Artigo
| Diterpenoids from the roots of Annona vepretorum Mart. (Annonaceae) |
|
Ana L. M. SouzaI; Jéssica F. AuzierII; Lívia M. DutraI; Victoria L. A. SantosIII I. Núcleo de Estudos e Pesquisas de Plantas Medicinais (NEPLAME), Universidade Federal do Vale do São Francisco, 56304-205 Petrolina - PE, Brasil Received: 02/27/2025 *e-mail: jackson.guedes@univasf.edu.br Five diterpenoids were isolated from the hexane extract obtained from the roots of Annona vepretorum Mart. (Annonaceae). The chemical structures were determined as ent-3b-acetoxy-kaur-16-ene (1), 17-nor-kaur-16-one (2), ent-kaur-16-en-19-oic acid (3), ent-3b-hydroxy-kaur-16-ene (4) and ent-16b-hydro-kauran-17-oic acid (5). Their structures were identified by spectroscopic analyses (1D and 2D nuclear magnetic resonance (NMR) data) and comparison with literature data. This is the first report on the isolation of compound 2 in the Annonaceae family. INTRODUCTION Annona vepretorum Mart. (Annonaceae) is a native tree from the Caatinga biome (semiarid region of Brazil) popularly known as "araticum da caatinga" and "pinha da caatinga", used in folk medicine for the treatment of inflammatory processes.1 - 4 Previous phytochemical investigation of A. vepretorum showed the presence of steroids, alkaloids, monoterpenoids, diterpenoids, triterpenes and flavonoids.5-7 The chemical constituents of the essential oils have also been reported from the leaves of A. vepretorum, mainly showing the presence of sesquiterpenes and monoterpenes.5,8 - 13 To determine the content of total kaurene diterpenes in extracts and fractions of the stems of this species, a quantification method was developed using the technique of quantitative nuclear magnetic resonance spectroscopy.14 In our continuous search for bioactive natural products and their chemophenetic significance for the Annonaceae family, we report herein a study showing the isolation and identification of kaurene diterpenoids from the roots of A. vepretorum. The compound 17-nor-kaur-16-one (2) have not been previously reported in the Annonaceae family. EXPERIMENTAL General experimental procedures The isolated compounds were identified by one-dimensional (1D) and two-dimensional (2D) nuclear magnetic resonance (NMR) spectral data and further supported by comparison with literature values. The 1D and 2D (NMR) experiments were performed in CDCl3 at 298 K using an ASCEND 400 (Bruker) (400 and 100 MHz for 1H and 13C, respectively). The NMR spectrometer was equipped with a 5 mm multinuclear direct detection probe (1D and 2D NMR experiments) with z-gradient. One-bond (heteronuclear single quantum correlation (HSQC)) and long-range (heteronuclear multiple bond correlation (HMBC)) 1H-13C NMR correlation experiments were optimized for average coupling constant 1J(C,H) and LRJ(C,H) of 140 and 8 Hz, respectively. All 1H and 13C NMR chemical shifts (d) are presented in ppm related to the tetramethylsilane (TMS) signal at 0.00 ppm as an internal reference, and the coupling constants (J) in Hz. Silica gel 60 (Sigma-Aldrich, 70-230 mesh) was used for the column chromatography (CC), while silica gel 60 F254 (Macherey-Nagel, 0.25 mm, aluminum) was used for analytical, and preparative thin-layer chromatography (PTLC) (Macherey-Nagel, 1.00 mm, glass). Compounds were visualized by exposure under UV 254/365 light, by spraying with a 1% solution of vanillin in concentrated sulfuric