Artigo
| A new prenylated guaiane from the brown seaweed Dictyota mertensii |
|
Neilyane de Paula FernandesI; Fábio do Nascimento ÁvilaI I. Departamento de Química Orgânica e Inorgânica, Universidade Federal do Ceará, 60021-970 Fortaleza - CE, Brasil Received: 02/28/2025 *e-mail: opessoa@ufc.br A chemical investigation of the brown seaweed Dictyota mertensii (Hoyt) Schnetter, Hörning & Weber-Peukert, a member of the Dictyotaceae, was carried out. The ethyl acetate extract led to the isolation of two prenylated guaiane, including a new one (1) and the known dictyol H (2). In addition, the dolabellane diterpene 10,18-dihydroxy-dolabella-2,7-diene (3) and two benzalkonium chlorides (4-5) were also isolated. The structures of all compounds were elucidated by an extensive analysis of their one- and two-dimensional nuclear magnetic resonance data (1D and 2D NMR), and high-resolution electrospray ionization mass spectrometry (HRESIMS). The cell viability and the anti-inflammatory activity of diterpenes 1 and 2 were evaluated against lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. INTRODUCTION The marine ecosystem boasts remarkable biodiversity, with approximately 242 thousand described animal species and representatives of nearly all known animal phyla, highlighting its taxonomic richness and the ongoing discovery of new marine taxa.1 Among the countless living beings inhabiting this ecosystem, algae play an essential role as leading primary producers.2 Algae have numerous applications, including the food, cosmetic, and pharmaceutical industries.3 The Dictyotaceae is a large family of brown seaweed comprising approximately 349 species composed by 19 genera.4 Brown macroalgae, particularly those of the family Dictyotaceae, are prolific producers of secondary metabolites, accounting for more than 60% of the known compounds isolated from marine macroalgae.5 Dictyotaceae species are key components of benthic communities in tropical and temperate regions, including the diverse and extensive Brazilian coastline. With its extensive littoral, Brazil holds a rich and diverse marine ecosystem with a wide range of biological species, including brown macroalgae.5 The genus Dictyota comprises more than 94 taxonomically authenticated species and several species with uncertain classification.6 Previous chemical studies show that the genus is a major producer of secondary metabolites, moreover, compound-derived terpenes are the most abundantly found.5 Antiproliferative, antimicrobial, antiviral, antioxidant, anti-inflammatory, antihyperpigmentation, and chemical defensive activities have been reported for compounds from the genus.5,7-9 This study aimed to investigate the chemical composition of the extracts from the brown alga Dictyota mertensii collected at the sea coast of Ceará State, Brazil, and, as part of the collaborative work of the organic chemistry and pharmacology departments at UFC (Universidade Federal do Ceará), to search for the biological activity of the isolated secondary metabolites from natural sources, the anti-inflammatory potential of the isolated compounds was evaluated.
