Artigo
| Bixa orellana lipids as a source of geranylgeraniol: extraction techniques and characterization |
|
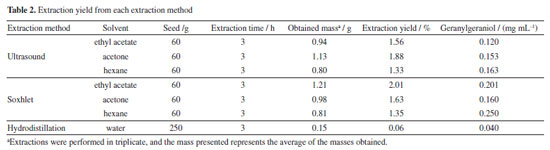
Miguel A. Martinez Gutierrez Laboratório de Pesquisa em Recursos Naturais (LPqRN), Universidade Federal de Alagoas, Campus de Engenharias e Ciências Agrárias (CECA), 57072-900 Maceió - AL, Brasil Received: 02/28/2025 *e-mail: aegs@ceca.ufal.br Bixa orellana L., known as annatto, is a plant in the Bixaceae family, widely used for its seeds rich in carotenoids, such as bixin (a natural dye) and geranylgeraniol (GGOH), an isoprenoid of interest for health and agriculture. This study evaluated the efficiency of annatto oil extraction using hydrodistillation, Soxhlet solvent extraction (conventional methods), and ultrasound-assisted extraction (modern method). Acetone, ethyl acetate, and hexane were used as extractor solvents. Gas chromatography coupled with flame ionization detection (GC-FID) and gas chromatography coupled with mass spectrometry (GC-MS) analyses revealed GGOH as the main compound in all methods, with concentration variations determined using 1-octadecanol as an external standard. Hydrodistillation showed the lowest yield (0.06%) but was more straightforward and sustainable. Extraction with organic solvents (Soxhlet and ultrasound) resulted in higher GGOH yields (1.35-2.01%), with hexane being the most efficient (0.250 mg mL-1). Soxhlet extraction with hexane is ideal for large quantities, while hydrodistillation is preferable for medicinal uses due to the absence of organic solvents. INTRODUCTION Bixa orellana L. is a tree belonging to the Bixaceae botanical family. It is native to tropical Latin America. It is known as urucum in Brazil, achiote in Spanish (derived from Nahuatl), and annatto in English. Annatto is cultivated in several regions of Brazil, with the states of São Paulo, Rondônia, and Pará being the most prominent producers.1 Annatto seeds have been used worldwide in the food, medicine, and cosmetics industries as a source of natural dye due to their intense color and lack of toxicity. Its characteristic color is orange-red, primarily due to its high carotenoid content, with bixin being the compound responsible for red. Bixin is the primary carotenoid found in this seed, comprising approximately 80%.2,3 In addition to its pigments, annatto has gained notoriety for containing other substances of great interest to health. Its essential oil is a source of some terpenes, with geranylgeraniol being the most abundant compound among its constituents. Geranylgeraniol (GGOH, Figure 1) is an isoprenoid found in plants, and its presence in B. orellana can represent up to 1% of the dry seed weight, making it the most abundant component.4 GGOH has also been observed in the plant popularly known as “sucupira", a tree native to Brazil belonging to the Fabaceae family. However, annatto is a more productive and easy-to-cultivate source.
The efficiency of oil extraction using different techniques was analyzed. The best-known method of extracting oil is the conventional steam distillation technique, also known as hydrodistillation.5 Another traditional method is continuous hot extraction using organic solvents, which is very efficient. Modern methods like microwave- and ultrasound-assisted extraction and supercritical fluid extraction have also proved efficient, including some that do not use solvents (solvent-free).6 These techniques are considered economically viable for extracting essential oils and are environmentally friendly.7 This work evaluated the yield and purity of obtaining GGOH from Bixa orellana seeds through oil extraction using the conventional Soxhlet extraction and hydrodistillation techniques and the modern ultrasound-assisted extraction method.