acid. Plant material Roots of A. vepretorum were collected in May 2012, at the Serra da Guia, Poço Redondo, state of Sergipe, Brazil (coordinates: 09º57'54" S and 37º51'46" W). The material was identified by Ana Paula do Nascimento Prata (a plant taxonomist of the Department of Biology of the Universidade Federal de Sergipe (UFS), São Cristovão, SE, Brazil), and a voucher specimen (ASE 23492) was deposited in the Herbarium of the Universidade Federal de Sergipe (Herbarium ASE) of the Department of Biology, UFS. All procedures for access in the genetic patrimony and associated traditional knowledge were carried out and the project was registered in SisGen (Register #ACAAA36). Extraction and isolation The dried and powdered roots of A. vepretorum (326.0 g) were subjected to exhaustive maceration with hexane (Hex) for 72 h at room temperature. The extractive solution was concentrated under reduced pressure (40-50 ºC), yielding the crude hexane extract (17.0 g). Subsequently, one part of hexane extract (7.24 g) was subjected to column chromatography (CC) over silica gel 60 (230-400 mesh), eluted with increasing polarity of solvent systems starting with hexane:chloroform (100:0 to 10:90, v/v), followed by chloroform:ethyl acetate (100:0 to 10:90, v/v) and ethyl acetate:methanol (100:0 to 0:100, v/v), affording 327 fractions of approximately 10 mL each. Column fractions were combined in 7 fractions (A-G), and grouped according to the chromatographic profile and similarity based on the retention factor (Rf). Fraction D (42.5 mg) was subjected to CC eluting with increasing concentrations of hexane:chloroform (80:20, v/v) to ethyl acetate (100:0, v/v), to give three subfractions (D1-D3) resulting in compound 1 (18.7 mg). Fraction E (409.5 mg) was subjected to a preparative TLC eluting with hexane:ethyl acetate (95:05, v/v, two elutions), to give four subfractions (E1-E4), and E3 gave compound 2 (1.7 mg). Fraction F (1156.7 mg) was subjected to CC eluting with increasing concentrations of hexane:chloroform (80:20, v/v) to ethyl acetate (100:0, v/v), to give thirteen subfractions (F1-F13). F7 was subjected to CC eluting with increasing concentrations of hexane:chloroform (80:20, v/v) to ethyl acetate (100:0, v/v), to give eleven subfractions (F7.1-F7.11). The subfraction F6 gave compound 3 (48.3 mg), and F7.8 were subjected to a preparative TLC eluting with hexane:ethyl acetate (95:05, v/v, two elutions), yielding compound 4 (8.7 mg). The subfraction F13 was subjected to CC eluting with increasing concentrations of hexane:chloroform (80:20, v/v) to chloroform (100:0, v/v), resulting in compound 5 (2.3 mg).
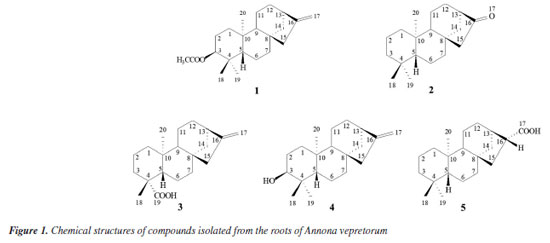
RESULTS AND DISCUSSION In this study, five compounds were isolated from the hexane extract from the roots of A. vepretorum, including one nor-diterpenoid (2), in addition to other four diterpenes (1, 3-5), metabolites commonly found in the genus Annona (Figure 1).
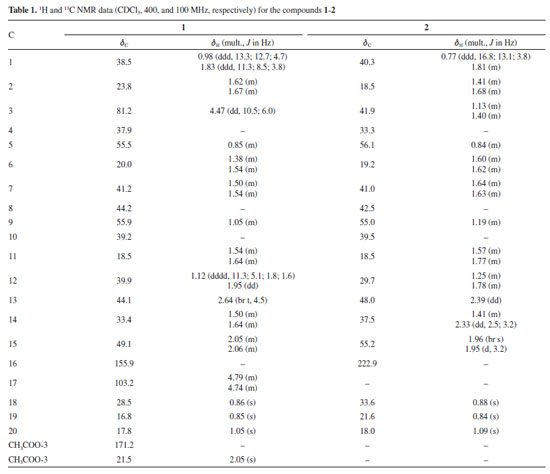