EXPERIMENTAL General experimental procedures Chromatographic separations were performed by column chromatography on silica gel 60 (0.063-0.200 mm, Merck) or Sephadex LH-20 (Pharmacia Biotech) with pure analytical solvents (Synth). Analytical thin layer chromatography (TLC) was carried out on precoated silica gel 60 F-254 (200 µm) aluminum plates (Silicycle) with a fluorescent indicator (254 nm), and the spots were visualized by UV detection after spraying with a cerium sulfate solution (0.1% w/v), followed by heating. High-performance liquid chromatography (HPLC) was performed on a Shimadzu apparatus equipped with an SPD-M20A UV-Vis diode detector, and an RP-C18 or Phenyl-Hexyl semi-preparative column (Phenomenex®, 250 × 10 mm, 5 μm) with a flow rate of 3 mL min-1 at 35 ºC and wavelength monitored at 210-400 nm. Optical rotations were measured with a Jasco P-2000 polarimeter, operating with a tungsten lamp at a wavelength of 589 nm at 22 ºC. High-resolution electrospray ionization mass spectra (HRESIMS) were recorded on a Waters Acquity UPLC system coupled to a quadrupole/time-of-flight (QTOF) system (UPLC/Qtof MSE spectrometer) in the positive mode and low-resolution mass spectra were recorded on a Shimadzu QP2010 (GC-MS), with electron impact and equipped with an RTX column (30 m × 0.25 mm). The nuclear magnetic resonance (NMR) spectra were performed on a Bruker AVANCE DPX-300 (300.13 MHz for 1H and 75.48 MHz for 13C), using either CDCl3 or CD3OD as deuterated solvents. Sample collection The brown seaweed D. mertensii (Hoyt) Schnetter, Hörning & Weber-Peukert was collected during the low tide (SisGen license number A86B918), on Flecheiras beach (03º13'S; 39º16'W) at Trairi County, at the west coastline of Ceará State, Brazil. A voucher specimen (HMAR 2999) has been deposited at the Herbário Professora Francisca Pinheiro at the Institute of Marine Sciences (LABOMAR). Extraction and isolation The brown seaweed D. mertensii was washed with distilled water, dried at room temperature, and crushed (56.9 g) followed by extractions with hexane (500 mL, 2×), EtOAc (500 mL, 2×), and EtOH (500 mL, 2×). The EtOAc extract (3.3 g) was fractionated over silica gel (30.0 g) gravity column chromatography (θ = 4.7 cm) by elution with hexane/EtOAc 75:25, 50:50, 25:75, EtOAc, and MeOH to provide the five respective fractions (A-E), after solvent evaporation. Fraction B (2.0 g) was further subjected to silica gel (25.0 g) column chromatography (θ = 4.7 cm) eluting with hexane/EtOAc 90:10, 80:20, 70:30, 60:40, 50:50, 25:75, EtOAc, and MeOH yielding eleven subfractions (BA-BK) after TLC analysis. Subfraction BC (343.5 mg) was fractionated on Sephadex LH-20 eluted with MeOH to give three subfractions BCA-BCC. The subfraction BCC (222.0 mg) was purified by HPLC using a reverse phase phenyl-hexyl semi-preparative column and a gradient solvent system of H2O (trifluoroacetic acid (TFA) 0.1%)/MeOH (55-100 (v/v)) for 0-15 min + 10 mim MeOH, at a flow rate of 3 mL min-1 to yield compounds 4 (retention time (tR) = 15.16 min; 17.0 mg), 5 (tR = 16.10 min; 9.1 mg), and 1 (tR = 20.20 min; 7.6 mg), and a fraction corresponding to the peak tR = 21.56 min. This fraction (53.7 mg) was further subjected to HPLC using a gradient solvent system of H2O (TFA 0.1%)/MeOH (85-100 (v/v)) for 0-5 min followed by MeOH for 15 min, at a flow rate of 3 mL min-1 to yield the compound 2 (tR = 10.42 min; 7.4 mg). Subfraction BF (214.5 mg) was