Artigo
| Microencapsulated powders of agraz (Vaccinium meridionale Swartz) fruit as a carrier for metabolites with in vitro antihypertensive activity |
|
Andrés Mauricio AmayaI; María Carolina OtáloraII; Coralia OsorioI,* I. Departamento de Química, Universidad Nacional de Colombia, 14490 Cundinamarca, Bogotá, Colombia Received: 03/09/2025 *e-mail: cosorior@unal.edu.co The agraz (Vaccinium meridionale Swartz) fruit is an anthocyanin source with antihypertensive in vitro activity. V. meridionale fruit powders were obtained by spray-drying (SD) with maltodextrin DE-20 (MD). A polar extract was obtained by acetone-water extraction and then partitioned with solvents of increasing polarity. The anthocyanin-rich extract was microencapsulated using two inlet temperatures (120 and 150 ºC) and two ratios between fruit (AGZ) and encapsulating agent (MD) (1:1 and 2:3, w/w). The four microencapsulated powders (M1-M4) were physicochemically characterized; the total phenolic content and the in vitro antihypertensive activity (inhibition of the angiotensin-I enzyme, ACE-I) were measured in the freeze-dried fruit and polar fractions. V. meridionale fruit has potential as an ACE-I inhibitor, with delphinidin and cyanidin derivatives being among the chemical compounds responsible for this activity. The physicochemical, morphological, thermal, and biofunctional properties of microencapsulates were evaluated, finding that the M1 and M3 samples (120 ºC, 1:1, and 150 ºC, 1:1, respectively) showed the highest antihypertensive activity value. These results suggest that V. meridionale could be used to develop microencapsulates with biofunctional properties, which can be used as additives for different food matrices. INTRODUCTION Agraz or Andean blueberry (Vaccinium meridionale Swartz) is a fruit belonging to the Ericaceae family grown in the mountain regions of Colombia, around 2100 m above sea level.1 Vacciniummeridionale fruit has low sugar content and high concentration of antioxidant compounds, vitamins B1, B2, B6, and C, and minerals such as potassium, calcium, and phosphorus.2 Several bioactivities have been attributed to the phytochemical composition of V. meridionale fruit, such as cytotoxic and antioxidant capacity in transformed leukemic cell lines,3 antiproliferative effects against colon-adenocarcinoma cells,4 and antimicrobial activity,5 among others. The bioaccessibility of bioactive compounds from V. meridionale berry juice against SW480 colon-adenocarcinoma cells has also been studied.4 An increase in antiproliferative activity was found during colonic fermentation, specifically from gallic acid, chlorogenic acid, caffeic acid, ellagic acid, rutin, raffinose, stachyose, and xylose. Some in vivo studies6,7 have evaluated the effect of V. meridionale regular consumption on protecting patients with metabolic syndrome. The role of osmodehydrated V. meridionale fruit consumption on different parameters associated with lifestyle diseases in overweight adults has been studied.8 These results showed a correlation between regular consumption and the positive and significant impact on the anthropometric parameters and blood pressure of the participants. The consumption of nectar obtained from V. meridionale lyophilized fruits and reconstituted in water showed positive effects in people with metabolic syndrome (60 people).9 Among different phytochemicals, anthocyanins are relevant metabolites in V. meridionale fruit. Garzón et al.10 reported the anthocyanin content as 329.0 ± 28.0 mg cyanidin 3-glucoside equivalents 100 g-1 (fresh weight (FW)) and the total phenolic content as 758.6 ± 62.3 mg gallic acid equivalents (GAE) 100 g-1 FW. Cyanidin 3-galactoside was the major anthocyanin, followed by cyanidin 3arabinoside, delphinidin 3-hexoside, delphinidin 3-pentoside, and cyanidin 3-glucoside. Otálora et al.11 reported also the presence of peonidin 3-O-α-arabinoside and delphinidin 3-(6-p-coumaroyl-glucoside) in minor proportion. Caffeoylquinic acids were the most abundant non-anthocyanin phenolic compounds, followed by quercetin derivatives. Hypertension is a risk factor relevant to cardiovascular diseases (CVDs) because it increases their incidence, mainly in older people.12 Genetic or environmental factors, high cholesterol, diabetes, or alcohol consumption contribute to it. The SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) pandemic has caused almost 6.8 million deaths since 2020, and hypertension was identified as the most prevalent comorbidity in patients infected with