Compound 1 was obtained as a white amorphous powder. The 1H NMR spectrum revealed 4 methyl groups [δH 0.85, 0.86, 1.05, 2.05 (3H each, s)], 4 methine hydrogens [δH 0.85 (m), 1.05 (m), 2.65 (1H, br t, J 4.5 Hz), and 4.47 (1H, d, J 6.0, 10.5 Hz)], 2 olefinic hydrogens [δH 4.74 (m) and 4.79 (m)], and 16 aliphatic hydrogens belonging to 8 methylene groups (δH 0.97-2.06) (Table 1). The methyl groups are representative for C-18eq, C-19ax and C-20ax, which are typical for kaurane diterpenoids. The hydrogens at δH 2.05 (s, 3H) and 4.47 (dd, J 10.5, 6.0 Hz, 1H) displayed the presence of an acetoxy group and an oxygenated carbon in the structure, respectively. The signals at δH 4.74 (m) and 4.79 (m) are characteristics of hydrogens with an exocyclic double bond. The 13C{1H} and distortionless enhancement by polarization transfer (DEPT)-135 NMR spectra, as well as HSQC and HMBC NMR experiments of 1 exhibited 22 carbon signals, which comprised of 5 nonprotonated (δC 37.9, 39.2, 44.2, one olefinic at δC 155.9 and one acetoxy group at 171.2), 9 methylenes, 4 methines, and 4 methyl carbons (Table 1). The signal at δH 4.47 (H-3) showed direct HSQC correlation with the carbon at δC 81.2 and long-range HMBC correlations with the carbons at δC 16.8 (C-19), 28.6 (C-18), 37.9 (C-4) and δC 171.2, supporting the substitution pattern with the acetoxy substituent at C-3. Additionally, both the hydrogens at δH 4.74 and 4.79 (H-17) revealed direct HSQC correlation with the signal at δC 103.2 and long-range HMBC correlations with the carbons at δC 44.1 (C-13) and 49.2 (C-15), justifying the exocyclic double bond at C-16-C-17. Detailed analysis of the 1H and 13C NMR data (Supplementary Material, Figures 1S-9S) are compatible with a substance of kaurene-type diterpene. These suggestions were further confirmed by the HSQC and HMBC spectra, as well as by comparison with data reported in the literature.1 Based on these spectroscopic results, compound 1 was determined as ent-kaurene diterpenoid known as ent-3b-acetoxy-kaur-16-ene.1
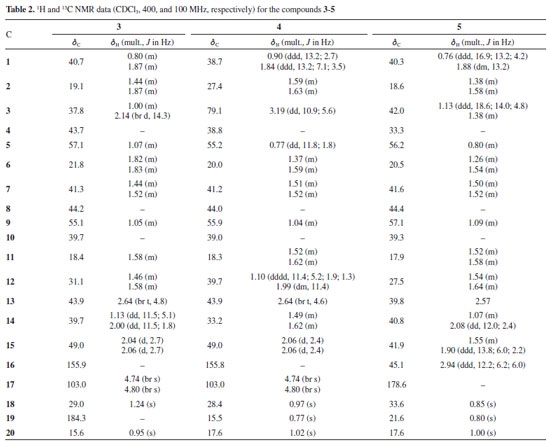
Compound 2 was obtained as a colorless solid. This compound presented 1H and 13C{1H} NMR spectra very similar to those of 1 (Table 1). Comparison of the NMR signals of 1 and 2 showed that the spectra of 2 showed signals similar to 3 methyl hydrogens [δH 0.84, 0.88 and 1.09 (each 3H, s)], characteristic H-18eq, H-19ax and H-20ax methyl groups of ent-kaurane diterpenes, while methyl signals (δH 2.05) and olefinic protons [δH 4.74 (m), 4.79 (m)] were not observed in the 1H NMR data. The 13C and DEPT-135 NMR spectra, as well 1H-13C as one-bond and long-range correlation maps from HSQC and HMBC NMR spectrum of compound 2 revealed 19 carbons, suggesting a diterpenoid with nor-kaurene skeleton, due to 4 nonprotonated (δC 33.3, 39.5, 42.5 and one ketone carbonyl group at 222.9), 9 methylenes, 3 methines (δC 48.0, 55.0 and 56.1), and 3 methyl carbons (δC 18.0, 21.6 and 33.6) (Table 1, and Figures 10S-17S, Supplementary Material). The methyl hydrogens at δH 0.88 (s) and δH 0.84 (s) were established in C-18 and C-19 due to the direct HSQC correlation with the carbons at δC 33.6 and 21.6, respectively. Furthermore, both the hydrogens showed long-range HMBC correlations with the carbons at δC 33.3 (C-4), 41.9 (C-3), and 56.1 (C-5) of the kaurane diterpene. In contrast, the hydrogen at C-18 also revealed long-range HMBC correlation with the carbon at δC 21.6 (C-19), while the hydrogen of the latter showed correlation with the carbon at δC 33.6 (C-18). Moreover, 1H-13C correlation map from HMBC were observed between the singlet at δH 1.09 (H-20) and the carbons at δC 39.4 (C-10), 40.3 (C-1), 54.9 (C-9), and 56.1 (C-4), supporting the methyl group at C-20. Finally, the ketone carbonyl group was attributed in C-16 due to the long-range HMBC correlations