Artigo
| Comparative study of the chemical composition of amapa latex from Parahancornia Amapa and Brosimum parinarioides BY HPLC-UV-DAD |
|
Rita Cynara de Oliveira SallesI,II,* I. Departamento de Química, Instituto de Ciências Exatas, Universidade Federal do Amazonas, 69077-000 Manaus - AM, Brasil Received: 02/27/2025 *e-mail: ritacynara@gmail.com; ritasnunomura@gmail.com A phytochemical study of the latex from P. amapa and B. parinarioides has led to the isolation of nine compounds. From the latex of B. parinarioides, six compounds were isolated: lupeol acetate (1), butyrospermol (2), and its epimer, tirucalla-7,24-dien-3β-ol (3), cycloartenol (4), cycloeucalenol (5), obtusifoliol (6). This is the first report of their occurrence in B. parinarioides. From the latex of P. amapa isolated lupeol acetate (1), a mixture of acetylated triterpenes was identified, including α-amyrin acetate, β-amyrin, and lupeol (MIST-2). Additionally, two mixtures of hydroxylated and non-hydroxylated lupeol esters (MIST-3) and (MIST-1), were isolated and fully characterized using gas chromatography-mass spectrometry (GC-MS) and nuclear magnetic resonance (NMR) techniques. A comparative study of the latex from both species has been conducted using high-performance liquid chromatography with ultraviolet detection using a diode array detector (HPLC-UV-DAD). This analysis has revealed common features, such as the presence of α- and β-amyrin acetates and lupeol. However, the major distinction has been that the mixture of hydroxylated lupeol esters has been found exclusively in P. amapa, while cycloeucalenol and obtusifoliol have been observed only in B. parinarioides. INTRODUCTION Amapa latex is a traditional medicine from the Brazilian Amazon, primarily used in the region as a tonic, healing agent, and anti-inflammatory. It serves as a valuable treatment for conditions like asthma, bronchitis, tuberculosis, trauma - especially in the chest area - general weakness, and intestinal diseases.1,2 This latex has a milky white appearance and is obtained by cutting the bark of specific trees. In the Amazon, amapa latex can be extracted from different plant species that are traditionally used for medicinal purposes. The "sweet amapa" latex is typically sourced from Brosimum parinarioides Ducke,2-5 while the "bitter amapa" latex comes from Parahancornia amapa (Huber) Ducke. Generally, the "sweet amapa" group includes species from the Brosimum genus within the Moraceae family, with Brosimum parinarioides Ducke being the most common. To date, there are only a few studies about the chemistry of this species, mostly conducted by our research group. For example, Sá et al.6 illustrate the identification of several compounds, including isoliquiritigenin, licoagrochalcone A, caffeic acid glycoside, and epicatechin glycoside in the bark of B. parinarioides.7 They also isolated and characterized parinarioidin C and identified additional substances - parinarioidin A, parinarioidin B, 3-O-caffeoylquinic acid, and canzonol C - based on the interpretation of tandem mass spectra (MS/MS). Furthermore, flavonoids such as licograchalcone and kanzonol were isolated from the bark of B. parinarioides. Another research group studied the "bitter amapa" group, which includes species from the Apocynaceae family, with Parahancornia amapa being the most common. Sobrinho et al.8 and Carvalho et al.9 conducted studies on less polar extracts derived from the bark, latex, and roots of P. amapa collected in Macapá, located in the eastern Amazon of Brazil. Their findings reported the presence of steroids such as β-sitosterol, stigmasterol, and β-sitosterone, along with the isolation of pentacyclic triterpenes including lupeol, α-amyrin, and β-amyrin, as well as their acetylated derivatives, friedelin, and various aliphatic acids and hydroxylated and non-hydroxylated esters of lupeol.8-10 Previous chemical studies10 identified in the methanol extracts a significant amount of a carbohydrate blend, phenylethanoid derivatives, and methyl myoinositol as the principal constituents. In another study conducted by Henrique et al.,11 the latex from Amazon "sweet amapa" was analyzed. Several compounds from the dichloromethane extract of the branches, including β-sitosterol, stigmasterol, and pentacyclic triterpenoids such as α-amyrin, β-amyrin, lupeol, and friedelin have been described. Additionally, they identified the indole alkaloid isositsirikine from the methanolic extract of the bark of P. amapa. Considering that the latex of amapa is used in traditional medicine and is sold in the Brazilian Amazon without any quality control, we conducted a study to compare the chromatographic profiles of different samples of amapa latex from B. parinarioides and P. amapa found in the Amazonas State. We aimed to isolate and identify the main chemical constituents and compare the chromatographic profiles of these latex samples using high-performance liquid chromatography (HPLC).
