Artigo
| In silico and in vitro activity of friedelin N-derivatives against promastigote forms of Leishmania amazonensis |
|
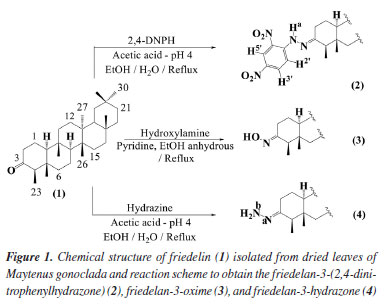
Mauro Lúcio Gonçalves de OliveiraI; Lucienir Pains DuarteI; Grácia Divina de FátimaI; Silvia Ribeiro de SouzaII; I. Departamento de Química, Instituto de Ciências Exatas (ICEx), Universidade Federal de Minas Gerais, 31270-901 Belo Horizonte - MG, Brasil Received: 04/03/2025 *e-mail: jlopes.ufmg@gmail.com Leishmaniasis is one of the six most important protozoal diseases worldwide. The potential antiprotozoal effect of pentacyclic triterpenes (TTPs), including friedelin (1), has been reported. Our goal is to create in silico models that can predict the effectiveness of TTPs against Leishmania species. We developed the Active-IT software to build these models using support-vector machine (SVM) and Naïve Bayes machine learning tools, high-quality datasets, and 3D compound structures. We synthesize N-derivatives (2-4) from friedelin to validate our theoretical results and evaluate their activity against the promastigote form of Leishmania amazonensis using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. Two compounds showed appreciable inhibitory effects on L. amazonensis. We found that friedelan-3-(2,4-dinitrophenylhydrazone) (2) had the most significant inhibitory activity and the lowest half-maximal inhibitory concentration (IC50) value, supporting our developed theoretical models. Our findings showed that theoretical models created from the 3D structures of active and inactive ligands (ligand-based drug design) can provide invaluable insights into complex biological processes. INTRODUCTION Leishmaniasis is considered one of the most severely neglected tropical diseases (NTDs) and is found in all continents except Oceania.1,2 It is a broad spectrum of diseases caused by species of Leishmania, a protozoan parasite belonging to the family Trypanosomatidae.3 Leishmaniasis is a severe and complex international health problem. The current drugs, such as pentavalent antimonials, are decades old. They can be problematic due to their toxic effects on the liver and heart, as well as other severe adverse reactions. The limitations include low efficacy, high cost, prolonged treatment regimens, and emerging drug resistance.4 Therefore, the limited availability of antiparasitic medicines on the market and the low interest of pharmaceutical industries in developing new products, as well as the increased drug resistance,5,6 indicate the urgent need to discover new substances that can advance towards the clinic. Different extracts and isolated phytochemicals have shown inhibitory effects against the amastigote and promastigote forms of Leishmania species.7,8 Among the metabolites found in plants, pentacyclic triterpenes have demonstrated inhibitory activity against different species of Leishmania sp.8,9 Friedelin has been studied by diverse research groups using different species or strains of Leishmania. In a study by Torres-Santos et al.,9 friedelin showed low activity (half-maximal inhibitory concentration (IC50) 91 µg mL-1) against promastigote forms of L. amazonensis (strain LV 79, MPRO/BR/72/M 1841). Also, Meneguetti et al.10 showed the activity of friedelin against promastigote forms of L. amazonensis (IFLA/BR/67/PH8), the compound showed 45.3 ± 4% inhibition (IC50 100 µg mL-1). Nour et al.11 evaluated the activity of friedelin against L. infantum amastigotes (MHOM/MA(BE)/67), for which the IC50 > 64.0 µg mL-1. Camacho et al.12 did not detect significant leishmanicidal activity when L. donovani promastigotes were exposed to friedelin. Alcazar et al.13 have explored the effects of various betulin derivatives on L. braziliensis promastigotes. The betulonic acid and one of the oxime derivatives were among the most active. The study of phytochemicals, including pentacyclic triterpenes, opens the way for developing sustainable medicines. In general, research on the potential antiprotozoal effect of phytochemicals, including pentacyclic triterpenes, contributes to identifying candidates for new drugs and their mode of action. Evaluating new potential drugs through in vitro and in vivo biological tests requires substantial financial support and time to achieve satisfactory