of the methylene hydrogens [δH 1.94 (br s) and 1.96 (d, J 3.2 Hz)] at C-15 with the carbons δC 37.5 (C-14), 41.0 (C-7), 42.5 (C-8), 54.9 (C-9) and 222.9 (C-16). Therefore, according to 1H and 13C NMR, as well as one- and two-bidimensional analysis, the compound 2 was identified as the kaurane diterpenoid, 17-nor-kaur-16-one (2).15,16 This compound is being described for the first time in the Annonaceae family. Previously, this compound has been reported for the first time in 2009; Pacheco et al.16 compiled the 13C NMR data of 57 diterpenoids described between 1981 and 2007 that were isolated from Aristolochia species, like Aristolochia triangularis. Nor-kaurane diterpenoids were already identified in Annona, as ent-3b,4b-dihydroxy-18-nor-kaur-16-ene that was reported in the stems A. vepretorum.4 The other compounds: ent-kaur-16-en-19-oic acid (kaurenoic acid) (3),1,2,16-18 ent-3b-hydroxy-caur-16-ene (4),1,2 and 16b-hydro-ent-kauran-17-oic acid (5)2,4,16,19 were identified based on the 1H, 13C{1H} and DEPT-135 NMR spectra, one-bond and long-range 1H-13C correlations from HSQC and HMBC NMR experiments (Table 2), as well as by comparison with data reported in the literature. The experimental data for compounds 3-5 are shown in Supplementary Material. The ent-kaurane diterpenes are metabolites commonly reported in the species of the Annonaceae family. The kaurane-type compounds (1, 3-5) were already isolated in A. vepretorum. Kaurenoic acid (3), was found in species from the genus Annona, such as A. vepretorum, A. squamosa, A. glabra, A. senegalensis, A. reticulata and A.cherimolia. This compound is widely reported in the Annonaceae, particularly in the genus Annona and Xylopia, and is considered a chemical marker for the genus Annona. The ent-3-hydroxy-caur-16-ene (4) was previously isolated from the stem bark and stems of A. vepretorum2,4 and compound 16b-hydro-ent-cauran-17-oic acid (5) was first isolated from the roots in this species2 and later reported in the stems.4 Species of this genus have proven biological activities, such as antitumor, antioxidant, antimicrobial, and antifungal activities.20,21 Chemically, these studies suggested that secondary metabolites (mainly alkaloids, acetogenins and terpenes) are responsible for the aforementioned activities.22 One of the chemophenetic markers for the family and Annona genus are the alkaloids, which have several biological activities described,22-28 and can also be highlighted the presence of terpenes, mainly kaurane-type diterpenes, that are reported in many species of the genus Annona with predominance of these compounds in the fruits, followed by the barks and, less frequently in the leaves.1,29-33
CONCLUSIONS Phytochemical investigation of A. vepretorum led to the isolation of five kaurane-type diterpenoids. The metabolites have been reported in the genus Annona, including A. vepretorum, and this is the first report on the isolation of compound 2 in A. vepretorum as well as in the Annonaceae family. These findings contribute to chemophenetic knowledge of the genus Annona, particularly in A. vepretorum.
SUPPLEMENTARY MATERIAL Supplementary data associated with this article (Figures 1S-40S) can be found in the online version at http://quimicanova.sbq.org.br, as PDF file, with free access.
DATA AVAILABILITY STATEMENT All data of this publication are available in the text and supplementary material.