EXPERIMENTAL Solvents Solvents with PA grade from Labsynth (São Paulo, Brazil) were purified by distillation prior to thin layer chromatography (TLC) and column chromatography (CC) analyses. The HPLC grade solvents were purchased from Sigma-Aldrich, acetonitrile (ACN) from Fluka (Germany), hexanes from JT Baker (USA) and methyl tert-butyl ether (MTBE) from Tedia (USA For NMR analysis, deuterated chloroform was purchased from Cambridge Isotope Laboratories Inc. (USA). General procedures The NMR analysis was performed in a Unity Inova - VARIAN at 500 MHz to hydrogen (1H) and 125 MHz to carbon (13C) and in a Bruker (Fourier 300) at 300 MHz to 1H and 75 MHz to 13C. The analysis by high resolution mass spectrometry were performed by a Bruker spectrometer MicroTOF-QII, with atmospheric pressure chemical ionization (APCI) at positive mode. The analysis by LC-MS was performed on a UFLC system from Shimadzu, equipped with binary pump LC-20 AD, DGU-20A 3R degasser, SPD-M 20A detector, CBM-20A controller, automatic injector SIL-20AC HT and oven column CTO-20A; coupled to a high-resolution mass spectrometer from Bruker model MicroTOF QII-with APCI source in positive mode. The parameters used for MS analysis were as follows: end plate -500 V; capillary 4000 V; corona 5000 nA; nebulizer 1.6 bar; dry gas 2.0 L min-1; dry temperature 250 ºC; vaporizer temperature 450 ºC; scan range 50-3000. Calibration was performed with a solution of Tuning mix APCI (Fluka). The isolation of compounds by HPLC was performed in a Shimadzu equipped with two LC-6 AD pumps, a DGU-14A degasser, a UV detector with two SPD-10Avp channels, SCL-10Avp controller and Rheodyne manual injector model 7725i. The data were processed by Class-VP program version 6.13 SP2 (Shimadzu, Japan). The qualitative analyses by HPLC-DAD (high-performance liquid chromatography-diode array detection) were performed in Shimadzu Prominence LC-20A, equipped with quaternary pump LC-10AT Vp, DGU 20A 5 degasser, SPD-M 20A DAD detector, automatic injector SIL-20A and CBM-20A controller. The analysis by GC-MS to confirm the mixture of triterpenes were performed in a gas chromatograph equipment from Shimadzu, model QP-2010, equipped with an automatic injector AOC-5000, and the analyses to determine the side chain of mixture of methyl esters were performed in a GC-MS Ultra Trace, Thermo Scientific DSQ-2, equipped with automatic injector AI/AS 3000. Material plant and extract Samples of latex from P. amapa and B. parinarioides were collected during both the dry and rainy seasons at the Adolpho Ducke Forest Reserve (INPA), located at km 26 on AM-010 highway. The specimens were previously identified by the Flora from the Reserva Ducke project. Dried exsiccates are deposited at the INPA herbarium in Manaus, AM, under voucher numbers 191.462, 191.463, and 191.478 for B. parinarioides, and number 189.608 for P. amapa (SisGen A04356A). For the species B. parinarioides, 212.0 mL was collected during the dry season and 164.0 mL during the rainy season. For P. amapa, a total of 350.0 mL of latex was collected. Latex from P. amapa was collected in October 2007, while latex from B. parinarioides was collected in October 2010 (dry season) and April 2011 (rainy season). Each latex sample was initially suspended in methanol (1:1 volume ratio), and the residue was then redissolved in dichloromethane with the assistance of an ultrasonic bath. The extracts of dichloromethane were obtained after the solvent was removed using a rotary evaporator under vacuum. Isolation of compounds Compounds from P. amapa