results. In this context, in silico evaluation tools are suitable for discovering and developing new anti-parasite medicines, reducing expenses, and accelerating the process. Virtual screening, an important technique in drug discovery, plays a crucial role in designing or selecting compounds with potential activity. Active-IT is an in-house advanced drug discovery and development approach under ligand-based virtual screening.14,15 It consists of four key modules: molecular descriptor generation, machine learning modeling and validation, a database of predictive models, and a prediction module for new molecular entities. This prediction system has proven to be a valuable tool for drug discovery and development over the last few years.14,16-18 The present study used the Active-IT system to predict and evaluate the effects of N-derivatives of pentacyclic triterpene friedelin against the Leishmania amastigote and promastigote forms. To validate the predictions, we synthesize three N-derivatives of friedelin (1), namely friedelan-3-(2,4-dinitrophenylhydrazone) (2), friedelan-3-oxime (3), and friedelan-3-hydrazone (4) (Figure 1) through semi-synthesis reactions and evaluate their activity against the promastigote forms of Leishmania amazonensis.
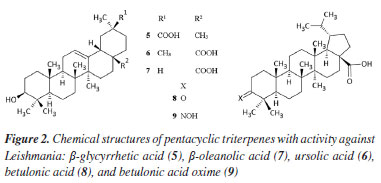
RESULTS AND DISCUSSION Pentacyclic triterpenes (TTPs) are natural compounds that show various biological activities, including antiprotozoal, anticancer, anti-inflammatory, and hepatoprotective effects.19,20 However, TTPs have some limitations that limit their therapeutic application in clinical use.21 Several triterpenoid derivatives with many skeletal modifications have been developed to improve the solubility and other pharmacokinetics (absorption, distribution, metabolism, excretion, and toxicity - ADMET) properties. One of these modifications involves forming analogues or derivatives of TTPs with the nitrogen group.22 Most of the structural modifications of friedelin occur in the C3 (A-ring) due to the 3-keto group, the only chemically active functional group in the molecule. Thus, using the Active-IT system, N-derivatives of friedelin were planned and evaluated in silico against the Leishmania amastigote and promastigote forms. Subsequently, the compounds were synthesized and tested in vitro to validate our approach further. In silico prediction of antileishmanial activities The Active-IT system utilizes information from the PubChem databases, specifically the BioAssay and Compound databases.23 The PubChem BioAssay covers a wide range of biological entities and tests, while the 3D structure of compounds is obtained from the PubChem Compound database.24 The 3D-Pharma module25 converts molecular structures into 3D pharmacophore fingerprints. The selected compounds were submitted to the Leishmania models we built using the ExCVBA module of Active-IT.26 For this work, we downloaded all 3D structures (up to ten conformations) of friedelin and its N-derivatives from the PubChem Compound database. For comparison purposes, the structures of oleanolic acid (5), β-glycyrrhetic acid (6), ursolic acid (7), betulonic acid (8), and betulonic acid oxime (9) were also downloaded (Figure 2). These pentacyclic triterpenes have already proven activity against Leishmania.13,27-30
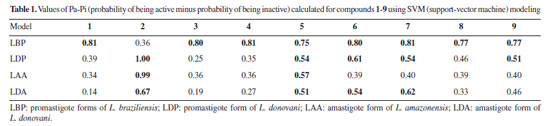
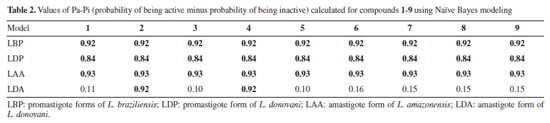
In summary, nine compounds were analyzed, four of the friedelin framework and five other TTPs. A total of 10 datasets were modeled, three for amastigote forms of L. amazonensis, L. donovani, and L. infantum. The other five datasets were associated with promastigote forms of L. brasiliensis, L. donovani, L. infantum, L. major, and L. mexicana. The last two datasets were associated with the enzymes pteridine reductase 1 of L. major and pyruvate kinase of L. mexicana. Tables 1 and 2 present the potential antileishmanial activity estimates from Pa-Pi (probability of being active minus probability of being inactive) values of the simulations for compounds 1-9 using support-vector machine (SVM) and Naïve Bayes modeling. According to Sudan et al.,17 more promising activities appear when Pa-Pi values exceed 0.5 and 0.8 for SVM and Naïve Bayes models, respectively. The values that exceed these limits are placed in bold type.
