Artigo
| Chemical and biological investigation of Cattleya nobilior Rchb. F. (Orchidaceae): targeting on phenanthrene and stilbenoid derivatives |
|
Caroline de Moura CostaI,#; Katyuce Souza FariasI,#; Izadora BonfimI,# I. Laboratório de Produtos Naturais e Espectrometria de Massas (LaPNEM), Universidade Federal de Mato Grosso do Sul (UFMS), 79070-900 Campo Grande - MS, Brasil Received: 03/01/2025 *e-mail: denise.brentan@ufms.br Orchidaceae family is a valuable source of bioactive compounds, particularly phenanthrenes and stilbenoids, which exhibit promising pharmacological properties. Cattleya nobilior Rchb. F. remains chemically and biologically unexplored, yet its potential bioactivity warrants investigation. This study aimed to identify phenanthrenes and stilbenoids from C. nobilior and evaluate their anti-Trypanosoma cruzi and cytotoxic activities. Extracts from leaves, pseudobulbs, roots, and rhizomes were obtained using sequential solvent extraction by accelerated solvent extraction. Their biological effects were assessed against T. cruzi epimastigotes and cancer cell lines (Kasumi-1, CCRF-CEM, HCT-116, and MCF-7). Metabolomic profiling (liquid chromatography-diode array detection-mass spectrometry (LC-DAD-MS)) and multivariate analyses correlated chemical composition with bioactivity. Rhizome extracts (CnRz-DM5, CnRz-DM50) inhibited HCT-116 cells (> 80%), while CnBb-DM5, CnRz-DM5, and CnRo-DM5 inhibited > 90% of leukemic cells. CnRo-DM5 also exhibited moderate anti-T. cruzi activity (half-maximal inhibitory concentration (IC50) = 33.3 µg mL-1). Metabolomic analysis suggested 67 phenanthrenes and stilbenoids associated with cytotoxicity. Guided by these findings, the most active extract was fractionated, leading to the isolation of gigantol and batatasin III. This study provides the first chemical and biological insights into C. nobilior, reinforcing its potential as a source of bioactive phenanthrenes and stilbenoids with anticancer and antiparasitic properties. INTRODUCTION Orchidaceae is one of the largest plant families, comprising approximately 30,000 species distributed in 700 genera, which are distributed worldwide, except in Antarctica and arid regions.1 The species from this family have been widely used in traditional medicine, especially for the treatment of infectious diseases and cancer,2,3 however, only a limited number of species have been studied in the literature.2 Phenanthrenes and stilbenoid derivatives are reported from several species of Orchidaceae, which are uncommon metabolites and restricted to the particular plant families. These compounds have great innateness about their chemical structures, and they have demonstrated biological potential, mainly for malaria4,5 and cancer.6 The chloroform fraction from orchid Maxillaria picta, for example, showed promising activity against ten cancer cell lines (glioma U251, melanoma UACC-62, breast MCF-7, ovary NCI-ADR/RES, kidney 786-0, lung NCI-H460, prostate PC-3, ovary OVCAR-3, colorectal HT29, leukemia K562), revealing half-maximal inhibitory concentration (IC50) between 3.5 and 26.5 µg mL-1. Additionally, a mixture of the bioactive stilbenes foiumbene B and C isolated from this plant also revealed an IC50 of 3.9 µg mL-1 against ovary NCI-ADR/RES cancer cell line.7 Promising anti-Trypanosoma cruzi results are also highlighted for phenanthrenes and stilbenoids, such as the results observed from extracts and fractions from the orchid Laelia marginata that exhibited in their composition phenanthrenes and stilbenoids.8 Cattleya genus is rich in species with high commercial value due to exuberant flowers. It is composed of 114 species with occurrence in South America9 and was scarcely studied. From Cattleya tigrina, stilbenoids, phenolic acids, steroids, and phenanthrenoids have been identified, including a phenanthrenequinone with cytotoxic activity against the HeLa cancer cell line.10 Similarly, phenanthrenes and stilbenoids were isolated from Cattleya intermedia, highlighting this genus as a promising source for the discovery of these bioactive compounds.11 Cattleya nobilior Rchb. F. (Orchidaceae) is a native Brazilian species known for its striking white to pink flowers. It is found in the states of Goiás, Mato Grosso, Mato Grosso do Sul, Pará, Rondônia, Tocantins, Maranhão, and the Distrito Federal.12 To date, no studies have been published on the chemical composition or biological properties of this plant. Therefore, this study aims to analyze the chemical composition of different parts of C. nobilior, with a particular focus on stilbenoids and phenanthrenes, and to evaluate its anti-Trypanosoma cruzi potential and cytotoxicity against cancer cell lines.
