7, 95. [Crossref] 23. Gélinas, M.; Lajeunesse, A.; Gagnon, C.; Gagné, F.; Ecotoxicol. Environ. Saf. 2013, 59, 94. [Crossref] 24. da Silva ‐ Junior, E. A.; Paludo, C. R.; Gouvea, D. R.; Kato, M. J.; Furtado, N. A. J. C.; Lopes, N. P.; Pupo, M. T.; J. Mass Spectrom. 2017, 52, 525. [Crossref] 25. Bernard, C. B.; Krishanmurty, H. G.; Chauret, D.; Durst, T.; Philogene, B. J. R.; Sanchez-Vindas, P.; Arnason, J. T.; J. Chem. Ecol. 1995, 21, 814. [Crossref] 26. Maleck, M.; Ferreira, B.; Mallet, J.; Guimaróes, A.; Kato, M.; J. Med. Entomol. 2014, 51, 463. [Crossref] 27. Rattan, R. S.; Crop Prot. 2010, 29, 920. [Crossref] 28. Ganesan, P.; Samuel, R.; Mutheeswaran, S.; Pandikumar, P.; Reegan, A. D.; Aremu, A. O.; Ignacimuthu, S.; J. S. Afr. Bot. 2023, 152, 49. [Crossref] 29. de Oliveira, A. C.; da Silva, F. M. A.; de Sá, I. S.; Leocadio, B. R. C.; Lima, S. C.; da Costa, M. L. L.; Nunomura, R. D. C. S.; Plants 2025, 14, 774. [Crossref] 30. Costa, M. B. S.; Simões, R. D. C.; Silva, M. D. J. A. D.; Oliveira, A. C. D.; Acho, L. D. R.; Lima, E. S.; Oliveira, C. M. D.; Revista da Sociedade Brasileira de Medicina Tropical 2022, 55, e0373. [Crossref] 31. Huang, W. Y.; Cai, Y. Z.; Zhang, Y.; Nutr. Cancer 2009, 62, 20. [Crossref] 32. Bodiwala, H. S.; Singh, G.; Singh, R.; Dey, C. S.; Sharma, S. S.; Bhutani, K. K.; Singh, I. P.; J. Nat. Med. 2007, 61, 421. [Crossref] 33. Bezerra, D. P.; Pessoa, C.; Moraes, M. O. D.; Silveira, E. R.; Lima, M. A. S.; Elmiro, F. J. M.; Costa-Lotufo, L. V.; Z. Naturforsch., C: J. Biosci. 2005, 60, 543. [Crossref] 34. Bezerra, D. P.; Militão, G. C. G.; de Castro, F. O.; Pessoa, C.; de Moraes, M. O.; Silveira, E. R.; Costa-Lotufo, L. V.; Toxicol. In Vitro 2007, 21, 8. [Crossref] 35. Kamalakannan, S.; Murugan, K.; Barnard, D. R.; J. Asia-Pac. Entomol. 2011, 14, 41. [Crossref] 36. Stalin, A.; Reegan, A. D.; Rajiv Gandhi, M.; Saravanan, R. R.; Balakrishna, K.; Hesham, A. E. L.; Ignacimuthu, S.; Zhang, Y.; Comput. Biol. Med. 2022, 146, 105535. [Crossref] 37. Castillo-Morales, R. M.; Carreño Otero, A. L.; Mendez-Sanchez, S. C.; da Silva, M. A. N.; Stashenko, E. E.; Duque, J. E.; Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2019, 221, 29. [Crossref] 38. Doorandishan, M.; Pirhadi, S.; Swilam, M. M.; Gholami, M.; Ebrahimi, P.; El-Seedi, H. R.; Jassbi, A. R.; J. Supramol. Struct. 2021, 1245, 131034. [Crossref] 39. Karak, S.; Acharya, J.; Begum, S.; Mazumdar, I.; Kundu, R.; De, B.; J. Appl. Res. Med. Aromat. Plants 2018, 10, 85. [Crossref] 40. Johnson, T. O.; Ojo, O. A.; Ikiriko, S.; Ogunkua, J.; Akinyemi, G. O.; Rotimi, D. E.; Oche, J. R.; Adegboyega, A. E.; Biochem. Biophys. Rep. 2021, 28, 101175. [Crossref] 41. Shahriari, M.; Zibaee, A.; Sahebzadeh, N.; Shamakhi, L.; Pestic. Biochem. Physiol. 2018, 150, 40. [Crossref] 42. de Lima, B. R.; Lima, J. M.; Maciel, J. B.; Valentim, C. Q.; Nunomura, R. C. S.; Lima, E. S.; Koolen, H. H. F.; de Souza, A. D. L.; Pinheiro, M. L. B.; Cass, Q. B.; da Silva, F. M. A.; Front. Chem. 2019, 7, 00629. [Crossref] 43. López, M. D.; Pascual-Villalobos, M. J.; Ind. Crops Prod. 2010, 31, 284. [Crossref] 44. de Oliveira, M. S.; da Cruz, J. N.; Silva, S. G.; da Costa, W. A.; de Sousa, S. H. B.; Bezerra, F. W. F.; Teixeira, E.; da Silva, N. J. N.; Andrade, E. H. A.; Chaves Neto, A. M. J.; de Carvalho, R. N.; J. Supercrit. Fluids 2019, 145, 74. [Crossref]
Guest Editor handled this article: Antonio E. M. Crotti Dedicated to Professor Raimundo Braz Filho for his pioneering work on Natural Products Chemistry in Brazil. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access