fractionated on Sephadex LH-20, eluted with MeOH, to give the subfractions BFA-BFC. The subfraction BFB (72.6 mg) was subjected to HPLC under the same conditions than peak tR = 21.56 to yield 3 (tR = 10.21 min; 8.4 mg). Dictyol N (1) Yellowish resin, +65.76º (c 0.1 MeOH); UV(MeOH) λmáx 199 nm; HRESIMS m/z 379.2479 calcd. for C22H35O5, [M + H]+ not observed; m/z 319.2274 [(M-CH3CO2H) + H]+ (calcd. for C20H31O3, 319.2268); m/z 301.2147 [(M-CH3CO2H-H2O) + H]+ (calcd. for C20H29O2, 301.2162); m/z 283.2012 [(M-CH3CO2H-2H2O) + H]+ (calcd. for C20H29O2, 283.2056); m/z 185.1220 [(M-C12H18O2) + H]+ (calcd. for C10H17O3, 185.1172). Pharmacological assays Cell culture RAW 264.7 cells (ATCC® TIB-71) were cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum, 100 IU mL-1 penicillin, and 100 µg mL-1 streptomycin, at 37 ºC, in 5% CO2 and 95% humidity. Cell viability assay in RAW 264.7 cells RAW 264.7 cells were seeded in 96-well microplates at a density of 1 × 105 cells well-1 and incubated for 24 h. Cells were treated with compounds 1 and 2 (12-200 µM) and Triton X-100 (1% v/v, cytotoxic standard) for 24 h. After this period, 3-(4,5-dimethylthazolk-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, 1 mg mL-1) was added to each well, and after 4 h, the medium was removed and dimethyl sulfoxide (DMSO, 100 µL) was added.10 Absorbance was read using a microplate reader (Asys UVM 340, Biochrom, USA) at a wavelength of 570 nm. Results are expressed as percentages of cell viability relative to the control group (100% viability). The half maximal inhibitory concentration (IC50) was calculated from concentration-response curves, and results were obtained from three independent experiments performed in triplicate. Nitric oxide (NO) production inhibition assay RAW 264.7 cells were seeded in 48-well microplates at a density of 5 × 105 cells well-1 and incubated for 24 h. Cells were pre-treated with compounds 1 and 2 (12-200 µM) for 1 h, followed by treatment with lipopolysaccharide (LPS, 1 μg mL-1) at 37 ºC for 24 h. Then, 100 μL of supernatant was mixed with 100 μL of Griess reagent (1% sulfanilamide, 0.1% N-[1-naphthyl]ethylenediamine dihydrochloride, 5% phosphoric acid) in a 96-well microplate for reading at 540 nm using a spectrophotometer. Dexamethasone (4 µM) was used as a standard, and nitrite quantification was calculated from a sodium nitrite standard curve. Results were obtained from three independent experiments performed in triplicate. Statistical analysis of the pharmacological assays was performed using GraphPad Prism.11 IC50 values are expressed as mean ± standard deviation, while cell viability percentages are presented as the mean ± standard error of the mean. Comparisons between means were analyzed using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. Differences were considered statistically significant at p < 0.05.
RESULTS AND DISCUSSION Chromatographic fractionation of the ethyl acetate extract from the brown alga D. mertensii led to the isolation and characterization of a new prenylated diterpene of guaiane skeleton (1), in addition to the already known diterpenes, dictyol H (2)12 and 10-acetoxy-18-hydroxy-dolabella-2,7-diene (3).13 Additionally, two ammonium quaternary salts (4 and 5)14 were also isolated (Figure 1).