ACKNOWLEDGMENTS The authors are grateful to Rede de Pesquisa em Biodiversidade e Sustentabilidade (Process CNPq 406399/2022-0) and Rede Baiana de Bioprospecção e Desenvolvimento de Fármacos - BioproFar-BA (Process PIE0001/2024) for financial support, and to Central Analítica - Centro de Apoio Multidisciplinar - Universidade Federal do Amazonas (CA/CAM/UFAM). AUTHOR CONTRIBUTIONS Ana L. M. Souza, Jéssica F. Auzier, Lívia M. Dutra, Victoria L. A. Santos: investigation, writing original draft, methodology, visualization, data curation; Emmanoel V. Costa, Jackson R. G. S. Almeida: data curation, supervision, writing review and editing, conceptualization. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
REFERENCES 1. Dutra, L. M.; Bomfim, L. M.; Rocha, S. L. A.; Nepel, A.; Soares, M. B. P.; Barison, A.; Costa, E. V.; Bezerra, D. P.; Bioorg. Med. Chem. Lett. 2014, 24, 3315. [Crossref] 2. dos Santos, T. B.; Meira, C. S.; Miranda, C. C.; Menezes, L. R. A.; Dutra, L. M.; Soares, L. N.; Barison, A.; Costa, E. V.; Guimarães, E. T.; Soares, M. B. P.; Int. J. Immunol. Immunother. 2016, 3, 021. [Crossref] 3. Flora e Funga do Brasil, Annona vepretorum Mart., http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB117277, acessado em julho 2025. 4. Araújo, C. S.; Nery, D. A.; Oliveira, A. P.; Oliveira-Júnior, R. G.; Rolim, L. A.; Lopes, N. P.; Silva, M. F. S.; Pessoa, C. Ó.; Braz-Filho, R.; Dutra, L. M.; Tavares, J. F.; Abreu, L. S.; da Silva, M. S.; Almeida, J. R. G. S.; Nat. Prod. Res. 2023, 37, 1565. [Crossref] 5. Dutra, L. M.; Costa, E. V.; Moraes, V. R. S.; Nogueira, P. C. L.; Vendramin, M. E.; Barison, A.; Prata, A. P. N.; Biochem. Syst. Ecol. 2012, 41, 115. [Crossref] 6. Teles, M. N. O.; Dutra, L. M.; Barison, A.; Costa, E. V.; Biochem. Syst. Ecol. 2015, 61, 465. [Crossref] 7. Silva, M. G.; de Oliveira, A. P.; Araújo, C. S.; de Lavor, E. M.; Silva, J. C.; Mendes, R. L.; Pessoa, C. Ó.; Costa, M. P.; Almeida, J. R. G. S.; Trop. J. Pharm. Res. 2017, 16, 597. [Crossref] 8. Araújo, C. S.; de Oliveira, A. P.; Lima, R. N.; Alves, P. B.; Diniz, T. C.; Almeida, J. R. G. S.; Pharmacogn. Mag. 2015, 11, 615. [Crossref] 9. Bomfim, L. M.; Menezes, L. R. A.; Rodrigues, A. C. B. C.; Dias, R. B.; Rocha, C. A. G.; Soares, M. B. P.; Neto, A. F. S.; Nascimento, M. P.; Campos, A. F.; Silva, L. C. R. C.; Costa, E. V.; Bezerra, D. P.; Basic Clin. Pharmacol. Toxicol. 2016, 118, 208. [Crossref] 10. Costa, E. V.; Dutra, L. M.; de Jesus, H. C. R.; Nogueira, P. C. L.; Moraes, V. R. S.; Salvador, M. J.; Cavalcanti, S. C. H.; dos Santos, R. L. C.; Prata, A. P. N.; Nat. Prod. Commun. 2011, 6, 907. [Crossref] 11. Meira, C. S.; Guimarães, E. T.; Macedo, T. S.; da Silva, T. B.; Menezes, L. R. A.; Costa, E. V.; Soares, M. B. P.; J. Essent. Oil Res. 2015, 27, 160. [Crossref] 12. Silva, J. C.; Araújo, C. S.; de Lima-Saraiva, S. R. G.; de Oliveira-Júnior, R. G.; Diniz, T. C.; Wanderley, C. W. S.; Palheta-Júnior, R. C.; Mendes, R. L.; Guimarães, A. G.; Quintans-Júnior, L. J.; Almeida, J. R. G. S.; BMC Complementary Altern. Med. 2015, 15, 197. [Crossref] 13. Silva, J. C.; Macedo, L. A. R. O.; Souza, G. R.; Oliveira-Júnior, R. G.; Lima-Saraiva, S. R. G.; Lavor, E. M.; Silva, M. G.; Souza, M. T. S.; Bonjardim, L. R.; Quintans-Júnior, L. J.; Mendes, R. L.; Almeida, J. R. G. S.; Z. Naturforsch., C: J. Biosci. 