Artigo
| Metabolic diversity of zoantharians collected at Brazilian oceanic islands |
|
Bianca Del B. SahmI,II,# I. Laboratório de Farmacologia de Produtos Naturais Marinhos, Instituto de Ciências Biomédicas, Universidade de São Paulo, 05508-000 São Paulo - SP, Brasil Received: 03/10/2025 *e-mail: costalotufo@usp.br Zoantharians are benthic marine organisms widespread in Brazilian tropical shallow waters. Recent evidence suggests that the metabolism of these cnidarians may be influenced by diverse environmental factors. We have previously shown that two species of Palythoa found on the Brazilian coast share metabolomic similarities related to their location. Herein, through gas chromatography coupled with mass spectrometry (GC-MS) and liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis, along with molecular networking, we assessed polar and apolar extracts from 28 samples of Palythoa spp. and Zoanthus spp. collected at different Brazilian oceanic islands - Saint Peter and Saint Paul Archipelago, Fernando de Noronha Archipelago, Rocas Atoll and Trindade Island - to describe and compare their metabolome in such isolated regions, under reduced interference of anthropic actions. The chemical profiles of zoantharians from Brazilian oceanic islands include fatty acids and sterol derivatives, in apolar fractions, and ecdysteroids, phospholipids, ceramides, mycosporine-like amino acids, and alkaloids in polar fractions. These chemical classes are similar to those described for the continental zoanthids, previously assessed. The results presented herein reveal a preserved metabolome across the analyzed species, Palythoa caribaeorum, Palythoa variabilis and Zoanthus spp., and between island and continental organisms, suggesting core species-specific metabolites and a portion of an environmentally-driven chemical expression. INTRODUCTION Zoantharians, also known as sea mat corals, are among the most common benthic marine organisms in Brazilian tropical shallow-water communities, including coral reefs. So far, thirteen different species have been reported in Brazil, where Palythoa caribaeorum, P. variabilis, and Zoanthus sociatus are among the most abundant ones.1 Both genera, Palythoa and Zoanthus, stands out not only for their abundance in marine ecosystems but also for their ability to biosynthesize secondary compounds with significant ecological and biotechnological potential.2-4 Data from reefs in the southwest Atlantic coast revealed that zoantharians, along with macroalgae, are organisms that rapidly occupy degraded areas where reef-building animals (such as scleractinian corals) have been lost,5 suggesting that these cnidarians are also important players in the community phase-shift process. In Todos os Santos Bay, for example, P. variabilis, accounts for up to 88.6% of shifted reef areas.6 This suggests that a high abundance of these animals does not necessarily indicate a healthy ecosystem, especially in coral reefs. Up to now, there have been no studies describing the chemical mediation of this coral-to-zoantharian shift. These characteristics make zoantharians relevant indicators of reef ecosystem health while also serving as a source of bioactive substances.7,8 Recent studies4,7,9,10 suggest that the metabolic profiles of these cnidarians may be influenced by a range of environmental factors, including temperature, salinity, and pollution, as well as by the composition of their associated microbiota. We have previously shown4 that two congeneric species of Palythoa found on the Brazilian coast share metabolomic similarities depending on the location in which they cohabit. Indeed, their chemical profile seems to be influenced by environmental variation and not only by specie-intrinsic specialized metabolism drivers. These changes in their metabolome serve as important cues as to the functional role of the microorganisms for acclimation or adaptation to variable environmental regimes. Hence, this dynamic reflects a complex balance between endogenous (organism-intrinsic) and exogenous (external) factors, which together can shape the adaptive capacity of Palythoa.4 Metabolomic approaches have proven to be valuable tools for characterizing the chemical variability of marine organisms, enabling the elucidation of adaptive mechanisms crucial for survival in challenging environments. Furthermore, this methodology has been effective in microbial identification, contributing to a deeper understanding of the symbiotic interactions that define these marine holobionts.11,12 Moving away from the coastline, the objective of this study is to assess the metabolomic profile of zoantharians holobionts found in Brazilian oceanic islands (Palythoa caribaeorum, P. variabilis and Zoanthus spp.), adding data to the significant variations in the metabolic profiles of zoantharians from coastal and isolated island environments. With the possible exception of Fernando de Noronha Archipelago, the Brazilian oceanic islands may be considered more pristine ecosystems, where the influence of natural environmental variations may be perceived with less interference from anthropic actions. Furthermore, to the best of our knowledge, this is the first metabolomic assessment of zoantharians found in the four Brazilian oceanic islands, which brings a valuable contribution to knowledge about the species. Through this analysis, we aimed to identify the chemical composition of these organisms from isolated regions, contributing to a better understanding of zoantharian adaptations and their responses to ecological changes. This research may provide valuable insights into the health of reef ecosystems and the roles of zoantharians in biodiversity conservation.