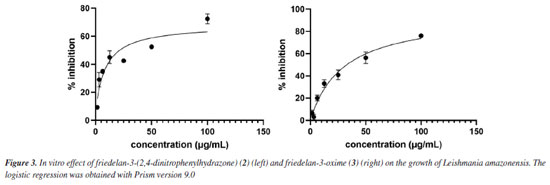
In the SVM modeling (Table 1), friedelin (1) and its oxime (3) and hydrazone (4) derivatives showed potential activity only for the L. braziliensis promastigote model, for which the hydrazone derivative 2 does not. The compound friedelan-3-(2,4-dinitrophenylhydrazone) (2) shows potential to be active for models of L. donovani promastigote, L. amazonensis amastigote, and L. donovani amastigote. β-Glycyrrhetic (5), ursolic (6), and oleanoic (7) acids showed potential activity against models of L. braziliensis promastigote, L. donovani promastigote, and L. donovani amastigote. However, only β-glycyrrhetic acid (5), like compound 2, showed potential activity against the L. amazonensis amastigote model. Finally, the betulonic acid (8) and its oxime derivative (9) showed potential activity against the L. braziliensis promastigote model, while oxime (9) was also potentially active in the L. donovani promastigote model. Naïve Bayes modeling (Table 2) shows little difference between compounds 1-9. All of them can potentially be active in models of L. braziliensis promastigote, L. donovani promastigote, and L. amazonensis amastigote. Only 2,4-dinitrophenylhydrazone (2) and hydrazone (4) derivatives showed potential activity for the L. donovani amastigote model. Therefore, based on our theoretical results on both SVM and Naïve Bayes modeling, we considered compound 2 (hydrazone derivative) the most potentially active, followed by β-glycyrrhizic acid (5). These compounds showed potential activity against seven Leishmania models. The oleanolic acid (7) and ursolic acid (6) showed potential activity against six models, considering both modeling methods. The following compounds are friedelin hydrazone derivative (4) and N-oxime derivative of betulonic acid (8) with five models, and friedelin (1), friedelin N-oxime (3), and betulonic acid (8) with four models. Considering all results, the order of potential activity for the evaluated pentacyclic triterpenes can be summarized as follows: 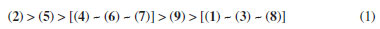 Six other models were built beyond the above four (Tables 3S to 7S in Supplementary Material). No compound showed potential activity against the models associated with the amastigote form of L. infantum (LIA), L. major pteridine reductase (LMjPR), and L. mexicana pyruvate kinase (LMxPK). There is just one occurrence of promising activity for promastigote forms of L. major, L. mexicana, and L. infantum. It is important to note that the order of potential activity aligns with known activities and the measured anti-leishmania activity in this work. However, one exception to this trend is the higher potential activity of hydrazone derivative (4), which was not confirmed in the present work. This could be due to the high reactivity of this functional group, and its decomposition during the biological tests cannot be discarded. The data found in the literature about the activity of some of these compounds against the promastigote forms of Leishmania were compared with our calculations (see Table 8S, Supplementary Material). From experimental data, we can state that the most active compounds are betulonic acid (8) (IC50 8.5 μM, L. brasiliensis promastigote),13 and β-glycyrrhetic acid (5) (IC50 9.77 μM, L. donovani),27 followed by ursolic acid (6) and oleanolic acid (7) (against L. amazonensis promastigote).9 These results are close to our predictions except for betulonic acid (8). However, we must consider that our predictions are qualitative, and compound 8 was predicted as potentially active in several Leishmania models. Following the theoretical prediction, the proposed compounds 2-4 were synthesized by adding a nitrogen compound to the carbonyl group of 1. The compounds 2 and 3 were produced with good yields. However, compound 4 was obtained with a lower yield, which can be explained