coronavirus disease (COVID-19), increasing the risk of hospitalization and death.13 Some studies14 indicated that renin-angiotensin-aldosterone system inhibitors might increase the risk of viral infection. Anthocyanins and other phenolic antioxidants have been reported15 to inhibit the development of CVDs and chronic diseases, including diabetes mellitus, cancer, and osteoporosis. Different mechanisms can explain their effect on blood pressure regulation; for instance, anthocyanins can increase the amount of nitric oxide by modulating the activity of the NO endothelial synthase and help the vasorelaxation of vascular smooth muscle.16 It has also been reported17 that anthocyanins reduced the synthesis of angiotensin II by inhibiting ACE-I (angiotensin-converting enzyme I) and decreasing its messenger ribonucleic acid (mRNA) production. Angiotensin II acts on the central nervous system and on venous and arterial smooth muscle to cause vasoconstriction, a phenomenon associated with increased blood pressure. Despite the sensory and biofunctional properties of V. meridionale, some of the production is lost due to difficulties in transporting fruit in good shape to big cities. Fruit drying is used as a strategy to increase their shelf-life time. Some attempts have been made to convert V. meridionale fruits into solid ingredients for the food industry. Estupiñan-Amaya et al.18 used maltodextrin to stabilize the freeze-dried fruit juice and to obtain powders with significant anthocyanin and polyphenol recoveries. More recently,19 V. meridionale pomace was used as a natural colorant in Greek-style yogurt with biofunctional properties. This study aimed to evaluate the antihypertensive activity of Vaccinium meridionale fruits, characterize some of the phytochemicals responsible for this bioactivity, and develop fruit microencapsulates by spray-drying with maltodextrin that preserve these characteristics. Thus, the physicochemical, thermal, morphological, and biofunctional properties of microencapsulates were determined.
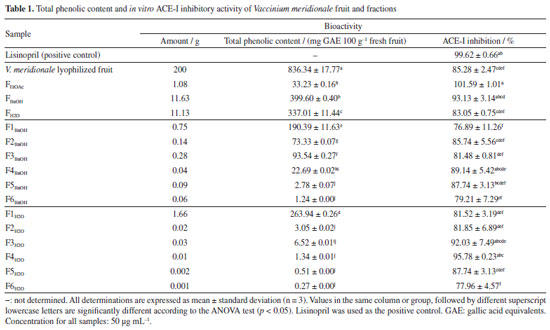
EXPERIMENTAL Chemicals Maltodextrin DE 20 (MD) (Casa Químicos, Bogotá, Colombia) was used as encapsulating agent. All solvents used during the fractionation process were of analytical grade (Panreac, Spain). For LC-MS analyses, acetonitrile, water, and formic acid were purchased from Honeywell Burdick and JacksonTM (Muskegon, USA). Water for chromatographic separation was purified using a Milli-Q water advantage A 10 water system (Millipore, Molsheim, France). Folin-Ciocalteu reagent and gallic acid were acquired from Merck (Darmstadt, Germany). The lyophilization process was carried out for 48 h in the Beta 1-8 LD plus lyophilizer equipment (CHRIST, Germany). Plant material Vaccinium meridionale Swartz fruits were acquired in local markets of Ráquira (Boyacá, Colombia). Their ripening stage was selected according to their soluble solid content (14.83 ± 0.97 ºBrix) and pH (3.04 ± 0.03). Bioguided fractionation of Vaccinium meridionale extract Whole fruits (2 kg) were lyophilized in batches of 500 g to obtain 200 g of dried fruit. Lyophilized fruits (101 g) were extracted with three volumes (300 mL) of acetone:water (7:3), and then, the extracts were combined and concentrated to a one-liter volume. This aqueous extract was submitted to successive partitions with pentane, dichloromethane, ethyl acetate, and n-butanol, following the procedure reported by Isaza et al.20 For extraction, 300 mL of each solvent was used in triplicate. In all fractions, the solvent was removed under reduced pressure, and the residue was freeze-dried, obtaining 0.69 g of Fpentane, 0.32 g of FDCM, 1.08 g of FEtOAc, and 11.63 g of FBuOH. The residual fraction was FH2O (11.13 g). Each of these fractions was analyzed by high-performance liquid chromatography coupled to mass spectrometry (HPLC-MS), and their total phenolic content (TPC) and ACE-I inhibition values were measured. Based on the results of ACE-I enzyme inhibition (Table 1), the fractions FBuOH (11.63 g) and FH2O (11.13g) were separately fractionated by SPE (solid phase extraction) using C18 EC cartridges (10 g Chromabond, Macherey-Nagel GmbH & Co., Düren, Germany) and a Manifold (Thermo Scientific HyperSep, USA). Each cartridge was loaded with 1.9 g of sample and eluted successively with MeOH:H2O (0:100, 20:80, 40:60, 60:40, 80:20, 100:0 v/v), with 60 mL of each to obtain six fractions (F1-F6) in each case. The solvent was removed from all the fractions, and the ACE-I inhibition was evaluated.