EXPERIMENTAL Chemicals High-performance liquid chromatography (HPLC)-grade MeOH, hexane and analytical-grade acetic acid were purchased from J.T. Baker (Phillipsburg, USA). Acetonitrile (ACN) was obtained from Merck (Darmstadt, Germany). Anhydrous Na2SO4 (P.A.-ACS) was obtained from Synth (Diadema, Brazil) and water was distilled and purified using a Millipore Milli-Q Plus system (Bedford, USA). Sample collection Expeditions to Brazilian oceanic islands took place between 2014 and 2015 with support from the Brazilian Navy and the Chico Mendes Institute for Biodiversity (ICMBio - Scientific authorization No. 44435-4 and No. 29953-7). Specimens of Palythoa caribaeorum, Palythoa variabilis and Zoanthus spp. were collected manually with the aid of a stainless-steel spatula at depths ranging from intertidal to 15 m. Collectors and divers wore gloves during all the procedures. In the field, the collected material was cased with aluminum foil and placed in a refrigerated styrofoam box containing ice packs. At the scientific stations, samples were screened for the presence of other organisms using sterile dissection forceps and placed in sterile Whirl Pak bags. The organisms were then immediately frozen in liquid nitrogen for metabolic quenching. The samples remained frozen in liquid nitrogen until processing in a fully equipped laboratory. In this work, we analyzed samples of P. caribaeorum collected in the Saint Peter and Saint Paul Archipelago - SPSPA (2015, n = 1), Fernando de Noronha Archipelago - FNA (2014, n = 3), Rocas Atoll - RA (2014, n = 2; 2015, n = 1) and Trindade Island - TR (2015, n = 3); and of P. variabilis from SPSPA (2015, n = 3), FNA (2015, n = 1) and RA (2014, n = 6); Zoanthus spp. from RA (2014, n = 6); and TR (2015, n = 2) (Table 1S, Supplementary Material). Extract preparation The thawed samples were cut with surgical scissors and extracted with 20 mL of MeOH under sonication (3 × 15 min). The resulting solutions were filtered and subjected to partition with n-hexane (3 × 10 mL for 20 min), where the organic phase was treated with anhydrous Na2SO4 followed by evaporation of the solvent by distillation at reduced pressure (35 ºC), providing the hexane fractions. The hydroalcoholic phase was distilled and subsequently lyophilized, then resuspended with MeOH (10 mL). After extraction, samples were dried, providing the methanolic fractions. Sea salt from the methanolic fractions was removed with a Discovery C18 3 mL 500 mg SPE cartridge (Supelco, Sigma-Aldrich, USA). Columns were activated with MeOH (3 × 1 mL), followed by equalization with ultrapure H2O (3 × 1 mL). Samples were diluted in 50 µL of MeOH and 950 µL of H2O prior to application into the cartridge. Salt was removed by washing with 1 mL of ultrapure H2O, extract was recovered with 1 mL of MeOH and dried once more in a concentrator at reduced pressure. A pooled quality control (QC) sample was prepared separately for each analytical platform by combining equal aliquots of hexane and methanol fractions. GC-MS analysis Analysis of apolar compounds was conducted using gas chromatography coupled with mass spectrometry (GC-MS) on a GCMS-QP2010 system (Shimadzu, Kyoto, Japan), following specific operational parameters. A Zebron ZB-5MS capillary fused chromatographic column (30 m × 0.25 mm, 0.25 μm film thickness) was employed, with helium as the carrier gas at a pressure of 72.8 kPa, a flow rate of 1.20 mL min-1, and a linear velocity of 40.0 cm s-1. The injector temperature was maintained at 250 ºC, and the column oven temperature was programmed to start at 60 ºC (1 min hold), increasing at a rate of 5 ºC min-1 until reaching 320 ºC (10 min hold), with a total runtime of 63 min. An injection volume of 1 μL was used with a split ratio of 1:10. Mass spectrometric detection was performed using electron ionization (EI) at 70 eV, operating in scan and mass range of m/z 35-600. The ion source temperature was set to 250 ºC, and the interface temperature was maintained at 320 ºC. Data acquisition was carried out with an event time of 0.3 s and a scan speed of 2500 u s-1. At the end of the sequence, the pooled QC sample was injected, and the n-alkane mixture (C8-C40) was analyzed to determine retention indices (RI) using the Kovats index. Solvent blanks (ethyl acetate) were injected every ten samples. Data was processed using Shimadzu GCMS-Solution software, version 4.20 (Shimadzu Corporation, Japan, 2017). LC-MS/MS analysis Liquid chromatography tandem mass spectrometry (LC-MS/MS) analyses were performed on an LC-QqTOF mass spectrometer (Bruker Daltonics, Billerica, USA) equipped with an Apollo-ESI source operating in positive ion mode. Chromatographic separation was carried out on a reverse-phase XB-C18 Kinetex core-shell column (100 × 2.1 mm, 2.6 µm) at a flow rate of 300 µL min-1. The mobile phase consisted of water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B), using a gradient from 5 to 100% B over 35 min at 40 ºC. The injection volume was 2 µL. The solvent blanks (MeOH) and QC samples were included at the beginning, and after every ten samples. Mass spectrometric detection was performed with a capillary voltage of 4.5 kV. Nebulizer gas pressure, dry gas flow rate, and dry gas temperature were set at 4.5 bar, 9.0 L min-1, and 200 ºC, respectively. Collision-induced dissociation (CID) was applied with collision energies ranging from 20 to 70 eV. Data were acquired in full-scan mode (m/z 100-2000), with MS/MS spectra collected in data-dependent acquisition (DDA) mode, selecting the five most intense precursor ions per cycle. The mass calibration was performed using NaTFA cluster ions in enhanced quadratic mode, with the calibration solution infused at 3 μL min-1 via a six-port divert valve at the end of the run. Data acquisition was carried out using Hystar Application software, version 3.2 (Bruker Daltonik GmbH, Germany, 2019) and Otof Control v. 3.4 (Bruker Daltonik GmbH, Germany, 2014), and mass spectra were processed using DataAnalysis software, version 4.3 (Bruker Daltonik GmbH, Germany, 2020). Data processing GC-MS and LC-MS/MS raw data were converted to mzXML format using Shimadzu GCMS-Solution software and Bruker DataAnalysis software, respectively. GC-MS data were processed using the MSHub-GC workflow on the Global