EXPERIMENTAL Plant material Green seeds of annatto (Bixa orellana L.) were collected in the forest area between the municipalities of União dos Palmares and Branquinha, in the state of Alagoas, Brazil (9º12'56.7"S 35º59'46.5"W), in January 2024. The seeds were removed from the freshly collected fruit, crushed, and then subjected to the following extraction processes: steam distillation extraction (hydrodistillation), Soxhlet extraction, and ultrasound-assisted extraction. For the Soxhlet and ultrasound-assisted extraction techniques, three solvents were used separately: (1) acetone, (2) ethyl acetate, and (3) hexane (solvent grade P.A., Química Moderna, São Paulo, Brazil). This work was registered in the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SisGen) under number A44AC04. Extraction methods Extraction by hydrodistillation An amount of 250 g of B. orellana L. seeds were crushed in a porcelain mortar and placed in a round-bottomed glass flask containing 1 L of distilled water. The flask was heated to 170 ºC with the aid of a heating mantle (Fisatom Equipamentos Científicos Ltda., model 102E, Perdizes, São Paulo, Brazil) for 3 h, and, after this period, the oil was collected, separated from the aqueous layer using a Pasteur pipette, stored in an amber glass vial with a polytetrafluoroethylene (PTFA) cap (Supelco Analytical Products, Bellefonte, PA, USA), and placed in a freezer (-20 ºC) until analysis. Extraction by Soxhlet organic solvent The B. orellana seeds (60 g) were crushed in a porcelain mortar and placed in a filter paper cartridge. The material was put into a Soxhlet-type extraction apparatus, and the extraction process was carried out for 3 h (on average, six cycles) using three types of solvent: acetone, ethyl acetate, and hexane. For each 60.0 g of seed, 500 mL of the solvent was used, and a new sample was used for each extraction. The extractions were performed in triplicate. The extracts were concentrated in a rotary evaporator. The crude oil was treated with a saturated aqueous solution of NaHCO3 to remove traces of organic acids and extracted with hexane. The organic fraction was dried over Na2SO4 and concentrated in a rotary evaporator, obtaining the seed oil stored in an amber glass bottle with a PTFA cap and stored in a freezer (-20 ºC) until analysis. Extraction by ultrasound The annatto seeds (60.0 g) were crushed in a porcelain mortar and placed in an Erlenmeyer flask with 200 mL of the solvent for extraction. As in the Soxhlet extraction, three solvents were used: acetone, ethyl acetate, and hexane. A new sample was used for each extraction; the experiment was performed in triplicate. The material was kept in a Branson 3510 ultrasonic bath for 1.5 h. After this period, the solvent was removed, and a fresh 200 mL was added, remaining for another 1.5 h. At the end of this period (3 h), the extract was pooled, and the solvent was removed. The obtained product was treated with a saturated aqueous solution of NaHCO3 to remove traces of organic acids and extracted with hexane. The organic fraction was dried over Na2SO4, and the solvent was removed in a rotary evaporator. The seed oil was stored in an amber glass bottle with a PTFA cap in a freezer (-20 ºC) until analysis. Analytical methods Gas chromatography with flame ionization detector (GC-FID) Gas chromatography analysis was performed on a gas chromatograph Shimadzu (model GC-2010 Plus, Kyoto, Japan) with a flame ionization detector and nitrogen (N2) as carrier gas with an NTS-5 capillary column (30 m × 0.25 mm × 25 µm). The heating ramp started at 60 ºC for 4 min and increased by 8 ºC per min until 300 ºC. The sample injection mode was manual. The solvents used for sample preparation were high-performance liquid chromatography (HPLC)-grade hexane or HPLC-grade dichloromethane from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Gas chromatography-mass spectrometry (GC-MS) The gas chromatography-mass spectrometry analysis was performed on a gas chromatograph Shimadzu, model GC-2010 Plus, coupled to a mass spectrometer Shimadzu, model GCMS-QP5050A, with an NTS-5 capillary column (30 m × 0.25 mm × 25 µm) and helium (He) as a carrier gas at a flow rate of 1 mL min-1. The mass spectra were obtained by electronic ionization (EI) at 70 eV. The heating ramp started at 60 ºC for 4 min and increased by 8 ºC per min until reaching 300 ºC, with the sample injected using an automatic injector (temperature 250 ºC) in split mode. The solvents used to prepare the samples were hexane or dichloromethane HPLC-grade from Sigma-Aldrich. Identification of compounds The compounds were identified by comparison with the synthetic standard when available. The linear retention index (linear RI or simply RI) was calculated for all components from a homologous series of n-alkanes (C7-C40, from Sigma-Aldrich), and its comparison with literature data was also used to aid in the identification of the compounds. In addition, the mass spectra of the oil components were compared with the mass spectral data from the National Institute of Standards and Technology (NIST) and Wiley (Wiley MS) databases, as well as with an authentic sample.8 Calculation of yield and quantification The essential oil yield (Nr) was calculated according to Equation 1, where Mo represents the mass of oil obtained in each extraction, and Ms is the mass of the seeds used in each extraction:  The quantification was performed by comparing the area of the external standard (1-octadecanol, 75-200 ppm, see Figure 31S in the Supplementary Material) according to Equation 2, in GC-FID analysis with the same parameters presented in the topic. 