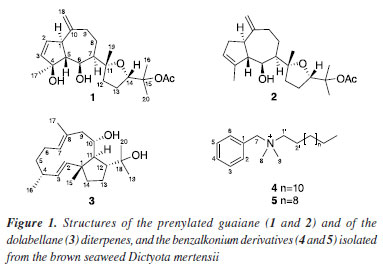
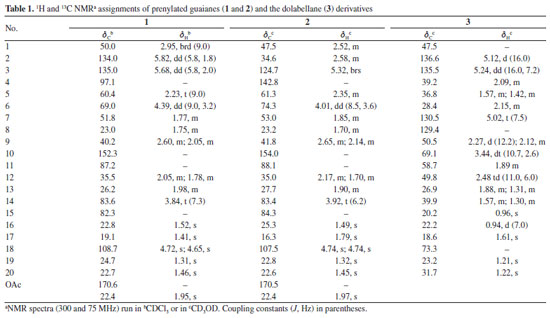
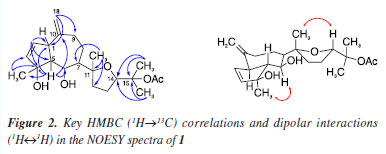
Diterpene 1 was isolated as a yellowish resin ( +65.76º c 0.1 MeOH). Its 1H NMR spectrum exhibited signals for olefinic protons corresponding to a carbon-carbon double bond having a cis-configuration dH 5.82 (dd, J 5.8, 1.8 Hz, H-2) and 5.68 (dd, J 5.8, 2.0 Hz, H-3) and an exocyclic double bond indicated by the methylidene proton singlets at dH 4.72 (s, H-18a) and 4.65 (s, H-18b), respectively. In addition, 1 showed resonances for two oxymethine protons dH 4.39 (dd, J 9.0, 3.2 Hz, H-6) and 3.84 (t, J 7.3 Hz, H-14), and two methine protons dH 2.95 (brd, J 9.0 Hz, H-1) and 2.23 (t, J 9.0 Hz, H-5) in a trans-diaxial arrangement characteristic of perhydroazulene fusion.15 In addition, signals for five methyl singlets, including one of an acetyl group at dH 1.95 (s) were observed. The 13C NMR spectrum showed signals corresponding to 22 carbon atoms compatible with an acetylated diterpene, commonly isolated from algae of the Dictyotaceae.16-18 The signals at dC 152.3 (C-10), 108.7 (C-18), 60.4 (C-5), and 50.0 (C-1) are typical of prenylated guaiane diterpenes.18,19 The combination of the 13C NMR CPD (composite pulse decoupling), and HSQC (heteronuclear single quantum correlation) techniques, revealed signals for a cis-disubstituted carbon-carbon double-bond dC/dH 135.0/5.68 (dd, J 5.8, 2.0 Hz, H-3) and 134.0/5.82 (dd, J 5.8, 1.8 Hz, H-2), and two oxymethine carbons 83.6/3.84 (t, J 7.3 Hz, H-14) and 69.0/4.39 (dd, J 9.0, 3.2 Hz, H-6). In addition, the 13C NMR spectrum exhibited signals for three oxygenated tertiary carbon atoms dC 97.1 (C-4), 87.2 (C-11), and 82.3 (C-15). The double bond at C2/C3 was assigned based on heteronuclear multiple bond correlations (HMBC) of H-2 (dH 5.82, dd, J 5.8, 1.8 Hz) with dC 97.1 (C-4), 60.4 (C-5) and 50.0 (C-1) while the hydroxyl groups at C-4 and C-6 was supported based on correlations of H-5 (dH 2.23, t, J 9.1 Hz) with dC 50.0 (C-10), 97.1 (C-4), 69.0 (C-6), 50.0 (C-1) and 22.8 (CH3-17). The relative stereochemistry of compound 1 was established through nuclear Overhauser effect spectroscopy (NOESY) correlations (Figure 2). Cross-peaks for H-6 and the α-oriented methyl group CH3-17, as well as for H-14 and CH3-19, indicate that these protons are located on the same face of the tetrahydrofuran ring.13 Additionally, the large coupling constants observed between H-1/H-5 and H-5/H-6 (J 9.0 Hz) support a trans-diaxial arrangement, reinforcing the proposed anti-relationship among these protons.
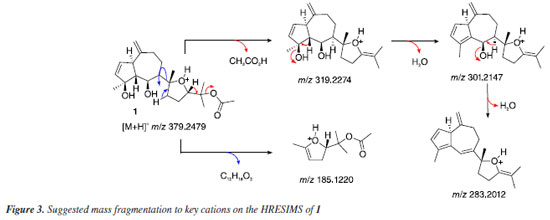
The molecular formula C22H34O5 (calcd. 378.2406 Da, six degrees of unsaturation) was determined based on m/z 319.2274 Da, corresponding to the loss of a neutral unity of acetic acid [(M-CH3CO2H) + H]+. The ion peaks at m/z 301.2147 Da [(M-H2O) + H]+ and 283.2012 Da [(M-2H2O) + H]+ showed a good match with the bicyclic moiety in agreement with the suggested structure (Figure 3). Thus, the structure of diterpene 1 was determined as a new diterpene of the prenylated guiane type, common in Dictyota species,20 and was named dictyol N due to its structural similarity to previously described dictyols and in accordance with the established naming sequence within this diterpene family.