Natural Products Social Molecular Networking (GNPS) platform, applying chromatographic and spectral deconvolution. LC-MS/MS data were analyzed using MZmine, version 4.4.3 (mzio GmbH, Germany, 2023) for peak detection, feature resolving, isotope filtering, and alignment. The processed data were exported in .mgf and .csv formats for GNPS analysis. MSHub deconvolution URL (uniform resource 20250178) data results are available at the Data Availability Statement section, and MZmine processing parameters are detailed in Table 2S of the Supplementary Material. The output feature table was processed by removing features with peak areas below threefold the mean of blank samples. Missing values were imputed by replacing zeros with one-fifth of the smallest observed peak area, and the feature matrix was normalized to the total peak area of each sample. To integrate GC-MS and LC-MS/MS data, concatenation was used as a method of data fusion to generate a comprehensive metabolic profile encompassing both apolar and polar metabolites. To prevent thedata blocks from being disproportionately weighted, unit variance scaling (1/σ) was applied to the variables. Additionally, block scaling was used, where each dataset was weighted to achieve an equal standard deviation sum (1/(∑σ) block). Molecular networking For GC-MS data, the library search/molecular networking GC workflow on GNPS (CCMS, USA, 2016) was used to generate a molecular network. The setup included MSHub as the quantification table source, a fragment ion mass tolerance of 0.5 Da, a cosine score threshold of 0.65, a minimum of six matched fragment ions, a Network TopK of 10, and a maximum molecular family size of 100. Searches in the GNPS library were performed using the bronze class, allowing up to 10 hits per spectrum. The library spectra were filtered identically to the input data to ensure valid matches. Unmatched features were manually annotated by comparing their mass spectra with reference databases, including Wiley MS Database, NIST8, and NIST14. Only matches with a minimum spectral similarity of 70% were considered. Additionally, the calculated retention indices were compared with literature values from NIST to further validate compound annotations. For LC-MS/MS data, feature-based molecular networking (FBMN) was conducted on the GNPS2 platform, release 2025.06.17 (Wang Bioinformatics Group, USA, 2025). Molecular networks were generated using a cosine score threshold of 0.65, a minimum of 5 matched fragment ions, and a maximum parent and fragment mass tolerance of 0.02. Feature pairs with similar fragmentation patterns were clustered, and spectral annotations were assigned by matching against GNPS spectral libraries. In silico molecular formula predictions were performed using SIRIUS, version 6.1.0 (Böcker, Germany, 2025) to support structural annotation. All molecular networks were visualized in Cytoscape, version 3.8.2 (Cytoscape Team, USA, 2020) to explore metabolite relationships. The library search/networking URL jobs for GC-MS and LC-MS are available at the Data Availability Statement section. Statistical analysis Multivariate statistical analyses were performed using SIMCA®, version 18.0 (Umetrics AB, Sweden, 2023). For non-fused datasets, the normalized peak table was Pareto-scaled and log-transformed before analysis. Principal component analysis (PCA) was used to assess data quality by evaluating the clustering of QC samples (Figure 1S, Supplementary Material). For the fused GC-MS and LC-MS/MS dataset, partial least squares discriminant analysis (PLS-DA) was applied to identify the metabolites that drive species differentiation. Model reliability was validated through permutation tests (n = 100) (Figure 2S, Supplementary Material), and discriminant metabolites were selected based on vector importance projection (VIP) scores (> 1) and adjusted p-values (< 0.05, Benjamini-Hochberg correction). Bar plots were generated to visualize species-specific variations. UpSet plots were created using R, version 4.0.0 (R Core Team, Austria, 2020) with UpSetR package, version 1.4.0 (Harvard Medical School, USA, 2019) and proportional Venn diagrams were generated with BioVenn (CMBI, Netherlands, 2008).
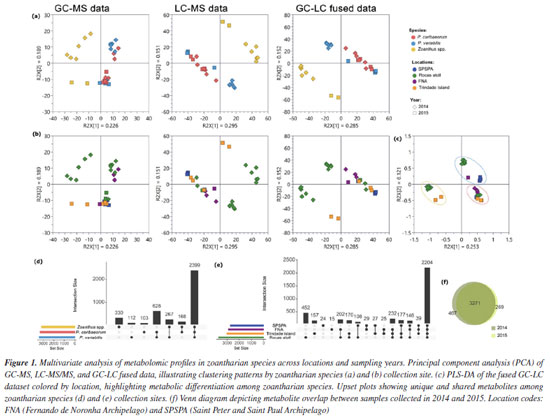
RESULTS AND DISCUSSION The metabolite expression of living organisms has been proposed as a valuable complementary tool in integrative systematics, connecting taxonomy to chemical signatures, but also allowing the understanding of some of the environmental drivers in chemically mediated interactions between the host and the microbiome.13-16 Although it has sparked controversy in recent years, there is increasing evidence that the metabolome may be divided into two main components: a core collection of metabolites related to primary metabolism and species-specific functions, and a dynamic collection of metabolites related to environmental conditions.17-19 Such division has also been proposed for the microbiome, which is, thus, reflected in the metabolome.20,21 Herein, 28 samples of zoantharians, Palythoa caribaeorum (n = 10), Palythoa variabilis (n = 10) and Zoanthus spp. (n = 8), collected at Brazilian oceanic islands during 2014 and 2015, were investigated using metabolomic tools. Crude extracts were partitioned to obtain apolar and polar fractions, which were analyzed by GC-MS and LC-MS/MS, respectively. Initially, data from apolar (GC-MS) and polar (LC-MS/MS) fractions were submitted to an exploratory principal component analysis (PCA) both separately and jointly, illustrating clustering patterns by zoantharian species (Figure 1a) and collection site (Figure 1b). There is a clear separation between samples from the two genera, Palythoa and Zoanthus, regardless of the analyzed fraction. However, LC-MS/MS seemed to have a higher discriminative power between the two groups, and fused data reinforced this pattern. GC-LC dataset was then analyzed by PLS-DA, highlighting metabolic differentiation among zoantharian species (Figure 1c). Most of the detected signals were shared by the three species (59.9%). In contrast, only a small fraction of the signals was species-specific for Zoanthus spp. (8.2%), P. caribaeorum (2.8%), and P. variabilis (2.6%) (Figure 1d). Shared signals between the two Palythoa species reached 15.7%.