Antihypertensive activity Whole fruits (100 g) were frozen at -4 ºC and lyophilized in a Beta 1-8 Ldplus lyophilizer (CHRIST, Germany) for 72 h, with a main drying phase of 60 h, at 1.0 mbar and -20 ºC. The final drying phase lasted 12 h, at 0.001 mbar and -76 ºC. After freeze-drying, the seeds were manually extracted from the fruits; and the pulp and peel were homogenized using a grinder (A11 basic, IKA, Staufen, Germany), obtaining a fine powder. The V. meridionale sample was prepared at a concentration of 50 µg mL-1, where 500 µg of fine powder was dissolved in 10 mL of deionized water, sonicated, and filtered using a polytetrafluoroethylene (PTFE) syringe filter of 0.45 µm (Agilent Technologies, USA). The fractions were prepared at a concentration of 50 µg mL-1. The in vitro antihypertensive activity of V. meridionale was determined by the ACE-I enzyme inhibition assay following the protocol provided by the manufacturer (Dojindo Molecular Technologies Inc., Kumamoto, Japan).21 Commercial lisinopril was used as a positive control prepared at 50 µg mL-1 in deionized water (ACE-I inhibition: 99.62 ± 0.66%). The absorbance of all samples was measured in a 96-well plate multireader using a BertholdTech TriStar2S spectrophotometer (Berthold Technologies GmbH & Co., Germany), and the data were processed using ICE software, version 1.0.9.0 (Synthego Performance Analysis, ICE Analysis, Redwood, CA, USA). The inhibition percentage of the samples was calculated considering the following Equation 1: 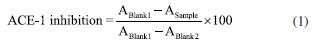 where, A is the absorbance value measured at λ 450 nm, blank 1 is the positive control and blank 2 is the blank of reaction (all reagents without sample). Determination of total phenolic content (TPC) Total phenolic content was determined by the Folin-Ciocalteu spectrophotometric method22 using gallic acid as a standard. A solution was prepared by diluting the Folin reagent 1:10 in distilled water. For each sample, 30 µL of sample or standard and 150 µL of Folin solution (Merck, Darmstadt) were placed in a 96-well plate. After 4 min, the reaction was stopped by adding 120 µL of saturated sodium carbonate solution. Then, the samples were left at 18 ºC in a dark place for 2 h. Finally, the absorbance was measured in an ultraviolet-visible (UV-Vis) spectrophotometer TriStar2S (Berthold Tech GmbH & Co., Germany) at 760 nm. All assays were performed in triplicate and expressed as total phenol content in mg GAE (g powder)-1. HPLC-MS analysis All samples were centrifuged at 15000 rpm for 3 min at room temperature, and 2 µL of the supernatant were analyzed in Bruker Impact II LC Q-TOF MS equipped with electrospray ionization (ESI) in positive mode, and an Intensity C18 column (2.1 × 100 mm, 1.8 μm) (Bruker Daltonik, Germany) at a temperature of 30 ºC and a flow rate of 250 mL min-1. The mobile phase consisted of water (A) and acetonitrile (B), each containing 0.1% formic acid. The gradient elution was: 0-1 min 5% B, 1-11 min 5-95% B, 11-13 min 95% B, and 13.1-15 min 95-5% B. The ESI source conditions (positive mode) were: end plate offset 500 V, capillary 3.2 kV, nebulizer 1.8 bar, dry gas nitrogen 600 L h-1, dry temperature 400 ºC. The scan mode auto MS/MS with spectral range of 20-1000 m/z, spectra rate of 2 Hz, and collision energy of 5.0 eV. Development of anthocyanin-rich microencapsulated powders from Vaccinium meridionale fruits Ripe fruits (4 kg) were used to develop microencapsulates using the spray-drying technique. A factorial design (2 × 2) was used to obtain four samples (M1-M4), using two inlet temperatures (120 and 150 ºC) and two ratios between fruit (AGZ) and encapsulating agent (maltodextrin, MD) (1:1 and 2:3, m/m). The spray-drying process was performed on a laboratory scale using a LabPlant SD-06 spray-dryer (LabPlant, Huddersfield, England) with an 1110 × 825 × 600 mm main spray chamber in the same conditions published by Villacrez et al.23 Thus, four microencapsulates were obtained: M1 (120 ºC, 1:1 AGZ:MD), M2 (120 ºC, 2:3 AGZ:MD), M3 (150 ºC, 1:1 AGZ:MD), and M4 (150 ºC, 2:3 AGZ:MD); collected in plastic containers, weighed, and stored in desiccators containing silica gel at 18 ºC. Each treatment was carried out in triplicate. Powder characterization Physicochemical parameters The water activity (Aw) of the microencapsulates was measured in a HygroPalm AW1 Rotronic (Rotronic Instruments, Huntington, NY, USA) at 20 ºC. The pH and soluble solid content (expressed in ºBrix) were measured using a 370-pH meter (Jenway, London, UK) and