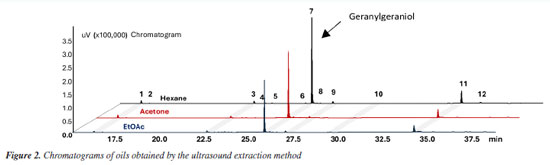
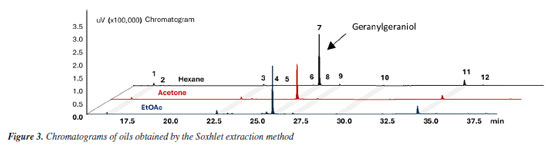
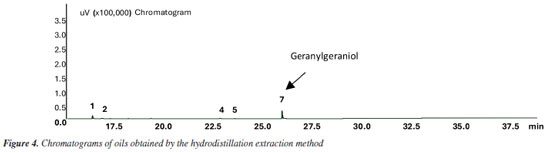
RESULTS AND DISCUSSION The extracts obtained from B. orellana seeds using the three techniques - hydrodistillation, Soxhlet, and ultrasound - were characterized by gas chromatography (GC-FID) under the same conditions. Figures 2-4 and 30S (in the Supplementary Material) show the chromatograms containing the profile of compounds obtained from annatto seeds oils from each technique, highlighting geranylgeraniol as the primary compound.
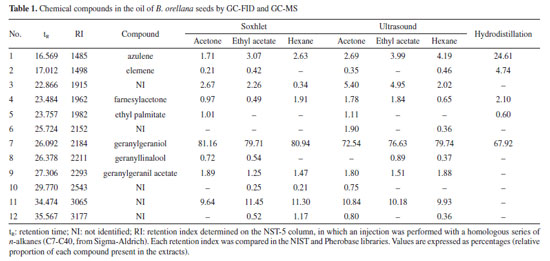
The analysis of the chromatograms indicates that each extract presented a different concentration of geranylgeraniol (Figures 2-4). However, the qualitative composition of the oils obtained was similar, with GGOH as the primary compound in all techniques used. In total, 12 compounds were identified in the oil of B. orellana seeds by GC-MS. Table 1 presents the retention index (RI) and retention time (tR) data of the oil components obtained by each technique. Each compound was identified by comparing the retention indices and mass spectra with spectral databases, literature data, and mass spectra analysis, and confirmed using a standard.
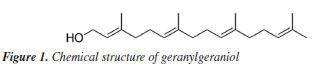
The oils obtained by Soxhlet and ultrasound-assisted extraction presented similar chemical composition, even in extractions using solvents of different polarities. In addition to GGOH, the main target component of the extraction, the following compounds were also obtained: azulene, farnesylacetone, and geranylgeranyl acetate (Table 1). Among the extracts, the main difference occurred in the extract obtained by hydrodistillation. Although it also had geranylgeraniol as the primary compound, it presented a higher concentration of azulene than the extracts obtained by the other techniques, approximately 25%, compared to a variation of 1 to 3% in the extracts with organic solvents. It also showed the presence of some minor constituents. In addition, it did not present the compound RI 3065 (Figures 4S, 8S, 12S, Supplementary Material), which is present in all extracts with organic solvents. This can be explained by the fact that hydrodistillation favors compounds with low molecular weight, which are more volatile.9,10 Due to this characteristic, the number of compounds in hydrodistillation is lower, with only four compounds identified (Figures 4 and 28S), as the heavier compounds are not easily dragged away, making the sample cleaner. However, the relative percentage of GGOH obtained is approximately 67%. In contrast, in extracts using organic solvents, the GGOH percentage varied from 72 to 79% in extracts obtained by the ultrasound technique and from 79 to 81% in extracts obtained by Soxhlet extraction (Table 1). Studies3 show that the presence of organic solvents significantly improves the extraction efficiency of bioactive compounds. Solvents with different polarities, such as hexane, ethyl acetate, and acetone, can obtain different compounds due to the stronger interaction between similar polarities. Despite this, the compounds obtained using the different solvents were practically the same, with terpenes and terpenoids generally being found. However, the amount of GGOH was higher in the Soxhlet and ultrasound techniques than in hydrodistillation, likely because these methods selectively extract specific compounds with organic solvents. Different solvents were also used to evaluate the oil yield obtained in each extraction. The extractions using organic solvents presented a higher oil yield than those carried out by hydrodistillation. For every 60.0 g of annatto seed, between 0.80 and 1.21 g of oil were obtained in the extractions carried out by Soxhlet and ultrasound, respectively, while in the hydrodistillation method using 200.0 g of seed, 0.15 g of oil was obtained. Table 2 shows the yields obtained for each extraction technique. It should be noted that the organic solvents acetone, ethyl acetate, and hexane increased the extraction efficiency by solubilizing lipophilic compounds like geranylgeraniol in a higher oil yield. The more polar solvents, ethyl acetate and acetone, presented better extraction yields; however, it was necessary to perform a treatment to remove bixin and norbixin with NaHCO3 solution, which, due to its alkalinity, can remove organic acid compounds. However, when hexane was used, with or without treatment, this removal procedure did not generate a difference in the extracted mass obtained, indicating that it is necessary to perform this process only with the more polar solvents.