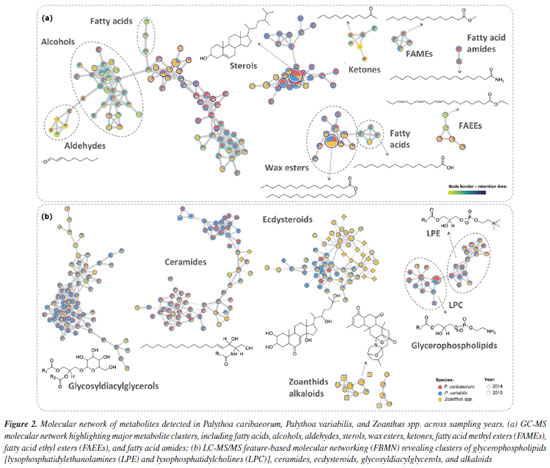
Considering the different collection sites, a core collection of metabolites could be observed, reaching 55% of the signals (Figure 1e), while samples from RA showed the highest number of unique signals (11.3%), followed by TR (3.9%), SPSPA (0.06%) and FNA (0.04%). Even though a reduced number of samples was assessed herein, it is possible to suggest a site-driven assortment of metabolites, reinforcing the influence of environmental conditions on metabolic fingerprinting. Furthermore, these findings align with those previously observed for P. caribaeorum and P. variabilis collected along the Brazilian coastline.4 The overlap of signals between samples collected in 2014 and 2015 is displayed on the Venn diagram (Figure 1f), showing that most metabolites (81.6%) were common to the organisms within the two collection years. Some differences between metabolites are discussed later in this section. Considering the metabolites annotated in the apolar extracts obtained for the three species in both years, analyzed by GC-MS, there is a predominance of fatty acids and their derivatives, as well as cholesterol analogues (Figure 2a and Table 3S in the Supplementary Material). These findings are consistent with our previous study,4 in which similar metabolite classes were identified across the species, underpinning a characteristic chemical profile of these extracts. The presence of fatty acids and sterol derivatives is somewhat expected due to their significant role in the structural and metabolic functions of the organisms, contributing to membrane integrity, energy storage,22,23 and bioactivity.24,25 The recurrence of these compounds in insular zoantharians highlights the chemical stability of apolar fractions and supports their relevance in biochemical and ecological contexts.
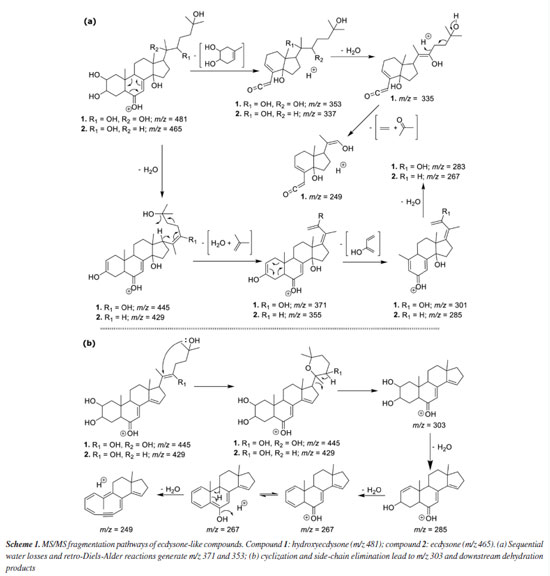
The characterization of the polar secondary metabolism was conducted using LC-MS/MS, leveraging high-accuracy mass measurements (< 10 ppm), MS/MS fragmentation patterns, and GNPS spectral library matching.26,27 The data allowed annotation of several metabolite classes, including ecdysteroids, phospholipids (glycerophosphoethanolamines and glycerophosphocholines), ceramides, mycosporine-like amino acids and various alkaloids, see Figure 2b and Table 4S (Supplementary Material), suggesting a similar chemodiversity, as previously observed4 for continental specimens of Palythoa spp. Representative MS/MS mirror plots illustrating spectral matches to GNPS library references are provided in Figures 4S-7S (Supplementary Material). In the case of the ecdysteroids-like family of compounds, the literature typically reports only numerical fragmentations, without a proper rationale for the fragmentation pathways that would allow manual structural interpretation, which can be useful to propose the analogues chemistry. Considering that the two major compounds in this series are ecdysone (Scheme 1, compound 1) and hydroxyecdysone (Scheme 1, compound 2), we opted to conduct a detailed analysis of their main fragments to infer the structures of related analogues. The protonated ions initially undergo the loss of six water molecules in the case of hydroxyecdysone (yielding ions at m/z 463, 445, 427, 409, 391, and 373), and five water molecules in the case of ecdysone (yielding ions at m/z 447, 429, 411, 393, and 375). These successive water losses are likely driven by remote hydrogen rearrangement mechanisms.28 Some of the ions, after the loss of one or two water molecules, can initiate additional fragmentation pathways. To investigate this, we examined all the groups of reactions depicted in Schemes 1a and 1b. These mechanistic proposals support the theoretical rationale for the formation of key ESI-MS/MS fragment ions observed for compounds 1 and 2. In both cases, a characteristic retro-Diels-Alder fragmentation (commonly reported for polycyclic systems) was observed, yielding ions at m/z 353 (1) and 337 (2).29 A charge migration fragmentation mechanism, analogous to the Grob-Wharton rearrangement described for polyethers,30 was observed exclusively in hydroxyecdysone, likely due to the inductive effect of the -OH group at the vinylic moiety. The same mechanism was more clearly observed in reverse order in both compounds (Scheme 1a), involving the elimination of the side chain from molecules that had lost at least one water molecule (the first loss necessarily occurring at the start of the side chain), leading to the formation of ions at m/z 445 (1) and 429 (2). The final ions in this portion of the scheme are generated via a retro-Diels-Alder reaction, this time involving the first ring, which becomes unsaturated after a neutral water elimination, producing ions at m/z 301 (1) and 285 (2).