EXPERIMENTAL Collection and identification of plant material Cattleya nobilior was collected in the municipality of Dois Irmãos do Buriti, MS, at coordinates 20º41'27.2"S and 55º17'16.1"W, on August 10, 2017. The plant material was identified by Prof. Dr. Flávio Macedo Alves, and a voucher specimen was deposited in the herbarium of the Universidade Federal de Mato Grosso do Sul under the registration number CGMS 75469. This study was officially registered in the National Genetic Heritage Management System (SisGen) under the number A1FB919. Preparation of plant material and extraction The collected plant materials were separated (leaves, roots, pseudobulb, and rhizome (Table 1S, Supplementary Material)), dried in a circulating air oven at 40 ºC for 48 h, and powdered by a knife mill. Extracts were obtained using an accelerated solvent extractor (ASE 150 DionexTM®) with the addition of silica gel (0.063-0.2 mm) at ratio 1:2 for the plant material. The solvents used in the successive extractions were the following: (i) hexane, (ii) dichloromethane and methanol 95:5, (iii) dichloromethane and methanol 1:1, and (iv) methanol. The parameters applied in the extractor were: extraction cycle of 4 min, wash volume of 80%, 1500 psi pressure, and nitrogen purge for 40 s. The extraction temperatures for hexane and other solvents were 70 and 100 ºC, respectively. The extracts were concentrated in a rotary evaporator and, subsequently, they were freeze-dried. The yields of extracts are summarized in Table 2S (Supplementary Material). Chemical analyses by LC-DAD-MS The extracts were analyzed by liquid chromatography coupled to diode array detector and mass spectrometry (LC-DAD-MS) using a Shimadzu UFLC chromatograph coupled to diode-array detector (DAD) and a mass spectrometer MicrOTOF-QIII (Bruker Daltonics, Billerica, USA) with electrospray ionization source (ESI) and analyzers quadrupole and time-of-flight. A chromatographic column Kinetex C18 (2.6 μm, 100 Å, 150 × 2.1 mm, Phenomenex) was used. The samples were prepared at 1 mg mL-1, filtered through syringe filters (PTFE Millex 0.22 mm × 13 mm filter, Millipore), and injected (1 μL) into the system via autoinjector. The mobile phase consisted of acetonitrile (B) and deionized water (A), both acidified with formic acid 0.1% (v/v). The elution gradient was 0-2 min, 3% B; 2-25 min, 3 to 25% B; 25-40 min, 25 to 80% B; and 40-43 min, 80% B. The chromatographic column was maintained at 50 ºC during the analyses. Nitrogen was applied as the nebulizer gas (4 bar), drying gas (9 L min-1), and collision gas. The DAD analyses were monitored between the wavelengths 220 and 800 nm, and the MS analyses were acquired in positive and negative ionization modes (m/z 120-1200), which was applied a capillary voltage of 2,500 kV. MS/MS spectra were acquired by an automatic method using a collision energy 45 to 65 eV, and nitrogen was applied as collision gas. The annotation of the metabolites was based on spectral data (fragmentation patterns, accurate mass, and UV spectra) compared to the literature data. Data processing and chemometric analyses The mass spectrometry analysis in positive mode was used for metabolomic analysis, since this ion mode revealed a higher number of ionized metabolites. The raw data were converted to .cdf by DataAnalysis 4.2 software (Bruker Daltonik GmcH, Germany, 2013) and they were aligned by Metalign (The Netherlands, 2009), resulting in 4,967 entries, the data was reduced by MSclust software for 164 entries, and considering peaks with intensities above 2,000, a total of 67 features were obtained. These processed data were for statistical analysis by Metaboanalyst 6.0 (The Netherlands, 2024). The values were log-transformed, and hierarchical clustering was organized using Euclidean distance, Ward clustering algorithm, and the metabolites selected by analysis of variance (ANOVA, p ≤ 0.05). Isolation and structural characterization of stilbenes The rhizome extract (CnRz_DM5) was submitted to column chromatography using Sephadex LH-20 and the elution solvent was methanol and ethyl acetate 1:1 (v/v). The fractions of 10 mL were collected and analyzed by thin-layer chromatography (silica gel, Macherey-Nagel®) applying 2-aminoethyl diphenylborinate (Natural Product reagent)/PEG and sulfuric acid 10% as chromogenic reagent, resulting 42 fractions. The fractions 22-27 were submitted to semi-preparative high performance liquid chromatography using a Shimadzu C18 Shim-pack ODS (H) column (25 cm × 20 mm i.d., 5 µm), the mobile phase consisted of deionized water (solvent A) and acetonitrile (solvent B), both containing 0.02% trifluoroacetic acid (TFA), and the flow used was 9 mL min-1. The following elution gradient was the following: 0-25 min, 25-40% B; 25-45 min, 40% B; 45-60 min, 40-70% B; 60-63 min, 70-100% B; 63-69 min, 100% B; 69-70 min, 100-25% B; and 70-75 min, 25% B. The fractions 17 and 19 (in about 6 mg) were analyzed by nuclear magnetic resonance (NMR) to confirm the structures of batatasin III (32) and gigantol (33). The NMR experiments were obtained in a deuterated chloroform solvent. Anti-Trypanosoma cruzi Epimastigote forms of T. cruzi Dm28c were maintained in LIT (liver infusion tryptose) medium, supplemented with 10% fetal bovine serum at 28 ºC. For the experiments, parasites of cultures in the exponential phase were applied. The evaluation of the extracts on the viability of epimastigote forms of the parasite was determined by the colorimetric assay with MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium). New cultures in 106 parasites mL-1 were added to wells and incubated for 72 h. The extracts were evaluated at six concentrations for the determination of IC50 and the assays were performed in triplicate. After incubation for 72 h, MTS/phenazine methosulfate (PMS) solution