by the fact that the hydrazone obtained is reactive and prone to degradation. Details about the obtention and identification of friedelin N-derivatives (2-4) are presented in the Supplementary Material (Figures 1S-20S). In vitro inhibition of L. amazonensis According to Corman et al.,4 much research has been focused on L. amazonensis, as this species is predominant in the New World and has the potential for chronic disease. For the first time, N-derivatives of friedelin (2-4) were evaluated in vitro against extracellular promastigote forms of L. amazonensis. The compounds 2 and 3 inhibited the growth of L. amazonensis, showing a % inhibition of 69.0 and 77.3 at 100 µg mL-1, respectively. They showed IC50 8.9 µg mL-1 (14.7 μM) and 30.0 µg mL-1 (65.8 μM), respectively (Figure 3). However, compound 4 showed no activity in the assay's conditions.
CONCLUSIONS This study aimed to discover new compounds with antileishmanial activity by conducting in silico studies. Three N-derivatives of pentacyclic triterpene friedelin were planned, and the compounds friedelan-3-(2,4-dinitrophenylhydrazone) (2), friedelan-3-oxime (3), and friedelan-3-hydrazone (4) were synthesized and tested in vitro against promastigote forms of Leishmania amazonensis. The planned compounds 2 and 3 showed higher activity than the base compound, friedelin (1). Compound 2 had more potent inhibitory activity, corroborating the in silico prediction results of the Active-IT system. Using in silico evaluation techniques in this research allows us to identify substances with a higher chance of success. It enables the selection of ligands with greater efficacy in treating these diseases, potentially reducing the attrition rate in the drug discovery process.
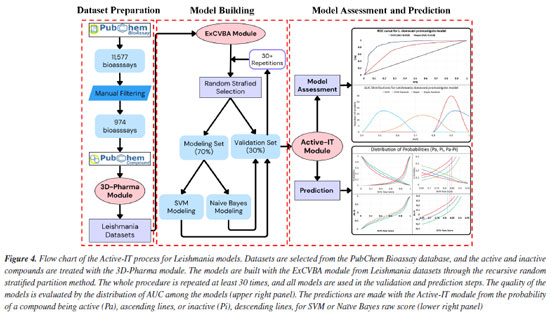
EXPERIMENTAL Plant material Leaves of Maytenus gonoclada (Celastraceae) were collected in "Serra da Piedade", Minas Gerais, Brazil. A voucher specimen (No. HBCB 60280) was preserved in the Herbarium of Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Brazil. The botanical material accessed was registered at Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado (SisGen), of Ministério do Meio Ambiente, Brazil, under number AFBF625. Obtention and identification of friedelin and its N-derivatives The friedelin (1) was isolated from leaves of Maytenus gonoclada following the procedure described by Silva et al.31 To obtain the N-derivative 2, friedelin (100.0 mg), 2,4-dinitrophenylhydrazine, and drops of acetic acid p.a. were added to a 50 mL round-bottom flask containing an ethanol/water mixture until pH 4. The reaction mixture was kept at reflux until the respective nitrogenous derivative was obtained. In this case, the reaction medium was adjusted to approximately 136 ºC to produce friedelan-3-dinitrophenylhydrazone (2) in syn conformation (synperiplanar) and not as a syn-anti mixture (2:1). The reaction was monitored by silica gel thin layer chromatography (TLC). After cooling, an aqueous NaHSO4 5% w/v solution was added until the product precipitated. The solid material was filtered and washed with distilled water, according to Rangaswami and Sambamurthy32 (yield 83%). For the synthesis of derivative 3, a mixture containing friedelin (100.0 mg), anhydrous ethanol, hydroxylamine, and pyridine was kept under reflux and monitored by TLC. After cooling, distilled water was added until the product precipitated. The solid material was filtered and washed with distilled water33 (yield 93%). To obtain derivative 4, a mixture containing friedelin (100.0 mg), hydrazine, and drops of acetic acid p.a. was added to a 50 mL round-bottom flask containing an ethanol/water mixture until pH 4. The reaction mixture was kept at reflux until the formation of the respective nitrogenous derivative was verified by TLC33 (yield 55%). The reaction conditions and yields of N-derivatives 2-4 