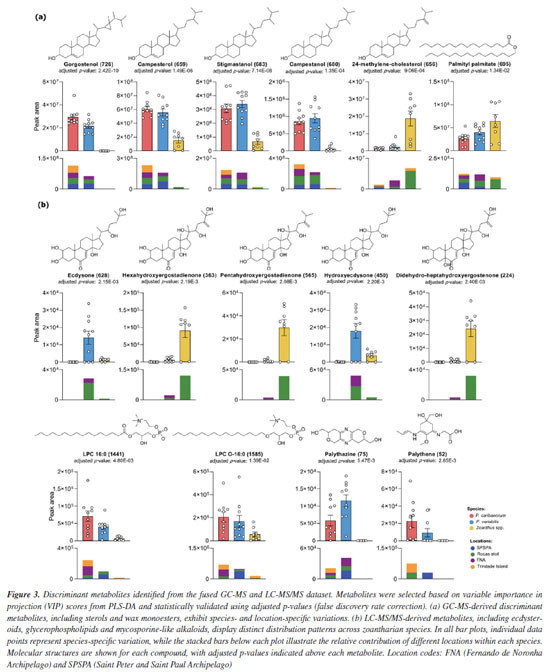
Scheme 1b shows the ions that rapidly eliminate the entire side chain, resulting in fragment ions of identical m/z for both compounds 1 and 2. The process is initiated by the loss of a water molecule from the beginning of the side chain, although additional neutral water losses may occur beforehand. A nucleophilic attack by the terminal -OH group on the unsaturation leads to a cyclization, forming a six-membered ring that immediately undergoes elimination of the entire cyclized side chain via a remote hydrogen rearrangement mechanism, generating the ion at m/z 303 in both structures. This ion then loses two additional water molecules through the previously described mechanism, and a third water molecule is eliminated upon ring opening, forming an alkyne - a process similar to that observed in sesquiterpene lactones.29 These fragmentation patterns further supported the propagation of structural annotations across the ecdysteroid cluster. Ecdysone and hydroxyecdysone differ by +16 Da, as shown in Scheme 1, consistent with the addition of a hydroxyl group on the side chain. Dihydroxymethylecdysone, which exhibited a +30 Da shift relative to hydroxyecdysone, produced the characteristic fragment at m/z 371, indicating that the modification is localized on the side chain. In contrast, acetyl-hydroxyecdysone showed a +42 Da shift and generated a fragment at m/z 413, while the presence of m/z 353 suggests that acetylation occurs on the steroid core. These compounds represent illustrative examples of the ecdysteroid-like family, with comparative MS/MS spectra shown in Figure 8S (Supplementary Material). Statistical analysis further supports a distinction between the two zoanthids studied genera, demonstrating that Zoanthus species differentiate from Palythoa species, and wax monoesters appear in slightly higher abundance in Zoanthus spp. Figure 3a showed that Palythoa species have a predominance of phytosterols, such as gorgosterol, campesterol, stigmasterol and campestenol, while Zoanthus spp. showed the predominance of 24-methylene-cholesterol and palmityl-palmitate. These results reinforce the genus-specific chemical signatures and suggest functional or ecological roles for these compounds, possibly related to structural integrity, environmental adaptation, or symbiotic interactions.31 The recurrence of these lipidic profiles across studies further underscores the stability and relevance of these metabolites within zoantharians, providing valuable insights into their biochemical and metabolic characteristics.
Ecdysone and related compounds accounted for a distinction between the species (Figure 3b). In fact, ecdysone related compounds were distinct in P. variabilis and Zoanthus spp., while absent in P.caribaeorum. Ecdysone and hydroxyecdysone were majorly detected in P. variabilis, mainly from RA but also in FNA specimens, while dihydro-ergostadienone-derivatives were observed in Zoanthus specimens sampled at RA. Ecdysone is a well-known hormone related to molting in arthropods and other Ecdysozoa and has also been described for cnidarians. It seems to be common in medusozoans, in which NR3E receptors were also described,32 pointing to a signaling function. For anthozoans, however, its presence does not seem to be so widespread, and its function remains unknown.2 Phosphocholines and the mycosporine-like amino acids, palythazine and palythene, were more abundant in Palythoa spp. Interestingly, these classes of compounds - that suggested, herein, some degree of species-driven variations - were correlated to environmental differences in the previous work4 carried out with the continental Palythoa spp. from the Brazilian coast. Moreover, it must be noted that no palythene derivatives were noted for FNA samples for any of the Palythoa spp., strengthening the hypothesis of an environmental component swaying the production of these compounds. Among the islands, metabolomic analyses revealed that organisms from FNA did not exhibit specific metabolite classes, unlike RA and TR, suggesting a more generalist metabolic production or prevalence of other metabolic pathways due to local environmental factors (Figure 2). Ecdysteroids were predominant in samples from Rocas Atoll, especially in Zoanthus spp., which exhibited species-characteristic ecdysteroids. Zoantharian alkaloids, such as kuroshina and zoanthamine, were detected exclusively in samples from Trindade Island, notably in Zoanthus specimens but also in Palythoa caribaeorum. It is important to point out that a previous analysis of the distribution of zoantharian species along the Brazilian coast and oceanic islands showed that Z. sociatus occurs on islands located in the northwestern portion of the South Atlantic Ocean (RA, FNA and SPSPA), while Z. pulchellus cohabits SPSPA along with its congeneric species, and Z. aff. pulchellus occurs exclusively on the southernmost island (TR).1 Furthermore, the differences in the metabolome for Zoanthus spp. collected at TR and AR reinforced the idea that there are different species occurring on these islands. Moreover, a previous investigation13 of the metabolome of six different species of zoantharians found on the eastern coast of