was added, and the microplates were then incubated at 28 ºC for 4 h. After this procedure, the optical density was read at 490 nm in a μQuant reader. The negative control was performed using 1% DMSO (dimethyl sulfoxide), and a replicate of each concentration, fixed with 4% paraformaldehyde prior to the addition of MTS/PMS (basal absorbance control). Cytotoxicity assays The cytotoxicity of orchid extracts was assessed using the Alamar Blue and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) assays. The tested cell lines included Kasumi-1 (acute myeloblastic leukemia), CCRF-CEM (human leukemic lymphoblasts), HCT-116 (colorectal carcinoma), and MCF-7 (breast adenocarcinoma). The Alamar Blue assay (AlamarBlue™, Biosource, Camarillo, CA, USA) was performed to evaluate the cytotoxic effects of the samples on leukemic cell lines. The orchid extracts were initially tested at concentrations of 30 and 100 µg mL-1. Samples were resuspended in DMSO at 10 mg mL-1, diluted in culture medium (final DMSO concentration of 0.4%), and added to 96-well microplates containing 1 × 105 cells mL-1 in a supplemented medium. After 24 h of incubation, 20 µL of resazurin solution (0.15 mg mL-1) was added, and the plates were briefly shaken. Following an additional 4-hour incubation at 37 ºC, absorbance was measured at 570 and 600 nm using a microplate reader (Thermo Scientific® Varioskan LUX). Each experiment was conducted in triplicate, and cell viability was analyzed using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA). For the colorectal and breast cancer cell lines (HCT-116 and MCF-7), cytotoxicity was evaluated using the MTT assay.13 Cells were seeded at a density of 1 × 104 cells per well in 96-well plates (5 × 104 cells mL-1 in 200 µL of culture medium) and incubated for 24 h. Samples were then added at final concentrations of 5 and 50 µg mL-1, each tested in triplicate. Doxorubicin served as a positive control, while DMSO was used as a negative control. After 72 h of incubation, the supernatant was replaced with culture medium containing MTT (0.5 mg mL-1), and the plates were incubated for an additional 3 h. The supernatant was then removed, and after drying, the resulting MTT formazan precipitate was dissolved in 150 µL of DMSO. Absorbance was measured at 570 nm. Statistical analysis All biological tests were analyzed using GraphPad Prism software version 7.04 (GraphPad Software, San Diego, CA, USA). The IC50 and CC50 (cytotoxic concentration 50%) values were obtained by nonlinear regression with variable slope and automatic withdrawal of outliers.
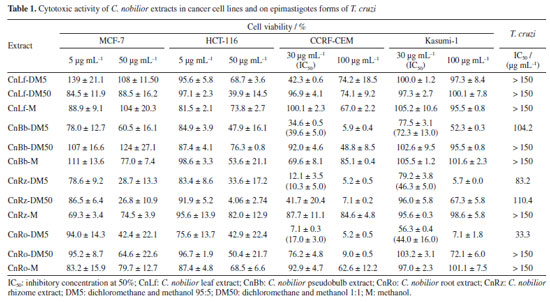
RESULTS AND DISCUSSION The extracts from the leaves, roots, rhizomes, and pseudobulbs of the orchid C. nobilior were obtained using an accelerated solvent extractor (ASE) with sequential extraction: dichloromethane and methanol 95:5 (DM5); dichloromethane and methanol 1:1 (DM50); methanol (M). ASE is an advanced extraction technique that uses controlled pressure and temperatures, and based on variations of parameters, such as the type of solvent, the result extract can be different,14 and the choice of this sequence of extraction was directed for the selective extraction of stilbenes and phenanthrenes. The yields of the extracts are summarized in Table 2S (Supplementary Material). The C. nobilior extracts were evaluated against colorectal carcinoma (HCT-116), breast adenocarcinoma (MCF-7), acute lymphoblastic leukemia (CCRF-CEM), and acute myeloblastic leukemia (Kasumi-1) cell lines, as well as epimastigote forms of T. cruzi. The biological results are presented in Table 1.
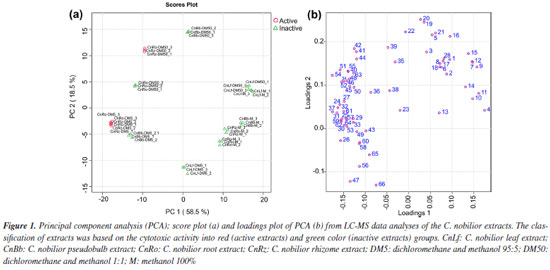
The extracts showed different percentages of inhibition for different cancer cell lines. The rhizome extracts CnRz-DM5 and CnRz-DM50, for example, exhibited a high inhibition rate against HCT-116, with over 80% cell inhibition, underscoring their promising cytotoxic potential. For the leukemia cell lines, a significant reduction in cell viability was observed with the CnBb-DM5, CnRz-DM5, and CnRo-DM5 extracts, derived from the pseudobulb, rhizome, and roots, respectively, showing inhibition greater than 90%. Notably, the extracts CnRz-DM5 and CnRo-DM5 exhibited IC50 values for the CCRF-CEM cell line of 10.3 and 17.0 µg mL-1, respectively, further emphasizing their potent cytotoxic activity. Regarding T. cruzi, the root extract CnRo-DM5 demonstrated the lowest IC50 value (33.3 μg mL-1), suggesting a moderate efficacy against the parasite. Overall, the CnRz-DM5, CnRz-DM50, and CnRo-DM5 extracts exhibited the highest activity against the evaluated cancer cell lines and were classified as active samples for statistical analysis based on LC-MS data. Consequently, the relationship between the chemical composition of the various C. nobilior extracts and their cytotoxic activity was investigated using multivariate statistical analyses. The principal component analysis (PCA) explained 77.3% of the variation data, representing 58.8 and 18.5% for PC1 and PC2, respectively (Figure 1). Extracts from the rhizome (CnRz), roots (CnRo), and pseudobulb (CnBb), particularly those obtained using dichloromethane and methanol mixtures (95:5 and 1:1) (DM5 and DM50), clustered closely together, indicating similarities in their chemical profiles. These extracts were predominantly associated with non-polar and moderately polar compounds. In contrast, extracts obtained with methanol (M), which are composed of more polar compounds, were positioned separately from those extracted with other solvents. However, despite originating from different plant parts, these methanol extracts exhibited greater similarity to each other, suggesting a comparable chemical composition regardless of the plant part used. However, they did not exhibit cytotoxic or anti-trypanosomal activity.