an Atago HSR-500 refractometer (Tokyo, Japan), respectively. Color parameters The color of the powders was measured by tristimulus colorimetry using a Hunterlab ColorQuest® XE d/8º colorimeter (1.00 inch light pass, nominal standard-ionization); and the CIELab (L*, a*, b*) coordinates were obtained. All the measurements were performed in triplicate. The parameters of chroma (C*ab) and hue (hab) were calculated according to Equations 2 and 3:24 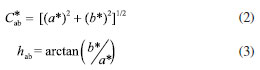 Morphology and particle size The morphology of the microcapsules was evaluated using a scanning electron microscope (SEM) Tesca Vega 3 SB (operating at 20 kV and 0.009 Pa), with the samples coated with graphite sputtering before examination. The particle size distribution was determined by measuring the diameter of 300 particles localized in a selected area using the analysis software ImageJ, version 1.49 (National Institute of Health, Bethesda, Maryland, USA). Thermal analysis Thermogravimetric analysis (TGA) were carried out on a simultaneous Analyser TGA 2050 (TA Instruments, precision ± 0.1%, resolution 0.2 μg). The apparatus was calibrated using high-purity air (Tm = 429.8 K, 100 mL min-1). The experiments were performed under nitrogen flow (50 mL min-1). The M2 and M4 powders (0.5 mg each) were heated from 40 to 400 ºC in aluminium crucibles with a linear heating rate of 10 ºC min-1, and an empty aluminium crucible was used as the reference material. Biofunctional properties TPC and ACE-I inhibition assays were performed as described above. For that purpose, each microencapsulate (10 mg) was dissolved in 10 mL of distilled water and filtered through 0.45 µm membrane filters (Millipore, USA). Statistical analysis All the experiments were performed in triplicate. Significant differences between means were assessed using one-way analysis of variance (ANOVA) followed by Tukey test at a significance level of p ≤ 0.05. The statistical analysis was performed using the Statgraphics software, version 2017.01 (Statistical Graphics Corp. Manugistics Inc., Cambridge, USA).
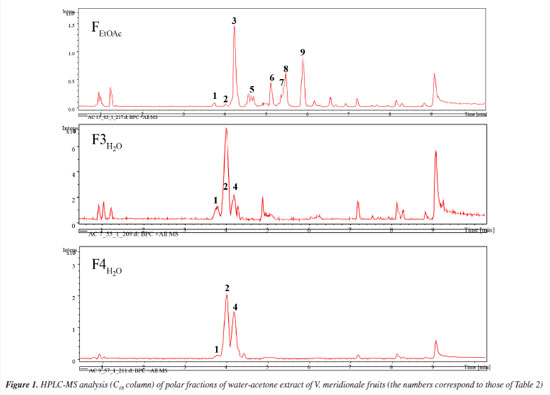
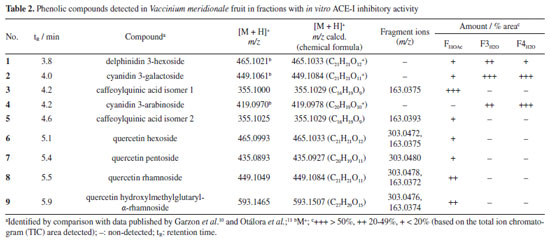
RESULTS AND DISCUSSION Bioguided fractionation of fruit polar extract and characterization of bioactive compounds The pH of V. meridionale fruits was similar to that obtained by Garzón et al.,10 and solid soluble content was in the range reported by Ligarreto.25 The TPC value was higher than that reported by Garzón et al.10 (758.6 ± 62.3 meq GAE 100 g-1) and lower than the related species Vaccinium floribundum (882 ± 38 meq GAE 100 g-1).26 Based on the high anthocyanin content of this fruit,10 the ACE-I inhibitory activity of lyophilized fruit was determined using an ACE inhibitory assay kit (ACE kit-WST, Dojindo Laboratories) (Table 1). This value was lower than the one exhibited by the positive control (lisinopril). However, the polar extract of V. meridionale fruit was successively fractionated with solvents of increasing polarity to obtain five fractions with good per cent recovery and different total phenolic content (TPC) and antihypertensive activity values (Table 1). These results showed that the most polar fractions (FH2O and FBuOH) exhibited the highest content of phenolic compounds; however, the FEtOAc showed the highest inhibition of ACE-I, higher than the value of lyophilized fruit. Based on the statistical analysis, there were no significant differences in the antihypertensive activity of FEtOAc and FBuOH, and between FBuOH and FH2O. The more polar fractions contain a high concentration of sugars and organic acids, which can interfere with the determination of phenolic compounds by the Folin-Ciocalteu method; thus, the ACE-I inhibition assay was selected to monitor the bioguided fractionation. For this purpose, the FBuOH and FH2O fractions were subjected to SPE to eliminate highly polar compounds that interfere with the HPLC-MS analysis. As shown in Table 1, differential values of ACE-I inhibition were obtained, with F3H2O and F4H2O being the most active fractions. To study the chemical composition of most active fractions, they were analyzed by HPLC-MS (Figure 1) and the compounds were identified by comparing the mass spectrum obtained in positive mode with those reported by Garzón et al.10 In the most active fraction (FEtOAc), two caffeoylquinic acid isomers (3 and 5) were identified, followed by four quercetin derivatives (6-9) and three anthocyanins, with delphinidin (1) and cyanidin (2 and 4) as aglycon (Table 2).