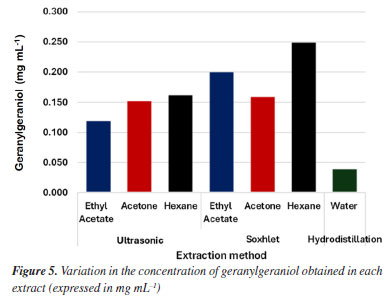
The relative percentage of GGOH in each sample was quantified from a calibration curve using an external standard, 1-octadecanol (Figure 31S), at different concentrations ranging from 25 to 200 ppm (R2 = 0.999). The extracts obtained by the various techniques showed a variation of 0.040 to 0.250 g of GGOH per mL of sample (Figure 5). Among the methods using an organic solvent, hexane presented the lowest yields in the extraction of oil. However, Soxhlet extraction using this solvent resulted in the highest concentration of GGOH in the oil obtained (0.250 g mL-1), indicating that, due to its characteristics, it may be a promising choice for the large-scale extraction of this component.
Despite having a lower yield, hydrodistillation extraction has the advantage of avoiding organic solvents. Furthermore, it has an extraction system that is quite simple and easy to handle. Although environmentally sustainable, it is less efficient in extracting compounds with higher molecular weights.11 On the other hand, extractions using organic solvents have higher yields but are more expensive and require environmental considerations. After each extraction, the solvent must be removed to obtain the oil. The use of GGOH has grown significantly, especially in the medicinal area, where it has been proven to have antinociceptive or analgesic properties.12 It is also a preventive agent for jaw osteonecrosis related to bisphosphonates.13 More recently, GGOH has been marketed as a dietary supplement in annatto extract and is generally recognized as safe, opening the door to potential clinical use as an endogenous nutrient.14 To this end, the most exciting technique is hydrodistillation, considered only for medicinal use, since it does not use organic solvents, making its consumption safer. In addition to its use in health fields, GGOH has generated interest in agriculture due to its efficient repellent and insecticidal properties, as reported for Helicoverpa armigera (Hübner), a potent inhibitor of farnesol dehydrogenase. When used in an artificial diet, geranylgeraniol acted as a competitive inhibitor of farnesol dehydrogenase and, as a result, reduced the growth and development of H. armigera larvae.15 Annatto extracts containing geranyl-α-terpinene and geranylgeraniol have vigorous larvicidal activity.16 In addition, GGOH has been reported in insects, mainly of the orders Hymenoptera and Coleoptera, promoting new research into potential use in formulations for the behavioral control of insects and as natural insecticides for integrated pest management (IPM). GGOH is a component of the pheromone of insects of the order Coleoptera, in the genus Agriotes. Their larvae are called spider worms, and the adults are click beetles, which are considered agricultural pests in Europe, Asia, and North America. In ants (soldiers) of the subfamily Ponerinae of the order Hymenoptera, GGOH acts as a trail pheromone for the mass recruitment of individuals. In bees, it is present in the secretion of the labial gland of male insects of Bombus morrisoni Cresson and Bombus rufocinctus from North America.17-19 GGOH derivatives, such as geranylgeraniol acetate, are present in several pheromone formulations, mainly for pests of the families Elateridae (Coleoptera), Apidae, and Formicidae (Hymenoptera).20
CONCLUSIONS Geranylgeraniol is an extensively studied compound that is mainly used for medicinal purposes. In agriculture, it has been studied as both an attractant and a pest repellent. For large-scale production, Soxhlet extraction using hexane as a solvent is an efficient way to obtain GGOH, since no post-extraction treatment is necessary. Better extraction yields with more polar solvents require a purification step. Ultrasound-assisted extraction is fast and efficient, with yields comparable to Soxhlet extraction. Acetone emerged as the most efficient solvent for this extraction technique. For direct consumption for medicinal purposes, the best extraction method is hydrodistillation, as it uses only water in the extraction process, avoiding organic solvents.
SUPPLEMENTARY MATERIAL Complementary material for this work is available at http://quimicanova.sbq.org.br/, as a PDF file, with free access.