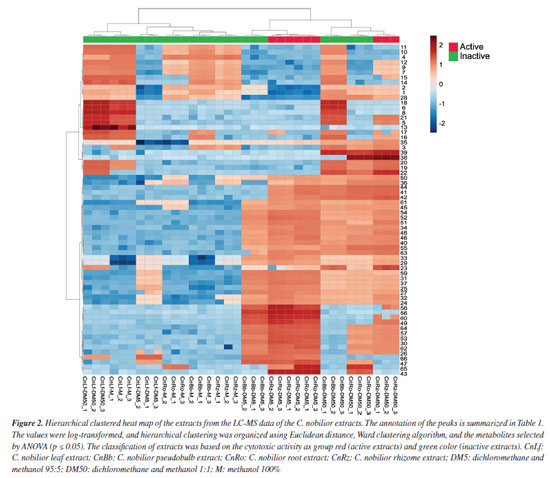
The hierarchically clustered heatmap (Figure 2) provides an overview of the chemical variation among C. nobilior extracts based on LC-MS data. Extracts from the rhizome (CnRz-DM5, CnRz-DM50), root (CnRo-DM5), and pseudobulb (CnBb-DM5) formed a distinct cluster, together with CnBb-DM50 and CnRo-DM50, suggesting the shared metabolites among these extracts, which could explain similarities in their chemical profiles and biological activities. These extracts, obtained with dichloromethane/methanol mixtures (95:5 and 1:1), were associated with non-polar and moderately polar compounds, which may contribute to their biological activity. For example, the root extract CnRo-DM50 exhibited 91% inhibition of the CCRF-CEM cancer cell line at 100 µg mL-1, but it showed a low cytotoxic activity at 30 µg mL-1.
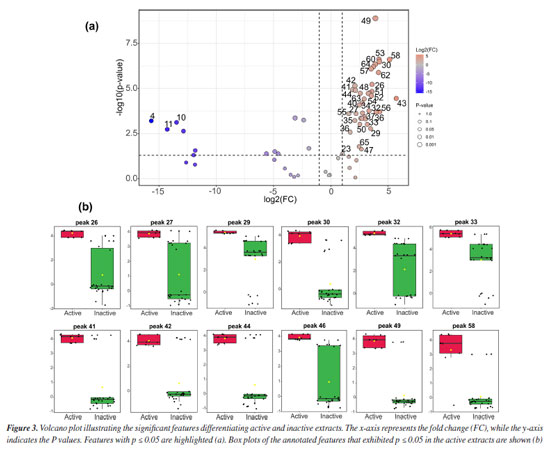
The heatmap suggests that the observed clustering patterns are driven by differences in metabolite composition, with specific compounds being more abundant in active extracts. These findings support the hypothesis that non-polar to moderately polar metabolites play a key role in the cytotoxic activity of C. nobilior extracts, highlighting the importance of solvent selection in influencing the distribution of extracted compounds. For instance, glycosylated flavonoids were predominantly found in the methanol extracts, which did not exhibit cytotoxic potential. The volcano plot (Figure 3a) highlights the significant metabolic features differentiating active and inactive extracts, with statistically relevant metabolites identified based on p ≤ 0.05. The x-axis represents the fold change (FC), indicating the relative abundance of metabolites between groups (active/red and inactive/green), while the y-axis denotes the P values, reflecting their statistical significance. The active extracts exhibited distinct metabolic profiles, with several metabolites significantly more abundant compared to the inactive extracts, such as the metabolites 23, 25-27, 29, 30, 32-35, 40-45, 46, 47, 48-49, 50-54, 56-58, and 62-65. These key metabolites may contribute to the cytotoxic activity observed in the bioassays.