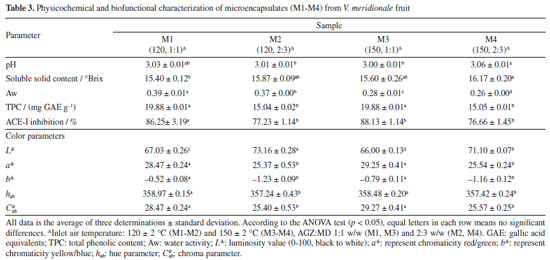
The high-resolution spectrum and fragment ions allowed to confirm the structures; in the case of anthocyanins, molecular ions were detected because these compounds are ionized in acid solution. The bioguided fractionation through SPE allowed the purification of two anthocyanin-rich fractions (F3H2O and F4H2O, Figure 1), which were the most active for in vitro inhibiting the ACE-I enzyme. Both fractions contained cyanidin 3-galactoside, cyanidin 3-arabinoside, and delphinidin 3-hexoside. This ACE-I inhibitory activity has been explained by their metal chelating activities related to the positive charge of C-ring. Parichatikanond et al.17 reported that the anthocyanins, delphinidin and cyanidin, as well as the flavonoid quercetin, regulate the renin-angiotensin system, which is involved in the process of hypertension. Delphinidin and cyanidin inhibit the ACE-I and decrease renin mRNA production, while quercetin inhibits ACE-II enzyme. Most of the fractions (ca. those derived from FBuOH) did not exhibit significant differences, such suggesting a synergistic effect between the polyphenols present in V. meridionale fractions. The most abundant anthocyanin in V. meridionale fruits, cyanidin 3-galactoside (Cy3Gal), is one of the most common anthocyanins in plants and has numerous health-promoting benefits.27 In vivo studies28 have shown that Cy3Gal remains present after human or animal digestion. For instance, dietary supplementation with Cy3Gal increased the levels of choline in plasma and taurine in the brain of mice, which is related to protection from cardiovascular and neurodegenerative diseases. Physicochemical and biofunctional characterization of anthocyanin-rich microencapsulated powders The physicochemical data of microencapsulates (M1-M4) allowed to study the effect of inlet temperature and feed mixture composition (Table 3). The pH values were similar between all samples and the lyophilized fruit (3.04 ± 0.03). The soluble solid content of the microencapsulates was comparable among all samples, ranging between 15.40 and 16.17 ºBrix, which was higher than the fruit value (14.83 ± 0.97) due to the addition of maltodextrin. All water activity values were below 0.60, the maximum value accepted to avoid food spoilage. The lowest values were obtained at the highest inlet temperature (150 ºC) during the spray-drying process.