DATA AVAILABILITY STATEMENT All data is available in the present text and supplementary material.
ACKNOWLEDGMENTS This study was supported by National Council of Humanities, Sciences and Technologies (CONAHCYT), National Institute of Science and Technology - Semiochemicals in Agriculture (CNPq proc. 465511/2014-7 and FAPESP proc. 2014/50871-0), Research Support Foundation of the State of Alagoas (FAPEAL), and the National Council for Scientific and Technological Development (CNPq).
REFERENCES 1. Dequigiovani, G.; Ramos, S. L. F.; Alves-Pereira, A.; Fabri, E. G.; Carvalho, P. R. N.; da Silva, M. G.; Abdo, M. T. V. N.; Martins, A. L. M.; Clement, C. R.; Veasey, E. A.; Genet. Resour. Crop Evol. 2017, 64, 1775. [Crossref] 2. Mercadante, A. Z. In Chemistry and Physiology of Selected Food Colorants; Ames, J. M.; Hofmann, T., eds.; American Chemical Society: Washington, 2001, ch. 6. [Crossref] 3. Alcázar-Alay, S. C.; Osorio-Tobón, J. F.; Forster-Carneiro, T.; Meireles, M. A. A.; Food Res. Int. 2017, 99, 393. [Crossref] 4. Jondiko, I. J. O.; Pattenden, G.; Phytochemistry 1989, 28, 3159. [Crossref] 5. Souiy, Z. In Essential Oils - Recent Advances, New Perspectives and Applications; Viskelis, J., ed.; IntechOpen: Rijeka, 2023, ch. 8. [Crossref] 6. Kant, R.; Kumar, A.; Sustainable Chem. Pharm. 2022, 30, 100829. [Crossref] 7. de Almeida-Couto, J. M. F.; Abrantes, K. K. B.; Stevanato, N.; da Silva, W. R.; Wisniewski, A.; da Silva, C.; Cabral, V. F.; Cardozo-Filho, L.; J. Supercrit. Fluids 2022, 180, 105432. [Crossref] 8. National Institute of Standards and Technology (NIST), NIST Chemistry WebBook, https://webbook.nist.gov/chemistry/, accessed in June 2025. [Crossref] 9. Tocchini, L.; Mercadante, A. Z.; Cienc. Tecnol. Aliment. (Campinas, Braz.) 2001, 21, 310. [Crossref] 10. Fagbemi, K. O.; Aina, D. A.; Adeoye-Isijola, M. O.; Naidoo, K. K.; Coopoosamy, R. M.; Olajuyigbe, O. O.; Sci. Rep. 2022, 12, 9432. [Crossref] 11. Pino, J. A.; Correa, M. T.; J. Essent. Oil Res. 2003, 15, 66. [Crossref] 12. Spindola, H. M.; Servat, L.; Denny, C.; Rodrigues, R. A. F.; Eberlin, M. N.; Cabral, E.; Sousa, I. M. O.; Tamashiro, J. Y.; Carvalho, J. E.; Foglio, M. A.; BMC Pharmacol. 2010, 10, 1. [Crossref] 13. Chin, K. Y.; Ekeuku, S. O.; Trias, A.; Front. Pharmacol. 2022, 13, 878556. [Crossref] 14. Miyawaki, A.; Rojasawasthien, T.; Hitomi, S.; Aoki, Y.; Urata, M.; Inoue, A.; Matsubara, T.; Morikawa, K.; Habu, M.; Tominaga, K.; Kokabu, S.; In Vivo 2020, 34, 2345. [Crossref] 15. Kumar, R.; Das, J.; Rode, S.; Kaur, H.; Shah, V.; Verma, P.; 3 Biotech 2023, 13, 175. [Crossref] 16. Barrie, T.; Jia, Z.; US pat. PCT /US2018/060229 B1 2018. 17. Oldham, N. J.; Morgan, E. D.; Gobin, B.; Schoeters, E.; Billen, J.; J. Chem. Ecol. 1994, 20, 3297. [Crossref] 18. Yatsynin, V. G.; Rubanova, E. V.; Okhrimenko, N. V.; J. Appl. Entomol. 1996, 120, 463. [Crossref] 19. Bestmann, H. J.; Janssen, E.; Kern, F.; Liepold, B.; Hölldobler, B.; Boveri, T.; Naturwissenschaften 1995, 82, 334. [Crossref] 20. The Pherobase, https://pherobase.com/, accessed in June 2025.
Editor handled this article: Jorge M. David |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access