obtained from friedelin (1) isolated from leaves of Maytenus gonoclada are shown in Table 1S (Supplementary Material). The chemical characterization of nitrogenated compounds 2, 3, and 4, obtained from friedelin (1) (Figure 1), was carried out through analyses of infrared (IR) and hydrogen and carbon nuclear magnetic resonance (1H and 13C, DEPT-135 and 2D NMR) spectral data (available at Supplementary Material, Figures 1S to 20S, and Table 2S). As a purity criterion of N-derivatives 2, 3, and 4, obtained from friedelin, was considered the visualization of a single spot in TLC carried out with eluents of different polarities and similarity of IR and 1H and 13C NMR results with already published data. In silico prediction of antileishmanial activity Selection of datasets related to Leishmania species and their biological targets We searched the PubChem Bioassay website for "Leishmania". After manual filtering, the remaining bioassay was classified and grouped according to Leishmania species, infecting form (amastigotes or promastigotes), and specific biological targets (Figure 4, Dataset Preparation). After filtering, 954 bioassays were analyzed and grouped into 10 datasets (Table 3S, Supplementary Material). The filtering process and composition of the Leishmania datasets are detailed in the Supplementary Material. Table 4S (Supplementary Material) includes the values of the area under the ROC (receiver operating characteristic) curve obtained in the validation process for each model built from those datasets. Most models have achieved a very good performance, as measured by the area under the ROC curve (AUC). Considering both methods, 70% of models had an AUC above 0.9, 15% between 0.8 and 0.9, and 15% between 0.7 and 0.8 (Table 4S).
Model building and prediction The NEQUIM Active-IT system was used to build and validate predictive models for Leishmania species and to predict the anti-Leishmania activity of pentacyclic triterpenes. The full details of the methodology are available elsewhere;14-17 thus, only a summary will be provided here. The molecular 3D structures of substances were converted into 3D pharmacophore fingerprints using the 3D-Pharma module.25 It categorizes atoms based on their interaction potential and calculates all sets of pharmacophore triplets and their distances to create a comprehensive vector of potential pharmacophores. The fingerprints of all conformations are combined to form a unique modal vector34 for each compound, incorporating dynamic conformational changes (4D pharmacophore fingerprints). The model corresponding to each dataset was constructed using the ExCVBA module26 and two supervised learning techniques: support vector machine using LibSVM35 and Naive Bayes.36 According to the Active-IT protocol, the models were developed using a recursive random stratified partition method to ensure reliability and robustness (Figure 4, Model Building). Active and inactive compounds are divided into subsets for model building (70%) and validation (30%), and the process is repeated at least 30 times. No model is discharged, and all of them will be used in the prediction phase. The average scores of each compound over the models where it appears in the validation set were used to assess the modeling performance with the area under the ROC curve (AUC), as well as for activity prediction of new compounds. The activity prediction of a new compound starts by building its modal fingerprint and submitting it to various models for each biological activity to calculate its raw score. The average scores were converted into probabilities through comparison with active and inactive compound score distributions, producing a measure of belonging to these two subsets.14,15,37 The Pa value is derived from the fraction of active compounds with a raw score smaller than the new compound. In contrast, the Pi value is calculated