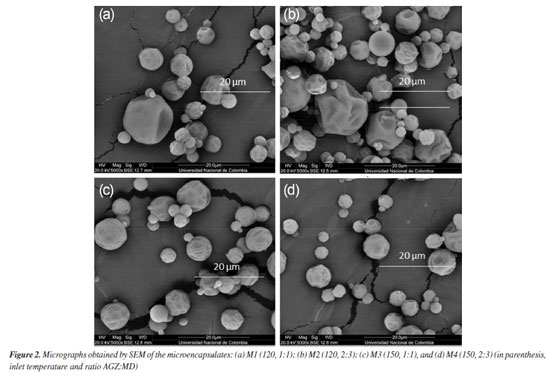
The color parameters in Table 3 shows how the luminosity value (L*) for the M2 and M4 powders was higher than that obtained in M1 and M3 powders. It was possibly due to the whiter color coming from the higher ratio of maltodextrin to the fruit (AGZ:MD ratio of 2:3 for M2 and M4 versus 1:1 for M1 and M3) used in the feed mixture for this process. On the other hand, M4 powder showed a lighter/brighter value than that obtained for M2 powder. These values are explained due to the inlet temperature used in the spray drying process (150 ºC for M4 and 120 ºC for M2), which suggests a decrease in the biological activity of anthocyanins microencapsulated or a transformation due to thermal treatment. The chroma parameter (Cab*) was higher for samples with less maltodextrin (M1 and M3). There were no significant differences in the effect of inlet temperature during spray-drying for the same ratio AGZ:MD (ca. M1 vs. M3, and M2 vs. M4). The a* and b* parameters were correlated with the red color exhibited by the microencapsulates. The increase in the hue (hab) and b* values in M1 and M3 in comparison to those of M2 and M4, is an indicator of a more intense red color related to a higher proportion of fruit (anthocyanin) in M1 and M3. This fact agrees with the highest phenolic content in M1 and M3. There was no significant effect of inlet temperature on TPC content. SEM micrographs with a magnification of 5000× for the microencapsulates are shown in Figure 2. The surface structure of the powders obtained with an AGZ:MD (1:1, m/m) ratio (M1 and M3, Figures 2a and 2c) shown a spherical, irregular, compact forms, with slightly rough morphology, homogeneous size and less agglomerate formation, in comparison to particles obtained with an AGZ:MD (2:3, m/m) ratio (M2 and M4, Figures 2b and 2d). The latter exhibited a rough, scaly, and dented morphology with heterogeneous size and adherence effects between the particles, adversely affecting their flowability and reconstitution properties.29 These types of morphologies have been attributed to a high sugar concentration in the fruit and the MD ratio in the preparation of feed mixtures.30 Thus, particles with a smoother surface, that is, with a lower maltodextrin ratio, are suitable for applications in the food industry.
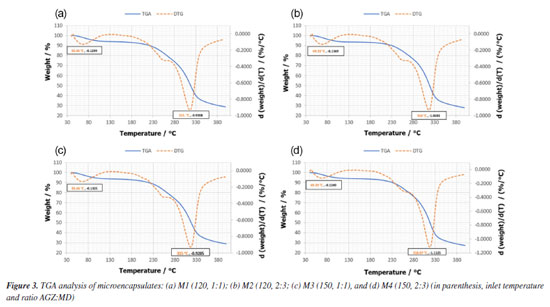
The effect of inlet temperature resulted in a higher number of small particles with slightly rough surfaces and bulging irregularities in microencapsulates obtained at 120 ºC (M1 and M2, Figures 2a and 2b), in contrast to those obtained at 150 ºC inlet air temperature (M3 and M4, Figures 2c and 2d). This fact means that as the drying temperature increases, a hard outer crust forms, resulting in large, rigid, smooth-surfaced particles. Thus, these characteristics are considered an advantage, as the capsules would have less permeability, thereby increasing the protection and retention of anthocyanins. The efficiency of the spray-drying process was evident in all cases, as semispherical particles were obtained, which are entirely different from the maltodextrin structure, characterized by an irregular shape.31 The particle size distribution showed that all samples (M1-M4) ranged between 2-30 µm, with a maximum of 27 µm for those obtained at 120 ºC (M1 and M2) and 21 µm for those obtained at 150 ºC (M3 and M4). These results confirmed that the spray-drying process allowed uniform particles with small particle sizes that effectively protect anthocyanins and other phenolic compounds, allow their controlled release, and improve their bioavailability.32 The thermograms of the microencapsulates showed a similar thermal behavior (Figures 3a-3d) with two endothermic events. The first event occurred between 40 and 220 ºC, with a weight loss of less than 12% (showing a narrow peak at 65.66, 69.33, 65.66, and 69.33 ºC for M1, M2, M3, and M4, respectively). These values are related to the AGZ:MD ratio, being higher when the amount of MD is greater. This behavior could be attributed to the evaporation of the water adsorbed and structurally incorporated into these samples as well as to the gelatinization of the encapsulating components (i.e., wall material and solids from fruit). The second thermal event occurred between 220 and 330 ºC (Tg 315, 318, 315, and 318 ºC for M1, M2, M3, and M4, respectively), with a mass loss of less than 60%. Additionally, the main MD content (M2 and M4) increases the Tg value in this case. This fact was attributed to the material decomposition and its subsequent volatilization. The Tg values represent the interaction and the crosslinking density between the components of the fruit and maltodextrin. This difference in thermal behavior between microencapsulates can be attributed to the stiffness, polymer chain structure, and ratio of the materials contained in the microcapsules. In general terms, the thermal analysis revealed that with a more significant proportion of maltodextrin in the feed mixtures, the powders exhibit higher thermal stability due to their higher melting and degradation temperatures. These results revealed that any of the four microencapsulates can be used in processed foods at high temperatures.