A detailed analysis of the 1H and 13C NMR spectra of compound 2 revealed a high structural similarity with compound 1, being the main difference related to the chemical shift at dH 5.32 (brs, H-3), associated with a vinyl proton, and the correspondents dC 124.7 (C-3) and 142.8 (C-4) corroborating with a trisubstituted double bond C-3/C-4, and the disappearance of the tertiary alcohol carbon atom (C-4) suggesting the dehydration to the formation of the C=C doublet bond. Comparison of the proton and carbon chemical shifts of 2 with those of the known dictyol H, previously isolated from the brown seaweed D. dentata,21 revealed a good match, thus confirming their identity. Analysis of the 1H NMR spectrum of 3 showed signals of scalar coupling vinyl protons at dH 5.24 (dd, J 16.0, 7.2 Hz, H-3) and 5.12 (d, J 16.0 Hz, H-2), for a trans carbon-carbon double bond, and at dH 5.02 (t, J 7.5 Hz, H-7) for a trisubstituted carbon-carbon double bond. Moreover, signals for an oxymethine proton dH 3.44 (dt, J 10.7, 2.6 Hz, H-10), four methyl groups at dH 1.61 (H-17), 1.22 (H-20), 1.21 (H-19), 0.96 (H-15) as singlets, and just one methyl at dH 0.94 (J 7.0 Hz, H-16) as a doublet were observed. The 13C NMR CPD spectrum showed 20 spectral lines indicating a diterpene structure. The olefinic carbons at dC 136.6 (C-2), 135.5 (C-3), 130.5 (C-7), and 129.4 (C-8) corroborate the presence of both double bonds, while the signals at dC 73.3 (C-18) and 69.1 (C-10) indicated a tertiary and secondary alcohol, respectively (Table 1).
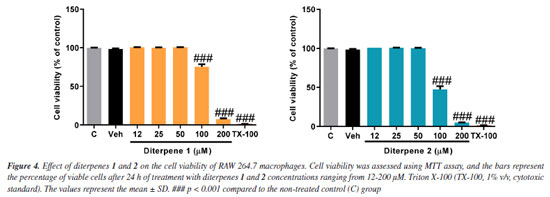
The 1H and 13C NMR data allowed to suggest the molecular formula C20H34O2 (four degrees of unsaturation) that was confirmed by the mass spectrum through the deprotonated molecule [M - H]+ m/z 305.2493 Da, and ion peaks at m/z 287.2357 Da [(M-H2O) - H]+ and m/z 269.2266 Da [(M-2H2O) - H]+, corresponding to the loss of two water molecules. If compound 3 possesses two carbon-carbon double bonds one can infer that compound 3 is bicyclic. Dictyota spp. around the globe produces ubiquitous [9.3.0] bicyclic diterpenes belonging to the dolabellane class. Indeed, search in the literature for dolabellane diterpenes from Dictyota revealed a structure similar to compound 3. The 1H and 13C NMR data comparison of compound 3 with those of the 10,18-dihydroxy-dolabella-2,7-diene,22,23 previously isolated from Dolabella californica24 and Dictyota dichotoma,13,19,25 permitted the identification of their structures. The 1H NMR spectrum of compound 4 exhibited a singlet at dH 7.57 with integration for five protons indicating a monosubstituted benzene ring. The signals at dH 4.52 (s, H-7) and 3.31 (m, H-1') were consistent with protons of methylene carbons bound to a positively charged ammonium salt. The singlet at dH 3.02, with integration corresponding to six protons, indicated two N-methyl groups, as well as the signals at dH 1.84-1.29 and 0.91 (t, J 6.6 Hz) indicated a saturated long-chain. The 13C NMR spectrum showed signals corresponding to an aromatic ring (dC 134.2 to 129.0) and four nitrogenous carbons, two of which dC 68.9 and 66.0 are methylene carbons and two dC 50.5 and 50.0 for methyl groups. The mass spectrum (70 eV) of 4 showed two key ion peaks, one at m/z 241 Da corresponding to an N, N-dimethyl-tetradecane-amine formed by the loss of the benzyl radical and another one at m/z 58 Da (base peak) corresponding to the fragment N, N-dimethyl-imine moiety which was formed by the loss of a tridecane radical (Figure 15S, Supplementary Material). Therefore, the molecular formula C23H42N (m/z 332), including the side chain size (C14), permitted the identification of compound 4 as the benzyldimethyltetradecylammonium cation. The 1H NMR spectrum of compound 5 was identical to that of 4 indicating a similar structure. Its mass spectrum (70 eV) exhibited ion peaks at m/z 213 and 58 Da compatible with the N, N-dimethyl-dodecane-1-amine and N, N-dimethyl-imine moieties, respectively. Thus, the structure of 5 was identified as the benzyldimethyldodecylammonium cation. These compounds are synthetic quaternary ammonium chloride salts known as benzalkonium chlorides (BKCs) which are widely used in the pharmaceutical industry due to their broad-spectrum antimicrobial properties against bacteria, fungi, and virus.26,27 Furthermore, they are present in several products for daily household use such as fabric softeners, personal hygiene products, and cosmetics. Reports of BKCs in natural sources are generally associated with the irregular disposal into the environment of large amounts of products containing these salts in their formulations, which are easily incorporated into the cellular tissue of terrestrial and marine organisms.14 Cell viability and nitric oxide (NO) production inhibition assays were performed for diterpenes 1 and 2. The compound profiles are presented in Figure 4. Cell viability is similar for diterpenes 1 and 2, as both substances exhibit comparable IC50 values, indicating that changing the position of the endocyclic double bond does not influence their cytotoxicity.