from the fraction of inactive compounds with better raw score (Figure 4, lower right panel). The difference between these probabilities, the Pa-Pi value, is fundamental in determining the activity potential of the compound. Previous studies17 have shown specific cutoff values for SVM (Pa-Pi ≥ 0.5) and Naive Bayes (Pa-Pi ≥ 0.8) models that indicate the most promising activities and targets for the compound. In vitro inhibition of promastigote forms of Leishmania amazonensis Sterile Schneider culture medium (Sigma-Aldrich®) (100 µL) was added to a 96-well tissue culture plate. Then, compounds 2 and 3 were added in serial dilutions. A suspension of 105 promastigote forms of L. amazonensis, previously isolated from infected tissue and counted in a Neubauer chamber, was added to each well. The final volume of each well was adjusted to 200 µL. The final concentration of the samples in the first dilution was 100.0 µg mL-1. The plate was incubated for 48 h at 22-26 ºC. After this period, in each well was added 100.0 μg of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution in PBS (phosphate-buffered saline (Sigma-Aldrich®)) at 5.0 mg mL-1, for 4 h at 37 ºC and protected from light. Then, 100.0 µL of DMSO (dimethyl sulfoxide, Merck) was added to dissolve the formazan crystals. In this experiment, L. amazonensis promastigotes were used in the log phase to improve the absorbance reading, measured in a Shimadzu UV/VIS spectrophotometer Model: UV 1800, at 570 nm, to quantify the metabolization rate of the colorimetric agent. L. amazonensis treated with amphotericin B (Sigma-Aldrich®) was used as a positive control, and L. amazonensis without any treatment was used as a negative control. The experiments were carried out in duplicate. After a five-day incubation period, the results were expressed as the concentrations inhibiting parasite growth by 50% (IC50), calculated using logistic regression with the GraphPad Prism version 9.0.0 (GraphPad Software, La Jolla, CA, USA).
SUPPLEMENTARY MATERIAL Supplementary material for this work (part A: obtention and identification of friedelin N-derivatives: 1H and 13C, DEPT-135, and 2D NMR spectra. Figures 1S to 20S and Tables 1S and 2S; part B: modeling antileishmanial activity. This section provides details on the selection and composition of the Leishmania datasets (Tables 3S-5S). Modeling results in SVM and Naïve Bayes are presented in Tables 6S and 7S., respectively. Table 8S presents the experimental inhibitory concentrations at 50% (IC50) for some pentacyclic triterpenes against promastigote forms of Leishmania species, compared with the literature data) is available at http://quimicanova.sbq.org.br/, as a PDF file, with free access.
DATA AVAILABILITY STATEMENT The authors confirm that the data supporting the findings of this study are available in the article and its supplementary materials. Contact the corresponding author for any additional information.
ACKNOWLEDGMENT The authors thank Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
AUTHOR CONTRIBUTIONS Mauro Lúcio Gonçalves de Oliveira was responsible for the investigation; writing original draft; Lucienir Pains Duarte for the conceptualization; formal analysis; data curation; writing original draft; Grácia Divina de Fátima for the conceptualization; supervision; funding acquisition; writing original draft; Silvia Ribeiro de Souza for the investigation; formal analysis; writing original draft; Misléia Rodrigues de Aguiar Gomes for the investigation; writing original draft; Sidney Augusto Vieira-Filho for the data curation; formal analysis; writing original draft; writing review and editing; Vera Lucia de Almeida for the data curation; writing review and editing; Júlio César Dias Lopes for the conceptualization; methodology; data curation; formal analysis; software; validation; writing original draft; writing review and editing.