The TPC content and ACE-I inhibition were measured in the four microencapsulates (Table 3). Interestingly, all the microencapsulates exhibited ACE-I inhibition activity, with the highest values in the samples with higher fruit amounts (M1 and M3). The amount of fruit was more significant in the ACE-I inhibition than the inlet temperature used during the spray-drying process.
CONCLUSIONS The results of this work confirmed the in vitro antihypertensive activity of Vaccinium meridionale fruits and their microencapsulates. The phytochemicals associated with this activity included anthocyanins (Cy3Gal, Cy3Ara, and Dp3-hexoside) and quercetin derivatives, exhibiting a synergistic effect. The spray-drying process proved to be beneficial in producing added-value products with ACE-I inhibition activity from V. meridionale fruits, thereby extending the shelf life of this fruit. The anthocyanins were present in the microencapsulates obtained by the spray-drying process, showing that this technique is effective for protecting these bioactive compounds.
DATA AVAILABILITY STATEMENT All data are available in the text of this manuscript.
ACKNOWLEDGMENTS The ANLA and Ministerio de Ambiente y Desarrollo Sostenible granted permission to collect samples and perform this research (Contrato Marco de Acceso de Recursos Genéticos y sus Productos Derivados No. 357, November 17th, 2022 suscribed by Ministerio de Ambiente y Desarrollo Sostenible and Universidad Nacional de Colombia). The authors thank to Diego Insuasty, Kevin Huertas and Prof. Dr. Zuly Rivera from LIF (Laboratorio Interfacultades) de Espectrometría de Masas Q-TOF and Cromatografía Líquida from Universidad Nacional de Colombia-Sede Bogotá for performing HPLC-MS analysis of fractions; also, thanks to Sandra Echeverry and Prof. Diana Marcela Aragón, from Departamento de Farmacia, Facultad de Ciencias, Universidad Nacional de Colombia-Sede Bogotá, for the support during the use of UV-Vis spectrophotometer TriStar2S. The authors thank to Fondo Nacional de Financiamiento para la Ciencia, la Tecnología y la Innovación, Francisco José de Caldas (contract No. 0459/2013), Red Nacional para la Bioprospección de Frutas Tropicales-RIFRUTBIO.
AUTHOR CONTRIBUTIONS Andrés Mauricio Amaya was responsible for methodology, formal analysis; María Carolina Otálora for formal analysis, writing (review and editing); Coralia Osorio for conceptualization, methodology, formal analysis, investigation, writing original draft.
REFERENCES 1. de Valencia, M. L. C.; de Lozano, N. B.; Acta Biol. Colomb. 1995, 2, 159. [Link] accessed in July 2025 2. Celis, M. E. M.; Tobón, Y. N. F.; Agudelo, C.; Arango, S. S.; Rojano, B. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health; Yahia, E. M., ed.; Wiley-Blackwell: Oxford, 2017. 