The box plots are illustrated in Figure 3b, revealing the intensity distribution of the most relevant annotated features (p ≤ 0.05) found in the active extracts. These plots provide insights into the variability and consistency of metabolite levels, reinforcing their potential role in the bioactivity of C. nobilior extracts, such as the observed for 26 (dihydro-hydroxy-methoxy phenanthrenedione), 27 (dihydro-dimethoxy phenanthrenediol), 29 (dihydro-methoxy-phenanthrenediol), 30 (dihydroxy-dimethoxy-bibenzyl), 32 (batatasin III), 33 (gigantol), 41 (bis-dihydro-methoxy phenanthrenediol), 42 (dihydro-methoxy phenanthrenetriol-dihydro-methoxy phenanthrenediol), 44 (methoxy phenanthrenetriol-dihydro-methoxy phenanthrenediol), 46 (dihydro-methoxy phenanthrenediol-dihydro-dimethoxy phenanthrenediol), 49 (dihydro-methoxy phenanthrenediol), and 58 (hydroxy-methoxy bibenzyl). The box plots from other metabolites are illustrated in Figure 1S (Supplementary Material). The results suggest that stilbenoid and phenanthrene derivatives are related to the cytotoxic effects. These findings underscore the importance of metabolic profiling in understanding bioactivity and support the selection of these extracts for further investigation into their potential pharmacological applications. The features applied in the multivariate statistical analysis and their annotation are summarized in Table 2, which totalized 67 metabolites, mainly two phenylpropanoid amides (22 and 23), a lignan (25), 12 glycosylated flavonoids (7, 9-17, 19, and 28), and 31 stilbenoid and phenanthrenes derivatives (24, 26-27, 29, 31-32, 35-37, 41-52, 54, and 56-64), including dimeric (35-36, 41-42, 44-46, 49, 51, 54, 56, 57, 59-62, and 64) and trimeric phenanthrenes derivatives (63). The chromatogram is presented in Figure 2S (Supplementary Material), and the annotation of metabolites is first presented with a focus on those selected through statistical analysis, as they may be associated with the cytotoxic properties observed in certain C. nobilior extracts.
Metabolites 32 and 33 exhibited an absorption band at 280 nm in the UV spectrum, consistent with bibenzyl units, such as stilbene derivatives.31 Both compounds yielded the fragment ion m/z 151, resulting from cleavage between the aromatic rings that confirmed the presence of hydroxyl and methoxyl substituents on one of the aromatic rings of the stilbenes. These stilbenes were isolated and identified by NMR experiments as batatasin III (32) and gigantol (33), as reported later in this study. The dihydrophenenthrenes showed absorption bands at the wavelengths ca. 280 and 308 nm, which are compatible with those reported in the literature.32 The dihydrophenanthrene 29 (m/z 243.1011 [M + H]+), for example, showed loss of water (18 u) or methanol molecules yielding the fragment ions at m/z 225 and 211, which confirmed the presence of hydroxyl and methoxyl substituents with a ring contraction to produce the product ion at m/z 181. Thus, it was annotated as dihydro-methoxy phenanthrenediol (29) and the spectral data are compatible with the data published in the literature.25 For dimeric dihydrophenanthrenes, additional fragmentation of the monomers was observed, which proved useful for annotation, as in the case of compounds 41 (bis-dihydro-methoxy-phenanthrenediol).29,33 In another case, compounds such as peaks 35 and 42 (dihydro-methoxy-phenanthrenetriol-dihydro-methoxy-phenanthrenediol isomers), 44, 56, and 59 (methoxy-phenanthrenetriol-dihydro-methoxy-phenanthrenediol isomers), 46, 57, 60, and 64 (dihydro-methoxy-phenanthrenediol-dihydro-dimethoxy-phenanthrenediol [bis-O-phenanthrene derivatives]), 62 (dihydro-dimethoxy-phenanthrenediol-methoxy-phenanthrenediol [bis-O-phenanthrene derivative]), and 63 (hexahydro-trimethoxy-terphenanthrene-hexol) present fragmentation data that are scarcely described in the literature. We emphasize that, although these compounds were annotated with a level 3 identification (as shown in Table 2), our report is particularly relevant. Mass spectrometry-based annotation can accelerate the study of these compounds, which have been identified as a promising class for the exploration of new targets against cancer cell lines and are recognized as chemotaxonomic markers of the Orchidaceae family. Notably, dimeric and trimeric phenanthrenes are rarely documented in literature. Compounds 22 and 23 exhibited absorption bands in the UV spectrum at λmax 285/310 and 285/320 nm, respectively, suggesting the presence of coumaric and caffeic acid chromophores. Additionally, these compounds displayed fragment ions at m/z 147 and 177, corresponding to the loss of the tyramine moiety.20 Based on these spectral characteristics, compounds 22 and 23 were annotated as N-coumaroyl tyramine and N-feruloyl tyramine. These metabolites have previously been reported in Orchidaceae, including Dendrobium species34 and Vanilla planifolia;35 however, this is the first report of their occurrence in the Cattleya genus. Compounds 7, 9-17, and 19 exhibited UV spectra characteristic of flavones (λmax ≈ 270 and 335 nm), while 28 displayed spectral features consistent with a flavanol (λmax ≈ 270 and 355 nm) and an additional absorption band at 310 nm, suggesting the presence of a coumaroyl group.36 The fragmentation patterns observed for the flavones were consistent with C-glycosylated flavonoids.37 Flavones 7, 11, 13, and 14 exhibited sequential losses of H2O (18 