2016, 71, 209. [Crossref] 14. Araújo, C. S.; de Oliveira, A. P.; Santos, A. D. C.; Guimarães, A. L.; Silva, N. D. S.; Queiroz, M. A. A.; Araújo, E. C. C.; Almeida, J. R. G. S.; Phytochem. Anal. 2019, 30, 83. [Crossref] 15. Kitajima, J.; Komori, T.; Kawasaki, T.; Chem. Pharm. Bull. 1982, 30, 3912. [Crossref] 16. Pacheco, A. G.; de Oliveira, P. M.; Piló-Veloso, D.; Alcântaca, A. F. C.; Molecules 2009, 14, 1245. [Crossref] 17. Vieira, G. H. F.; Mourão, J. A.; Ângelo, A. M.; Costa, R. A.; Vieira, R. H. S. F.; Rev. Inst. Med. Trop. Sao Paulo 2010, 52, 129. [Crossref] 18. Lima Neto, J. S.; Gramosa, N. V.; Silveira, E. R.; Quim. Nova 2008, 31, 1078. [Crossref] 19. Hsieh, T.-J.; Wu, Y.-C.; Chen, S.-C.; Huang, C.-S.; Chen, C.-Y.; J. Chin. Chem. Soc. 2004, 51, 869. [Crossref] 20. Leite, D. O. D.; Nonato, C. F. A.; Camilo, C. J.; de Carvalho, N. K. G.; da Nóbrega, M. G. L. A.; Pereira, R. C.; da Costa, J. G. M.; Curr. Pharm. Des. 2020, 26, 4056. [Crossref] 21. Tundis, R.; Xiao, J.; Loizzo, M. R.; Ann. N. Y. Acad. Sci. 2017, 1398, 30. [Crossref] 22. Costa, E. V.; Dutra, L. M.; Nogueira, P. C. L.; Moraes, V. R. S.; Salvador, M. J.; Ribeiro, L. H. G.; Gadelha, F. R.; Nat. Prod. Commun. 2012, 7, 265. [Crossref] 23. Rabêlo, S. V.; Costa, E. V.; Barison, A.; Dutra, L. M.; Nunes, X. P.; Tomaz, J. C.; Oliveira, G. G.; Lopes, N. P.; Santos, M. F. C.; Almeida, J. R. G. S.; Rev. Bras. Farmacogn. 2015, 25, 419. [Crossref] 24. Rocha, G. N. S. A. O.; Dutra, L. M.; Lorenzo, V. P.; Almeida, J. R. G. S.; Chem.-Biol. Interact. 2021, 336, 109390. [Crossref] 25. Alfaia, D. P. S.; Almeida, S. S. M. S.; Biota Amazônia 2022, 6, 26. 26. Alho, C. J. R.; Estudos Avançados 2012, 26, 151. [Crossref] 27. Chen, B.-H.; Chang, H.-W.; Huang, H.-M.; Chong, I.-W.; Chen, J.S.; Chen, C.-Y.; Wang, H.-M.; J. Agric. Food Chem. 2011, 59, 2284. [Crossref] 28. Omara, T.; Kiprop, A. K.; Ramkat, R. C.; Cherutoi, J.; Kagoya, S.; Nyangena, D. M.; Tebo, T. A.; Nteziyaremye, P.; Karanja, L. N.; Jepchirchir, A.; Maiyo, A.; Kiptui, B. J.; Mbabazi, I.; Nakiguli, C. K.; Nakabuye, B. V.; Koske, M. C.; Evidence-Based Complementary and Alternative Medicine 2020, 2020, 3529081. [Crossref] 29. Almeida, J. R. G. S.; Rev. Virtual Quim. 2019, 11, 379. [Crossref] 30. Almeida, J. R. G. S.; Araújo, C. S.; Pessoa, C. Ó.; da Costa, M. P.; Pacheco, A. G. M.; Rev. Bras. Frutic. 2014, 36, 258. [Crossref] 31. Chen, Y.-Y.; Ma, C.-Y.; Wang, M.-L.; Lu, J.-H.; Hu, P.; Chen, J.-W.; Li, X.; Chen, Y.; Nat. Prod. Res. 2020, 34, 2243. [Crossref] 32. Leboeuf, M.; Cavé, A.; Bhaumik, P. K.; Mukherjee, B.; Mukherjee, R.; Phytochemistry 1980, 21, 2783. [Crossref] 33. Miyashita, H.; Nishida, M.; Okawa, M.; Nohara, T.; Yoshimitsu, H.; Chem. Pharm. Bull. 2010, 58, 765. [Crossref]
Guest Editor handled this article: Ivo José C. Vieira |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access