3. González, M.; Samudio, I.; Sequeda-Castañeda, L. G.; Celis, C.; Iglesias, J.; Morales. L.; J. Appl. Pharm. Sci. 2017, 3, 24. [Crossref] 4. Agudelo, C. D.; Luzardo-Ocampo, I. R.; Campos-Vega, R. G.; Loarca-Piña, G.; Maldonado-Celis, M. E.; J. Agric. Food Chem. 2018, 66, 7358. [Crossref] 5. Garzón, G. A.; Soto, C. Y.; López, M.; Riedl, K. M.; Browmiller, C. R.; Howard, L.; Heliyon 2020, 6, 03845. [Crossref] 6. Espinosa-Moncada, J.; Marín-Echeverri, C.; Galvis-Pérez, Y.; CiroGómez, G.; Aristizábal, J. C.; Blessom, C. N.; Fernandez, M. L.; Barona-Acevedo, J.; Nutrients 2018, 10, 1639. [Crossref] 7. Marín-Echeverri, C.; Blesso, C. N.; Fernández, M. L.; Galvis-Pérez, Y.; Ciro-Gómez, G.; Núñez-Rangel, V.; Aristizábal, J. C.; Barona-Acevedo, J.; Antioxidants 2018, 7, 185. [Crossref] 8. Torres, D.; Reyes-Dieck, C.; Gallego, E.; Gómez-García, A.; Posada, G.; Maldonado-Celis, M. E.; Vitae 2018, 25, 141. [Crossref] 9. Quintero-Quiroz, J.; Galvis-Pérez, Y.; Galeano-Vásquez, S.; MarínEcheverri, C.; Franco-Escobar, C.; Ciro-Gómez, G.; NúñezRangel, V.; Aristizábal-Rivera, J. C.; Barona-Acevedo, J.; Food Sci. Technol. 2019, 39, 573. [Crossref] 10. Garzón, G. A.; Narváez, C. E.; Riedl, K. M.; Schwartz, S. J.; Food Chem. 2010, 122, 980. [Crossref] 11. Otálora, M. C.; Wilches-Torres, A.; Gómez Castaño, J. A.; Foods 2023, 12, 1811. [Crossref] 12. Haldar, R. N.; Indian Journal of Physical Medical Rehabilitation 2013, 24, 2. [Link] accessed in July 2025 13. Peng, M.; He, J.; Xue, Y.; Yang, X.; Liu, S.; Gong, Z.; J. Cardiovasc. Pharmacol. 2021, 78, 648. [Crossref] 14. Altuntas, M.; Yilmaz, H.; Guner, A. E.; Virol. J. 2021, 18, 57. [Crossref] 15. Panchal, S. K.; John, O. D.; Mathai, M. L.; Brown, L.; Nutrients 2022, 14, 2161. [Crossref] 16. Bell, D. R.; Gochenaur, K.; J. Appl. Physiol. 2006, 100, 1164. [Crossref] 17. Parichatikanond, W.; Pinthong, D.; Mangmool, S.; Planta Med. 2012, 78, 1626. [Crossref] 18. Estupiñan-Amaya, M.; Fuenmayor, C. A.; López-Córdoba, A.; Molecules 2020, 25, 5635. [Crossref] 19. Garzón, G. A.; Montana, T. L. M.; Sánchez, M.; Novoa, C. F.; Gutiérrez, L. F.; J. Food Sci. 2021, 86, 3896. [Crossref] 20. Isaza, J. H.; Ito, H.; Yoshida, T.; Phytochemistry 2004, 65, 359. [Crossref] 21. Dojindo, ACE Inhibitory Activity Assay, https://www.dojindo.com/products/A502/, accessed in July 2025. 22. Singleton, V. L.; Rossi, J. A.; Am. J. Enol. Vitic. 1965, 16, 144. [Crossref] 23. Villacrez, J. L.; Carriazo, J. G.; Osorio, C.; Food Bioprocess Technol. 2014, 7, 1445. [Crossref] 24. Comission Internacionale de L'eclairage (CIE); Technical Report - Colorimetry 15, 3rd ed.; CIE Divison: Rochester, 2004. [Link] accessed in July 2025 25. Ligarreto, G. A.; Perspectivas del Cultivo de Agraz o Mortiño (Vaccinium meridionale Swartz) en la Zona Altoandina de Colombia, 1st ed.; Gente Nueva Editorial: Bogotá, 2009. 26. Vasco, C.; Rihinen, K.; Ruales, J.; Kamal-Eldin, A.; J. Agric. Food Chem. 2009, 57, 8274. [Crossref] 27. Liang, Z.; Liang, H.; Guo, Y.; Yang, D.; Int. J. Mol. Sci. 2021, 22, 2261. [Crossref] 28. Yang, H.; Pang, W.; Lu, H.; Cheng, D.; Yan, X.; Cheng, Y.; Jiang, Y.; J. Agric. Food Chem. 2011, 59, 2069. [Crossref] 29. Ferro, D. M.; Müller, C. M. O.; Ferreira, S. R. S.; Biocatal. Agric. Biotechnol. 2020, 27, 101716. [Crossref] 30. Braga, V.; Rocha Guidi, L.; de Santana, R. C.; Zotarelli, M. F.; Powder Technol. 2020, 375, 409. [Crossref] 31. Osorio, C.; Forero, D. P.; Carriazo, J. G.; Food. Res. Int. 2011, 44, 1174. [Crossref] 32. Kwak, H. S.; Mijan, M. A.; Ganesan, P. In Nano- and Microencapsulation for Foods; Kwak, H. S., ed.; John Wiley & Sons: USA, 2014, p. 273. [Crossref]
Associate Editor handled this article: Eduardo H. S. Sousa |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access