u) and common fragmentation patterns indicative of a C-hexoside moiety (m/z 473 [M - H - 120]- and 383 [M - H - 120 - 90]-), leading to their annotation as 6,8-di-C-hexosyl apigenin. Compounds 10 and 12 showed fragment losses consistent with C-pentoside cleavage ([M - H - 60]- and [M - H - 90]-), while compounds 9 and 12 additionally exhibited the loss of a methyl radical (15 u), supporting their identification as 6,8-di-C-glucopyranosyldiosmetin (9), 6,8-C-hexosyl pentosyl apigenin (10), and 6,8-C-hexosyl C-pentosyl O-methyl apigenin (12).18,19 Compounds 15-17 and 19 displayed fragmentation patterns similar to C-pentoside and/or methyl losses, but they also showed the loss of an O-hexoside unit (162 u).18,19 Based on these characteristics, they were identified as O-hexosyl C-hexosyl O-methyl luteolin (15 and 16), C-hexosyl O-methyl luteolin (17), and O-hexosyl tetrahydroxy-O-methyl flavone (19). Additionally, flavonoid 28 (m/z 623.1405 [M - H]-, C31H28O14) exhibited the loss of a coumaroyl group (146 u), yielding a fragment ion at m/z 477. This was followed by the sequential loss of a hexosyl unit, resulting in the ion m/z 315 [M - H - 146 - 162]-, with subsequent loss of a methyl radical (15 u) to yield the fragment ion m/z 300 relative to aglycone. The identification of quercetin was supported by the presence of fragment m/z 163 ion formed via Retro-Diels-Alder (RDA) cleavage.24 Based on these fragmentation patterns, compound 28 was annotated as O-hexosyl-coumaroyl O-methyl quercetin. From CnRz-DM5, the stilbenes 32 and 33 (Figure 4) were isolated and analyzed by NMR to confirm their chemical structures. The 1H NMR spectra of 32 revealed signals in the aromatic region at δH 6.24 (2H) and 6.30 (1H), and signals at δH 6.65 (2H), 6.75 (1H), and 7.13 (1H). This last signal (δH) appeared as a doublet of doublets with a coupling constant (J) of 7.9 and 7.5 Hz, consistent with ortho-coupled hydrogens (Table 3S, Figure 3S, Supplementary Material). The hydrogens of methylene groups were observed at δH 2.80 (4H) and, in addition, a singlet at δH 3.73 (3H) was observed that confirmed the methoxy substituent. The 13C NMR spectrum showed 15 carbons signals, including 12 aromatics, two methylenes, five quaternary, and one methoxyl. Two hydroxyl groups at C-3 and C-3' and the methoxyl at C-5 were assigned based on the chemical shifts at δC 156.5, 155.5, and 161, respectively. The spectral data of 32 were similar with data described for batatasin III.38,39
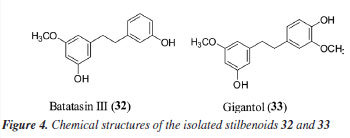
The 1H NMR of 33 revealed signals of six aromatic hydrogens and hydrogens of two methoxyl substituents (δH 3.73 and 3.82) (Table 4S, Figure 3S, Supplementary Material). In the 13C NMR, 16 carbons were observed, including 12 aromatics, two methylenes, six quaternary, and two methoxyl. The oxygenated substituents were suggested from the characteristic chemical shifts of que carbons at δC 143.1, 145.8, 156.0, and 160.3 relative to C-4', C-3', C-3, and C-5, respectively. The correlations observed in HMBC (heteronuclear multiple bond correlation) spectrum between hydrogens H-6 and the carbons C-5 and C-4, and H-2' with C-4', C-3', C-2', C-1', and C-6' were useful for the attribution of the signals. Thus, 33 was characterized as gigantol and their chemical shifts were similar with the described in the literature.38,39 The isolated metabolites of batatasin III (32) and gigantol (33) were also evaluated against HCT-116 and MCF-7 cancer cell lines at 10 µg mL-1. For the HCT-116 cells, the metabolites 32 and 33 revealed an inhibition of 39.1 ± 18.6% and 39.5 ± 0.8%, while for the MCF-7 cancer cells an inhibition of 30.1 ± 12.8% and 30.5 ± 9.1%. These stilbenoids have been described for several biological activities, mainly anticancer.40 Gigantol, for example, has already shown efficacy in the treatment of different cancer cell lines, such as hepatocellular, breast cancer, bladder cancer, and cervical cancer.41 Batatasin III showed similar aspects, with anticancer activity against lung cancer cells.40 Therefore, this study was significant as it described, for the first time, the chemical constituents of C. nobilior. Additionally, it stimulates future research focused on phenanthrene derivatives to identify new targets against cancer cell lines.
CONCLUSIONS This study systematically evaluated extracts from different parts of Cattleya nobilior for their chemical composition and biological activity, with a focus on phenanthrene and stilbenoid derivatives. The results demonstrated a strong correlation between phenanthrene and stilbenoids and cytotoxic effects on colorectal (HCT-116), breast (MCF-7), and leukemic (CCRF-CEM, Kasumi-1) cells, as well as moderate inhibition of Trypanosoma cruzi. Among the extracts, those from rhizomes (CnRz-DM5 and CnRz-DM50) exhibited the highest cytotoxic potency, leading to further fractionation and the isolation of two major stilbenoids, gigantol and batatasin III. These findings represent the first report on the phytochemical and pharmacological potential of C. nobilior, significantly expanding scientific knowledge of this species. The strong bioactivity observed reinforces the relevance of phenanthrenes and stilbenoids as potential anticancer agents, opening new avenues for research. Future studies should focus on the isolation and characterization of additional bioactive compounds, as well as in vivo evaluations to better understand their therapeutic potential.