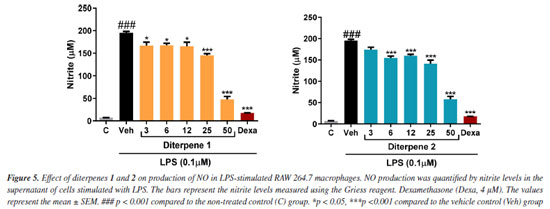
In the NO production inhibition assay, diterpenes 1 and 2 significantly reduced NO production. diterpene 1 effectively inhibited NO production at all tested concentrations (Figure 5). Conversely, for diterpenes 1 and 2 the most effective inhibition occurred at a concentration of 50 µM, achieving inhibition rates of 75 and 70%, respectively. Dexamethasone, used as a reference control, achieved a remarkable 91.18% reduction in NO production compared to the vehicle group.
CONCLUSIONS The marine algae of the genus Dictyota, including the Brazilian species, as rich sources of miscellaneous diterpenes, particularly those of the prenylated guaiane and dolabellane skeletons, are corroborated by the now reported results. The new dictyol N (1) and the knowns dictyol H (2) and the 10,18-dihydroxy-dolabella-2,7-diene (3) are being described for the first time for Dictyotamertensii. Both prenylated guaianes, 1 and 2, demonstrated anti-inflammatory potential, inhibiting NO production in LPS-stimulated macrophage cells. Despite their moderate activities, currently another paper covering the anti-inflammatory action of these kinds of diterpenes has just been published.9 Finally, the benzalkonium salts, recognized as environmental contaminants, and isolated from marine organisms are indicators of the pollution levels of the sites where the algae survive.
SUPPLEMENTARY MATERIAL Supplementary data are available for free access at http://quimicanova.sbq.org.br, in PDF format.
DATA AVAILABILITY STATEMENT All data are available in the text.
ACKNOWLEDGMENTS This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (No. 406119/2021-0 and 310183/2020-0), INCT BioNat (No. 465637/2014-0), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Finance Code 001), and Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (Funcap).
AUTHOR CONTRIBUTIONS Neilyane de Paula Fernandes was responsible for the extraction and isolation; Fábio do Nascimento Ávila for the extraction and isolation, and structure determination; Kesya Amanda D. Rocha for the discussion of the results and write the main manuscript; Pedro Bastos de Mâncedo Carneiro for the identification of biological material; Greyce L. Sasahara for the pharmacological assays; Flavia A. Santos for the pharmacological assays; Edilberto R. Silveira for the critical review; Otília Deusdenia L. Pessoa for the data interpretation, discussion of the results, and write the manuscript.