REFERENCES 1. Garza-Tovar, T. F.; Sacriste-Hernández, M. I.; Juárez-Durán, E. R.; Arenas, R.; Fac. Rev. 2020, 9, 28. [Crossref] 2. Wamai, R. G.; Kahn, J.; McGloin, J.; Ziaggi, G.; Journal of Global Health Science 2020, 2, e3. [Crossref] 3. Teles, C. B. G.; Moreira, L. S.; Silva, A. A. E.; Facundo, V. A.; Zuliani, J. P.; Stábeli, R. G.; Silva-Jardim, I.; J. Braz. Chem. Soc. 2011, 22, 936. [Crossref] 4. Corman, H. N.; McNamara, C. W.; Bakowski, M. A.; Microorganisms 2023, 11, 2845. [Crossref] 5. Capela, R.; Moreira, R.; Lopes, F.; Int. J. Mol. Sci. 2019, 20, 5748. [Crossref] 6. World Health Organization (WHO), Neglected Tropical Diseases, https://www.who.int/data/gho/data/themes/neglected-tropical-diseases, accessed in July 2025. 7. Rocha, L. G.; Almeida, J. R. G. S.; Macêdo, R. O.; Barbosa-Filho, J. M.; Phytomedicine 2005, 12, 514. [Crossref] 8. Hassan, A. A.; Khalid, H. E.; Abdalla, A. H.; Mukhtar, M. M.; Osman, W. J.; Efferth, T.; Molecules 2022, 27, 7579. [Crossref] 9. Torres-Santos, E. C.; Lopes, D.; Oliveira, R. R.; Carauta, J. P. P.; Falcao, C. A. B.; Kaplan, M. A. C.; Rossi-Bergmann, B.; Phytomedicine 2004, 11, 114. [Crossref] 10. Meneguetti, D. U. O.; Lima, R. A.; Hurtado, F. B.; Passarini, G. M.; Macedo, S. R. A.; de Barros, N. B.; Oliveira, F. A. S.; de Medeiros, P. S. M.; Militão, J. S. L. T.; Nicolete, R.; Facundo, V. A.; Rev. Soc. Bras. Med. Trop. 2016, 49, 579. [Crossref] 11. Nour, S.; Salama, M.; Mahrous, E.; El-Askary, H.; Hifnawy, M.; Kawy, M. A. A.; Jordan J. Pharm. Sci. 2023, 16, 18. [Crossref] 12. Camacho, M. R.; Mata, R.; Castaneda, P.; Kirby, G. C.; Warhurst, D. C.; Croft, S. L.; Phillipson, J. D.; Planta Med. 2000, 66, 463. [Crossref] 13. Alcazar, W.; López, A. S.; Alakurtti, S.; Tuononen, M.-L.; Yli-Kauhaluoma, J.; Ponte-Sucre, A.; Bioorg. Med. Chem. 2014, 22, 6220. [Crossref] 14. Rocha, M. P.; Campana, P. R. V.; Scoaris, D. O.; de Almeida, V. L.; Lopes, J. C. D.; Shaw, J. M. H.; Silva, C. G.; Molecules 2018, 23, 3303. [Crossref] 15. Almeida, V. L.; dos Santos, O. D. H.; Lopes, J. C. D.; Biomed. Khim. 2024, 70, 435. [Crossref] 16. Rocha, M. P.; Campana, P. R. V.; Scoaris, D. O.; de Almeida, V. L.; Lopes, J. C. D.; Silva, A. F.; Pieters, L.; Silva, C. G.; Phytother. Res. 2018, 32, 2021. [Crossref] 17. Sudan, C. R. C.; Pereira, L. C.; Silva, A. F.; Moreira, C. P. S.; de Oliveira, D. S.; Faria, G.; dos Santos, J. S. C.; Leclercq, S. Y.; Caldas, S.; Silva, C. G.; Lopes, J. C. D.; de Almeida, V. L.; Planta Med. 2021, 87, 1045. [Crossref] 18. da Silva, R. G.; Almeida, T. C.; Reis, A. C. C.; Vieira Filho, S. A.; Brandão, G. C.; da Silva, G. N.; de Sousa, H. C.; de Almeida, V. L.; Lopes, J. C. D.; de Souza, G. H. B.; Nat. Prod. Res. 2021, 35, 5918. [Crossref] 19. Ghante, M. H.; Jamkhande, P. G.; Journal of Pharmacopuncture 2019, 22, 55. [Crossref] 20. de