the tropical Pacific Ocean revealed a high concentration and diversity of zoanthamine-type alkaloids found exclusively in Zoanthus cf. pulchellus, while ecdysteroids were widespread in all other species, including Z. sociatus, but excluding Z. cf. pulchellus. It is also important to note that, although Palythoa and Zoanthus may co-occur at the same depths and sites, the latter is usually restricted to the intertidal or subtidal fringe. At the same time, Palythoa may be found further deep, sometimes at 10 m depth. Hence, some of the differences attributed to taxonomy may, in fact, reflect distinct micro-environmental conditions at the same site. As suggested before, the metabolic profile revealed minor chemical divergences between the Palythoa species,4 which may be expanded to zoantharians in general, although to a lower extent, with species-specific variations,13 as shown here. Overall, the chemical variations were a consequence of skeleton specialization, and such metabolic diversification primarily results in the biosynthetic functionalization of consistent classes of compounds. This hypothesis has been described and suggests zoantharians, like Parazoanthus axinellae, modify the same molecular backbone in different manners to generate molecular diversity.13,33 These findings suggest that modifications of precursor chemical structures may constitute a key driving force of metabolite variability and adaptive competence. Here, the limited sampling precludes more definite conclusions regarding the influence of environmental factors on the metabolome of insular zoantharians. Also, as environmental conditions change seasonally, this portion of the metabolome would vary accordingly, demanding further replicates at different times of the year. When changes on a global scale are considered, these relationships become more relevant, especially in winner/loser scenarios for shallow reef communities, in which zoantharians may replace coral or, even, be replaced by macroalgae.5,6 In this context, establishing chemical markers may help forecast changes in the functioning of these communities. Based on the data presented here, long-term monitoring studies related to seasonal variations or global climate change could provide valuable information on metabolites production by zoanthids in response to different environmental scenarios.
CONCLUSIONS The chemical diversity of zoantharians from Brazilian oceanic islands mostly includes fatty acids and sterol derivatives, as well as ecdysteroids, phospholipids, ceramides, mycosporine-like amino acids, and various alkaloids. These are, in general, chemical classes comparable to those previously described for the continental zoantharians collected along the Brazilian coast. The consistency of the metabolome in the different species analyzed herein, Palythoa caribaeorum, Palythoa variabilis and Zoanthus spp., and between island and continental organisms, suggests core species-specific metabolites and a dynamic fraction of environmentally driven chemodiversity.
SUPPLEMENTARY MATERIAL Some images of the systems used in this work are available at http://quimicanova.sbq.org.br, as a PDF file, with free access.
DATA AVAILABILITY STATEMENT MSHub deconvolution results are available at https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=efb48dec0adf4e7ab9ff5be87f4f15a3. The library search and networking jobs for GC-MS are available at https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=b8ed675271c640ccaf2aec0948895236 and for LC-MS/MS at https://gnps2.org/status?task=e3357317a6134042825c1fa21d0d6870. Additional data are available upon request to the corresponding author.
ACKNOWLEDGMENTS We thank the Brazilian Navy and the Chico Mendes Institute for Biodiversity (ICMBio-MMA) for logistical support and the team of the Long-Term Ecological Program in Brazilian Oceanic Islands (PELD-ILOC) for collecting samples. We are also grateful to the agencies that financed this work: Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPQ (grants No. 458548/2013-8, No. 443281/2019-0, No. 441820/2024-7, No. 403140/2012-6, No. 441241/2016-6, No. 441327/2020-6, No. 446005/2024-0, No. 440472/2022-9, No. 443318/2019-0) and Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP (grants No. 2014/50926-0, No. 2015/17177-6 and No. 2022/12654-4).
AUTHOR CONTRIBUTIONS Bianca Del B. Sahm was responsible for conceptualization, investigation, data curation, writing (original draft, review and editing); Ana C. Zanatta for investigation, data curation, formal analysis, writing (original draft, review and editing); Francisco da Chagas L. Pinto for investigation, data curation; Otília D. L. Pessoa for investigation, data curation; Diego V. Wilke for investigation, data curation, project administration, writing original draft; Paula C. Jimenez for investigation, writing original draft; Norberto P. Lopes for conceptualization, investigation, data curation, formal analysis, resources, writing (original draft, review and editing); Tito M. C. Lotufo for investigation, formal analysis; Letícia V. Costa-Lotufo, for conceptualization, investigation, data curation, project administration, funding acquisition, resources, writing (original draft, review and editing).