SUPPLEMENTARY MATERIAL Supplementary information (yields of extracts, 1H and 13C NMR data) for this work is available at http://quimicanova.sbq.org.br/, as a PDF file, with free access.
DATA AVAILABILITY STATEMENT All data are available in the text and supplementary material.
ACKNOWLEDGMENTS The authors are thankful to Universidade Federal de Mato Grosso do Sul (UFMS), Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT), Instituto Nacional de Ciência e Tecnologia em Áreas Úmidas (INAU), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico for financial support.
AUTHOR CONTRIBUTIONS Caroline de Moura Costa was responsible for the formal analysis, investigation; Katyuce Souza Farias for the data curation, validation, original draft writing, writing review and editing; Izadora Bonfim for the formal analysis, investigation; Heron F. V. Torquato for the formal analysis, investigation; Samara Requena Nocchi for the formal analysis, investigation; Laís Azevedo Rodrigues for the validation, original draft writing; Júlio Mentha Almeida for the data curation, investigation in the anti-T. cruzi assay, validation; Flávio Macedo Alves for the identification of material and harvest; Alda Maria Teixeira Ferreira for the conceptualization, and anti-T. cruzi assay; Edgar J. Paredes-Gamero for the conceptualization, funding acquisition, resource; Helori Vanni Domingos for the cytotoxity activity and formal analysis; Letícia Costa Lotufo for the conceptualization, funding acquisition, resource; Carlos A. Carollo for the funding acquisition, resource, validation, writing review and editing; Denise Brentan Silva for the conceptualization, funding acquisition, project administration, resource, validation, original draft writing, writing review and editing.
REFERENCES 1. Wang, Y.; Wang, H.; Ye, C.; Wang, Z.; Ma, C.; Lin, D.; Jin, X.; Plant Diversity 2024, 46, 425. [Crossref] 2. Bazzicalupo, M.; Calevo, J.; Smeriglio, A.; Cornara, L.; Plants 2023, 12, 257. [Crossref] 3. Nainwal, L. M.; Alam, M. M.; Shaquiquzzaman, M.; Marella, A.; Kamal, A.; Expert Opin. Ther. Pat. 2019, 29, 703. [Crossref] 4. Giacomini, E.; Rupiani, S.; Guidotti, L.; Recanatini, M.; Roberti, M.; Curr. Med. Chem. 2016, 23, 2439. [Crossref] 5. Tóth, B.; Hohmann, J.; Vasas, A.; J. Nat. Prod. 2018, 81, 661. [Crossref] 6. Shukla, M. K.; Monika; Thakur, A.; Verma, R.; Lalhlenmawia, H.; Bhattacharyya, S.; Bisht, D.; Singh, A.; Parcha, V.; Kumar, D.; S. Afr. J. Bot. 2022, 150, 69. [Crossref] 7. de Almeida, T. L.; Monteiro, J. A.; Lopes, G. K. P.; Chiavelli, L. U. R.; Santin, S. M. O.; da Silva, C. C.; Kaplum, V.; Scariot, D. B.; Nakamura, C. V.; Ruiz, A. L. T. G.; Carvalho, J. E.; de Faria, R. T.; Pomini, A. M.; Quim. Nova 2014, 37, 1151. [Crossref] 8. Belloto, A. C.; Souza, G. K.; Perin, P. C.; Schuquel, I. T. A.; Santin, S. M. O.; Chiavelli, L. U. R.; Garcia, F. P.; Kaplum, V.; Rodrigues, J. H. S.; Scariot, D. B.; Delvecchio, R.; Machado-Ferreira, E.; Aguiar, R. S.; Soares, C. A. G.; Nakamura, C. V.; Pomini, A. M.; Nat. Prod. Res. 2018, 32, 2916. [Crossref] 9. van den Berg, C.; Phytotaxa 2014, 186, 75. [Crossref] 10. Ferreira, N. P.; Lucca, D. L.; Diniz, B. V.; Negri, M. F. N.; Milaneze-Gutierre, M. A.; de Oliveira, S. M.; Pomini, A. M.; Biochem. Syst. Ecol. 2021, 97, 104303. [Crossref] 11. Ghiraldi, D. M.; Perin, P. C.; Lucca, D. L.; Pilau, E. J.; de Oliveira, S. M.; Diniz, B. V.; Negri, M. F. N.; Milaneze-Gutierre, M. A.; Martinelli, F. T. R.; Berlinck, R. G. S.; Pomini, A. M.; Nat. Prod. Res. 2025, 39, 769. [Crossref] 12. Barros, F.; Vinhos, F.; Rodrigues, V. T.; Barberena, F. F. V. A.; Fraga, C. N.; Pessoa, E. M.; Foster, W.; Orchidaceae in Lista de Espécies da Flora do Brasil; Jardim Botânico do Rio de Janeiro: Rio de Janeiro, 2013. [Link] accessed in July 2025 13. Mosmann, T.; J. Immunol. Methods 1983, 65, 55. [Crossref] 14. Višnjevec, A. M.; Barp, L.; Lucci, P.; Moret, S.; TrAC, Trends Anal. Chem. 2024, 173, 117620. [Crossref] 15. Dai, W.; Qi, D.; Yang, T.; Lv, H.; Guo, L.; Zhang, Y.; Zhu, Y.; Peng, Q.; Xie, D.; Tan, J.; Lin, Z.; J. Agric. Food Chem. 2015, 63, 9869. [Crossref] 16. Liang, Z.