REFERENCES 1. Bouchet, P.; Decock, W.; Lonneville, B.; Vanhoorne, B.; Vandepitte, L.; Front. Mar. Sci. 2023, 10, 929989. [Crossref] 2. Sahoo, D.; Seckbach, J.; The Algae World: Cellular Origin, Life in Extreme Habitats and Astrobiology, 1st ed.; Springer: Jerusalem, 2015. 3. Øverland, M.; Mydland, L. T.; Skrede, A.; J. Sci. Food Agric. 2019, 99, 13. [Crossref] 4. dos Santos, T. C.; Obando, J. M. C.; Cavalcanti, D. N.; Martins, R. C. C.; Rev. Biodiversidade 2023, 22, 55. [Link] accessed in June 2025 5. Georgii, A. D. N. P.; Teixeira, V. L.; Mar. Drugs 2023, 21, 484. [Crossref] 6. AlgaeBase, http://www.algaebase.org, accessed in June 2025. 7. Rushdi, M. I.; Abdel-Rahman, I. A. M.; Attia, E. Z.; Saber, H.; Saber, A. A.; Bringmann, G.; Abdelmohsen, U. R.; Molecules 2022, 27, 672. [Crossref] 8. Chen, J.; Li, H.; Zhao, Z.; Xia, X.; Li, B.; Zhang, J.; Yan, X.; Mar. Drugs 2018, 16, 159. [Crossref] 9. Shiiba, N.; Kumagai, M.; Endo, H.; Tsuruta, T.; Nishikawa, K.; Morimoto, Y.; Biosci. Biotechnol. Biochem. 2025, 89, 224. [Crossref] 10. Mosmann, T.; J. Immunol. Methods 1983, 65, 55. [Crossref] 11. GraphPad Prism, version 8.0; Intuitive Software for Science, San Diego, CA, 2019. 12. König, G. M.; Wright, A. D.; Sticher, O.; Tetrahedron 1991, 47, 1399. [Crossref] 13. Amico, V.; Oriente, G.; Piattelli, M.; Tringali, C.; Fattorusso, E.; Magno, S.; Mayol, L.; Tetrahedron 1980, 36, 1409. [Crossref] 14. Russo, C.; Kundi, M.; Lavorgna, M.; Parrella, A.; Isidori, M.; Arch. Environ. Contam. Toxicol. 2018, 74, 546. [Crossref] 15. Saito, F.; Becker, J.; Schreiner, P. R.; J. Org. Chem. 2020, 85, 4441. [Crossref] 16. De Paula, J. C.; Vallim, M. A.; Teixeira, V. L.; Rev. Bras. Farmacogn. 2011, 21, 216. [Crossref] 17. Vallim, M. A.; De Paula, J. C.; Pereira, R. C.; Teixeira, V. L.; Biochem. Syst. Ecol. 2005, 33, 1. [Crossref] 18. Cheng, S.; Zhao, M.; Sun, Z.; Yuan, W.; Zhang, S.; Xiang, Z.; Cai, Y.; Dong, J.; Huang, K.; Yan, P.; J. Nat. Prod. 2014, 77, 2685. [Crossref] 19. Othmani, A.; Bouzidi, N.; Viano, Y.; Alliche, Z.; Seridi, H.; Blache, Y.; El Hattab, M.; Briand, J.-F.; Culioli, G.; J. Appl. Phycol. 2014, 26, 1573. [Crossref] 20. Pinheiro, A. D. N.; Lopes-Filho, E. A. P.; De-Paula, J. C.; Pereira Netto, A. D.; Teixeira, V. L.; Biochem. Syst. Ecol. 2019, 86, 103926. [Crossref] 21. Alarado, A. B.; Gerwick, W. H.; J. Nat. Prod. 1985, 48, 132. [Crossref] 22. Ireland, C.; Faulkner, D. J.; J. Org. Chem. 1977, 42, 3157. [Crossref] 23. Piattelli, M.; Tringali, C.; Neri, P.; Rocco, C.; J. Nat. Prod. 1995, 58, 697. [Crossref] 24. Ireland, C.; Faulkner, D. J.; Finer, J.; Clardy, J.; J. Am. Chem. Soc. 1976, 98, 4664. [Crossref] 25. Durán, R.; Zubía, E.; Ortega, M. J.; Salvá, J.; Tetrahedron 1997, 53, 8675. [Crossref] 26. Pereira, B. M. P.; Tagkopoulos, I.; Appl. Environ. Microbiol. 2019, 85, e00377-19. [Crossref] 27. Qian, Y.; He, Y.; Li, H.; Yi, M.; Zhang, L.; Zhang, L.; Liu, L.; Lu, Z.; Environ. Pollut. 2022, 292, 118305. [Crossref]
Guest Editor handled this article: Ivo José C. Vieira |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access