REFERENCES 1. Santos, M. E. A.; Kitahara, M. V.; Lindner, A.; Reimer, J. D.; Marine Biodiversity 2016, 46, 547. [Crossref] 2. Guillen, P. O.; Jaramillo, K. B.; Genta-Jouve, G.; Thomas, O. P.; Nat. Prod. Rep. 2019, 37, 515. [Crossref] 3. Hines, D. E.; Pawlik, J. R.; Mar. Biol. 2012, 159, 389. [Crossref] 4. Costa-Lotufo, L. V.; Carnevale-Neto, F.; Trindade-Silva, A. E.; Silva, R. R.; Silva, G. G. Z.; Wilke, D. V.; Pinto, F. C. L.; Sahm, B. D. B.; Jimenez, P. C.; Mendonça, J. N.; Lotufo, T. M. C.; Pessoa, O. D. L.; Lopes, N. P.; Chem. Commun. 2018, 54, 1952. [Crossref] 5. Cruz, I. C. S.; Waters, L. G.; Kikuchi, R. K. P.; Leão, Z. M. A. N.; Turra, A.; Mar. Pollut. Bull. 2018, 135, 551. [Crossref] 6. Cruz, I. C. S.; Meira, V. H.; de Kikuchi, R. K. P.; Creed, J. C.; Mar. Environ. Res. 2016, 115, 28. [Crossref] 7. Soares, M. O.; Kitahara, M. V.; Santos, M. E. A.; Bejarano, S.; Rabelo, E. F.; Cruz, I. C. S.; Mar. Environ. Res. 2022, 173, 105535. [Crossref] 8. Wilke, D. V.; Jimenez, P. C.; Branco, P. C.; Rezende-Teixeira, P.; Trindade-Silva, A. E.; Bauermeister, A.; Lopes, N. P.; Costa-Lotufo, L. V.; Planta Med. 2021, 87, 49. [Crossref] 9. Kise, H.; Santos, M. E. A.; Fourreau, C. J. L.; Iguchi, A.; Goto, R.; Reimer, J. D.; Mol. Phylogenet. Evol. 2023, 182, 107732. [Crossref] 10. Campos, P.; Pires, A.; Figueira, E.; Environ. Res. 2020, 186, 109504. [Crossref] 11. Pilon, A. C.; Selegato, D. M.; Fernandes, R. P.; Bueno, P. C. P.; Pinho, D. R.; Carbevale Neto, F.; Freire, R. T.; Castro-Gamboa, I.; Bolzani, V. S.; Lopes, N. P.; Quim. Nova 2020, 43, 329. [Crossref] 12. Zuffa, S.; Schmid, R.; Bauermeister, A.; Paulo, P. W.; Caraballo-Rodriguez, A. M.; El Abiead, Y.; Aron, A. T.; Gentry, E. C.; Zemlin, J.; Meehan, M. J.; Avalon, N. E.; Cichewicz, R. H.; Buzun, E.; Terrazas, M. C.; Hsu, C. Y.; Oles, R.; Ayala, A. V.; Zhao, J.; Chu, H.; Kuijpers, M. C. M.; Jackrel, S. L.; Tugizimana, F.; Nephali, L. P.; Dubery, I. A.; Madala, N. E.; Moreira, E. A.; Costa-Lotufo, L. V.; Lopes, N. P.; Rezende-Teixeira, P.; Jimenez, P. C.; Rimal, B.; Patterson, A. D.; Traxler, M. F.; Pessotti, R. C.; Alvarado-Villalobos, D.; Tamayo-Castillo, G.; Chaverri, P.; Escudero-Leyva, E.; Quiros-Guerrero, L. M.; Bory, A. J.; Joubert, J.; Rutz, A.; Wolfender, J. L.; Allard, P. M.; Sichert, A.; Pontrelli, S.; Pullman, B. S.; Bandeira, N.; Gerwick, W. H.; Gindro, K.; Massana-Codina, J.; Wagner, B. C.; Forchhammer, K.; Petras, D.; Aiosa, N.; Garg, N.; Liebeke, M.; Bourceau, P.; Kang, K. B.; Gadhavi, H.; de Carvalho, L. P. S.; Santos, M. S.; Pérez-Lorente, A. I.; Molina-Santiago, C.; Romero, D.; Franke, R.; Brönstrup, M.; de León, A. V. P.; Pope, P. B.; La Rosa, S. L.; La Barbera, G.; Roager, H. M.; Laursen, M. F.; Hammerle, F.; Siewert, B.; Peintner, U.; Licona-Cassani, C.; Rodriguez-Orduña, L.; Rampler, E.; Hildebrand, F.; Koellensperger, G.; Schoeny, H.; Hohenwallner, K.; Panzenboeck, L.; Gregor, R.; O'Neill, E. C.; Roxborough, E. T.; Odoi, J.; Bale, N. J.; Ding, S.; Sinninghe Damsté, J. S.; Guan, X. L.; Cui, J. J.; Ju, K. S.; Silva, D. B.; Silva, F. M. R.; da Silva, G. F.; Koolen, H. H. F.; Grundmann, C.; Clement, J. A.; Mohimani, H.; Broders, K.; McPhail, K. L.; Ober-Singleton, S. E.; Rath, C. M.; McDonald, D.; Knight, R.; Wang, M.; Dorrestein, P. C.; Nat. Microbiol. 2024, 9, 336. [Crossref] 13. Jaramillo, K. B.; Reverter, M.; Guillen, P. O.; McCormack, G.; Rodriguez, J.; Sinniger, F.; Thomas, O. P.; Sci. Rep. 2018, 8, 7138. [Crossref] 14. Stabili, L.; Parisi, M. G.; Parrinello, D.; Cammarata, M.; Mar. Drugs 2018, 16, 296. [Crossref] 15. Edison, A. S.; Hall, R. D.; Junot, C.; Karp, P. D.; Kurland, I. J.; Mistrik, R.; Reed, L. K.; Saito, K.; Salek, R. M.; Steinbeck, C.; Sumner, L. W.; Viant, M. R.; Metabolites 2016, 6, 8. [Crossref] 16. Martucci, M. E. P.; De Vos, R. C. H.; Carollo, C. A.; Gobbo-Neto, L.; PLoS One 2014, 9, e93149. [Crossref] 