-Y.; Zhang, J.-Y.; Huang, Y.-C.; Zhou, C.-J.; Wang, Y.-W.; Zhou, C.-H.; Xing, S.-P.; Shun, Q.-S.; Xu, Y.-X.; Wei, G.; J. Sep. Sci. 2019, 42, 1088. [Crossref] 17. Caristi, C.; Bellocco, E.; Gargiulli, C.; Toscano, G.; Leuzzi, U.; Food Chem. 2006, 95, 431. [Crossref] 18. Becchi, M.; Fraisse, D.; Biomed. Environ. Mass Spectrom. 1989, 18, 122. [Crossref] 19. Cuyckens, F.; Claeys, M.; J. Mass Spectrom. 2004, 39, 1. [Crossref] 20. Caroli, C.; Brighenti, V.; Cattivelli, A.; Salamone, S.; Pollastro, F.; Tagliazucchi, D.; Pellati, F.; J. Pharm. Biomed. Anal. 2023, 236, 115723. [Crossref] 21. Sim, H.-J.; Kim, J. H.; Lee, K. R.; Hong, J.; Molecules 2013, 18, 5235. [Crossref] 22. Yoshikawa, K.; Ito, T.; Iseki, K.; Baba, C.; Imagawa, H.; Yagi, Y.; Morita, H.; Asakawa, Y.; Kawano, S.; Hashimoto, T.; J. Nat. Prod. 2012, 75, 605. [Crossref] 23. Leong, Y.-W.; Kang, C.-C.; Harrison, L. J.; Powell, A. D.; Phytochemistry 1997, 44, 157. [Crossref] 24. De Rosso, M.; Panighel, A.; Dalla Vedova, A.; Gardiman, M.; Flamini, R.; Molecules 2015, 20, 18095. [Crossref] 25. Ju, Z.; Tang, X.; Liao, Q.; Guan, H.; Yang, L.; Wang, Z.; J. Pharm. Biomed. Anal. 2021, 195, 113836. [Crossref] 26. Zhao, Y.; Zhu, S.; Li, Y.; Niu, X.; Shang, G.; Zhou, X.; Yin, J.; Bao, B.; Cao, Y.; Cheng, F.; Li, Z.; Wang, R.; Yao, W.; J. Pharm. Biomed. Anal. 2024, 243, 116077. [Crossref] 27. Kong, F.; Wang, T.; You, W.; Rapid Commun. Mass Spectrom. 2022, 36, e9361. [Crossref] 28. Cakova, V.; Urbain, A.; Antheaume, C.; Rimlinger, N.; Wehrung, P.; Bonté, F.; Lobstein, A.; Phytochem. Anal. 2015, 26, 34. [Crossref] 29. Wang, X.; Zhong, X.-J.; Zhou, N.; Cai, N.; Xu, J.-H.; Wang, Q.-B.; Li, J.-J.; Liu, Q.; Lin, P.-C.; Shang, X.-Y.; Molecules 2020, 25, 898. [Crossref] 30. Cioffi, G.; Montoro, P.; De Ugaz, O. L.; Vassallo, A.; Severino, L.; Pizza, C.; De Tommasi, N.; Molecules 2011, 16, 2527. [Crossref] 31. Majumder, P. L.; Guha, S.; Sen, S.; Phytochemistry 1999, 52, 1365. [Crossref] 32. Savchenko, E. V.; Kostjukov, V. V.; J. Mol. Model. 2024, 30, 24. [Crossref] 33. Di Fabio, G.; Zarrelli, A.; Chem. Biodiversity 2023, 20, e202201068. [Crossref] 34. Peng, L.; Yu, J.; Fang, J.; Yin, F.; Abirami, G.; Wu, J.; Lou, G.; Li, H.; Yang, L.; Xia, J.; Yang, D.; Liang, Z.; Zhang, X.; Arabian J. Chem. 2024, 17, 105922. [Crossref] 35. Beer, F.; Weinert, C. H.; Wellmann, J.; Hillebrand, S.; Ley, J. P.; Soukup, S. T.; Kulling, S. E.; Phytochem. Anal. 2025, 36, 30. [Crossref] 36. Markham, K. R.; Techniques of Flavonoid Identification; Academic Press: London, 1982. 37. Geng, P.; Sun, J.; Zhang, M.; Li, X.; Harnly, J. M.; Chen, P.; J. Mass Spectrom. 2016, 51, 914. [Crossref] 38. Yang, J. Z.; Jiang, H.; Wang, W. J.; Zhang, Y. M.; Liu, Y.; Chen, Y. G.; Trop. J. Pharm. Res. 2014, 13, 533. [Crossref] 39. Juneja, R. K.; Sharma, S. C.; Tandon, J. S.; Phytochemistry 1987, 26, 1123. [Crossref] 40. Śliwiński, T.; Kowalczyk, T.; Sitarek, P.; Kolanowska, M.; Cancers 2022, 14, 754. [Crossref] 41. Patel, K.; Singh, G. K.; Husain, G. M.; Prasad, S. K.; Patel, D. K.; Curr. Drug Ther. 2024, 19, 445. [Crossref]
Guest Editor handled this article: Lucas S. Abreu |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access