Jesus, J. A.; Laurenti, M. D.; Antonangelo, L.; Faria, C. S.; Lago, J. H. G.; Passero, L. F. D.; J. Immunol. Res. 2021, 2021, 6671287. [Crossref] 21. Khwaza, V.; Mlala, S.; Oyedeji, O. O.; Aderibigbe, B. A.; Molecules 2021, 26, 2401. [Crossref] 22. Nistor, G.; Trandafirescu, C.; Prodea, A.; Milan, A.; Cristea, A.; Ghiulai, R.; Racoviceanu, R.; Mioc, A.; Mioc, M.; Ivan, V.; Șoica, C.; Molecules 2022, 27, 6552. [Crossref] 23. Kim, S.; Bolton, E. E. In Open Access Databases and Datasets for Drug Discovery; Daina, A.; Przewosny, M.; Zoete, V., eds.; Wiley‐VCH GmbH, 2024, ch. 2. [Crossref] 24. Bolton, E. E.; Chen, J.; Kim, S.; Han, L.; He, S.; Shi, W.; Simonyan, V.; Sun, Y.; Thiessen, P. A.; Wang, J.; Yu, B.; Zhang, J.; Bryant, S. H.; J. Cheminf. 2011, 3, 32. [Crossref] 25. Domingues, B. F.; Martins-José, A.; Lopes, J. C. D.; ChemRxiv, 2024. [Crossref] 26. dos Santos, F. M.; De Winter, H.; Augustyns, K.; Lopes, J. C. D.; Annals of OPENTOX EURO 2015 - OpenTox InterAction Meeting - Innovation in Predictive Toxicology; Dublin, Ireland, 2015. [Crossref] 27. Ukil, A.; Biswas, A.; Das, T.; Das, P. K.; J. Immunol. 2005, 175, 1161. [Crossref] 28. Gupta, P.; Das, P. K.; Ukil, A.; Antimicrob. Agents Chemother. 2015, 59, 2531. [Crossref] 29. Jesus, J. A.; Fragoso, T. N.; Yamamoto, E. S.; Laurenti, M. D.; Silva, M. S.; Ferreira, A. F.; Lago, J. H. G.; Gomes, G. S.; Passero, L. F. D.; Int. J. Parasitol.: Drugs Drug Resist. 2017, 7, 1. [Crossref] 30. Raimundo, V. D.; Carvalho, R. P. R.; Machado-Neves, M.; Marques-da-Silva, E. A.; Pharmacol. Res. 2022, 177, 106117. [Crossref] 31. Silva, F. C.; de Oliveira, M. L. G.; Rodrigues, V. G.; Carvalho, S. M.; Duarte, L. P.; Silva, G. D. F.; Miranda, R. R. S.; Figueiredo, R. C.; Morais, J. C.; Filho, S. A. V.; Chem. Nat. Compd. 2013, 49, 571. [Crossref] 32. Rangaswami, S.; Sambamurthy, K.; Proc. Natl. Acad. Sci., India, Sect. A 1961, 54, 99. [Link] accessed in July 2025 33. Sarkar, A.: Synthesis, Characterization and Biocidal Activities of the Derivatives of Steroids and Pentacyclic Triterpenoids; PhD Thesis, University of North Bengal, 2015. [Link] accessed in July 2025 34. Shemetulskis, N. E.; Weininger, D.; Blankley, C. J.; Yang, J. J.; Humblet, C.; J. Chem. Inf. Comput. Sci. 1996, 36, 862. [Crossref] 35. Chang, C.-C.; Lin, C.-J.; ACM Transactions on Intelligent Systems and Technology 2011, 2, 27. [Crossref] 36. Williams, K., Algorithm::NaiveBayes - Bayesian Prediction of Categories, https://metacpan.org/pod/Algorithm::NaiveBayes, accessed in July 2025. 37. Filimonov, D. A.; Lagunin, A. A.; Gloriozova, T. A.; Rudik, A. V.; Druzhilovskii, D. S.; Pogodin, P. V.; Poroikov, V. V.; Chem. Heterocycl. Compd. 2014, 50, 444. [Crossref]
Guest Editor handled this article: Antonio E. M. Crotti |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access