17. Farag, M. A.; Al-Mahdy, D. A.; Meyer, A.; Westphal, H.; Wessjohann, L. A.; Sci. Rep. 2017, 7, 648. [Crossref] 18. West, A. K. R.; Bailey, C. B.; Bioorg. Med. Chem. Lett. 2023, 91, 129377. [Crossref] 19. Zhang, X.; Gao, X.; Liu, B.; Wang, J.; Shan, J.; Wang, J.; Zhang, Y.; Li, G.; Jia, Y.; Wang, R.; BMC Plant Biol. 2024, 24, 1128. [Crossref] 20. King, K. C.; Curr. Biol. 2019, 29, R78. [Crossref] 21. Flórez, L. V.; Biedermann, P. H. W.; Engl, T.; Kaltenpoth, M.; Nat. Prod. Rep. 2015, 32, 904. [Crossref] 22. Imbs, A. B.; Russ. J. Mar. Biol. 2013, 39, 153. [Crossref] 23. Hamoutene, D.; Puestow, T.; Miller-Banoub, J.; Wareham, V.; Coral Reefs 2008, 27, 237. [Crossref] 24. Ermolenko, E. V.; Imbs, A. B.; Gloriozova, T. A.; Poroikov, V. V.; Sikorskaya, T. V.; Dembitsky, V. M.; Mar. Drugs 2020, 18, 613. [Crossref] 25. Pinto, F. C. L.; Almeida, J. G. L.; Silveira, E. R.; Costa, A. M.; Guimarães, L. A.; Wilke, D. V.; Costa-Lotufo, L. V.; Torres, M. D. C. M.; Pessoa, O. D. L.; J. Braz. Chem. Soc. 2017, 28, 485. [Crossref] 26. Wang, M.; Carver, J. J.; Phelan, V. V.; Sanchez, L. M.; Garg, N.; Peng, Y.; Nguyen, D. D.; Watrous, J.; Kapono, C. A.; Luzzatto-Knaan, T.; Porto, C.; Bouslimani, A.; Melnik, A. V.; Meehan, M. J.; Liu, W. T.; Crüsemann, M.; Boudreau, P. D.; Esquenazi, E.; Sandoval-Calderón, M.; Kersten, R. D.; Pace, L. A.; Quinn, R. A.; Duncan, K. R.; Hsu, C. C.; Floros, D. J.; Gavilan, R. G.; Kleigrewe, K.; Northen, T.; Dutton, R. J.; Parrot, D.; Carlson, E. E.; Aigle, B.; Michelsen, C. F.; Jelsbak, L.; Sohlenkamp, C.; Pevzner, P.; Edlund, A.; McLean, J.; Piel, J.; Murphy, B. T.; Gerwick, L.; Liaw, C. C.; Yang, Y. L.; Humpf, H. U.; Maansson, M.; Keyzers, R. A.; Sims, A. C.; Johnson, A. R.; Sidebottom, A. M.; Sedio, B. E.; Klitgaard, A.; Larson, C. B.; Boya, C. A. P.; Torres-Mendoza, D.; Gonzalez, D. J.; Silva, D. B.; Marques, L. M.; Demarque, D. P.; Pociute, E.; O'Neill, E. C.; Briand, E.; Helfrich, E. J. N.; Granatosky, E. A.; Glukhov, E.; Ryffel, F.; Houson, H.; Mohimani, H.; Kharbush, J. J.; Zeng, Y.; Vorholt, J. A.; Kurita, K. L.; Charusanti, P.; McPhail, K. L.; Nielsen, K. F.; Vuong, L.; Elfeki, M.; Traxler, M. F.; Engene, N.; Koyama, N.; Vining, O. B.; Baric, R.; Silva, R. R.; Mascuch, S. J.; Tomasi, S.; Jenkins, S.; Macherla, V.; Hoffman, T.; Agarwal, V.; Williams, P. G.; Dai, J.; Neupane, R.; Gurr, J.; Rodríguez, A. M. C.; Lamsa, A.; Zhang, C.; Dorrestein, K.; Duggan, B. M.; Almaliti, J.; Allard, P. M.; Phapale, P.; Nothias, L. F.; Alexandrov, T.; Litaudon, M.; Wolfender, J. L.; Kyle, J. E.; Metz, T. O.; Peryea, T.; Nguyen, D. T.; VanLeer, D.; Shinn, P.; Jadhav, A.; Müller, R.; Waters, K. M.; Shi, W.; Liu, X.; Zhang, L.; Knight, R.; Jensen, P. R.; Palsson, B.; Pogliano, K.; Linington, R. G.; Gutiérrez, M.; Lopes, N. P.; Gerwick, W. H.; Moore, B. S.; Dorrestein, P. C.; Bandeira, N.; Nat. Biotechnol. 2016, 34, 828 [Crossref]; Global Natural Products Social (GNPS), https://gnps.ucsd.edu, accessed in July 2025. 27. Aksenov, A. A.; da Silva, R.; Knight, R.; Lopes, N. P.; Dorrestein, P. C.; Nat. Rev. Chem. 2017, 1, 0054. [Crossref] 28. Demarque, D. P.; Crotti, A. E. M.; Vessecchi, R.; Lopes, J. L. C.; Lopes, N. P.; Nat. Prod. Rep. 2016, 33, 432. [Crossref] 29. Crotti, A. E. M.; Lopes, J. L. C.; Lopes, N. P.; J. Mass Spectrom. 2005, 40, 1030. [Crossref] 30. Lopes, N. P.; Stark, C. B. W.; Gates, P. J.; Staunton, J.; Analyst 2002, 127, 503. [Crossref] 31. Sikorskaya, T. V.; Mar. Drugs 2023, 21, 335. [Crossref] 32. Khalturin, K.; Billas, I. M. L.; Chebaro, Y.; Reitzel, A. M.; Tarrant, A. M.; Laudet, V.; Markov, G. V.; J. Steroid Biochem. Mol. Biol. 2018, 184, 11. [Crossref] 33. Cachet, N.; Genta-Jouve, G.; Ivanisevic, J.; Chevaldonné, P.; Sinniger, F.; Culioli, G.; Pérez, T.; Thomas, O. P.; Sci. Rep. 2015, 5, 8282. [Crossref]
# The authors made an equal contribution to this work. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access