The dichloromethane extract from the latex of P. amapa (PAL-D) (5.0 g) was subjected to open column chromatography using silica gel 60 (63-200 µm) as the stationary phase, and a gradient system of n-hexane and ethyl acetate (Hex/EtOAc) with increasing polarity as the mobile phase. The fractions obtained were combined based on TLC analysis. Fraction F5 displayed crystal formation, which, after recrystallization, yielded a mixture of hydroxylated esters of lupeol, designated as MIST-3-3a'-d' (129.0 mg). The third combined fraction (F3, 1.5 g) was rechromatographed on a silica gel column using a mixture of hexane, dichloromethane, and ether as the eluent. The first fraction eluted from this column was further purified by column chromatography with silica gel 60 (40-63 µm) as the stationary phase. A solvent gradient consisting of hexane, hexane/chloroform, hexane/chloroform/ethyl acetate, and finally, ethyl acetate was used as the mobile phase. This process resulted in a mixture of acetylated triterpenes including α-amyrin, β-amyrin, and lupeol (MIST-2) (118.2 mg), as well as a second mixture containing non-hydroxylated esters of lupeol (MIST-1-1a'-d') (315.4 mg). After recrystallization, pure lupeol acetate (1) (13.0 mg) was also obtained. Compounds from B. parinarioides The dichloromethane extract of B. parinarioides (BPL-D) (9.8 g) was also fractionated by several separations by open-column chromatography. Two fractions (DF5 and DF6) were obtained from open-column chromatography with reverse phase RP-18 (40-63 µm) as the stationary phase and using ACN 100% as the mobile phase. Both fractions were purified by semi-preparative HPLC using a Shim Pack Prep-ODS H (250 × 20 mm, 5 µm) column and isocratic system of ACN 100%, flow of 16 mL min-1. The samples (DF5 and DF6) were solubilized in ACN/iPrOH (80:20), and the separation was monitored with a UV detector at wavelengths of 210 nm. From fraction DF5, butyrospermol (2) (6.5 mg) and its epimer, tirucalla-7,24-dien-3-β-ol (3) (12.5 mg), were isolated. From fraction DF6, cycloartenol (4) (3.5 mg) was obtained. Additionally, after several fractionations of the extract via open-column chromatography, lupeol acetate (1) (1.0 mg) was also isolated. A fifth fraction (FS), obtained from the second open-column separation, was further purified by HPLC in normal phase with Phenomenex Luna Si 100 A column (250 mm × 10 mm, 5 µm), isocratic system Hex/ MTBE (80:20) for 40 min at a flow of 3 mL min-1. The sample was solubilized in Hex/MTBE (90:10) resulting in the isolation of cycloeucalenol (5) (31.1 mg) and obtusifoliol (6) (26.6 mg). The compounds were identified by 1H NMR, 13C NMR, distortionless enhancement by polarization transfer 135 (DEPT 135), heteronuclear single quantum correlation (HSQC), heteronuclear multiple bond correlation (HMBC), and GC-MS analysis, compared with the literature. Mixture of non-hydroxylated esters of lupeol (MIST-1)8 1H NMR (500 MHz, CDCl3) δH 4.47 (1H, dd, J 5.5, 11.0 Hz, H-3), 0.87 (3H, s, H-23), 1.03 (3H, s, H-24), 0.89 (3H, s, H-25), 0.85 (3H, s, H-26), 0.94 (3H, s, H-27), 0.78 (3H, s, H-28), 4.68 (1H, d, J 2.0 Hz, H-29), 4.50 (1H, dd, J 2.0, 1.5 Hz, H-29), 1.68 (3H, s, H-30), 1.25 (3H, H-3'), 1.25 (CH2)n; 13C NMR (125 MHz, CDCl3) δC 38.3 (C-1), 23.9 (C-2), 80.8 (C-3), 38.0 (C-4), 55.6 (C-5), 18.2 (C-6), 34.5 (C-7), 41.1 (C-8), 50.6 (C-9), 37.3 (C-10), 21.2 (C-11), 25.4 (C-12), 38.6 (C-13), 43.1 (C-14), 27.7 (C-15), 35.8 (C-16), 43.2 (C-17), 48.5 (C-18), 48.2 (C-19), 151.1 (C-20), 29.7 (C-21), 40.2 (C-22), 28.2 (C-23), 16.2 (C-24), 16.4 (C-25), 16.8 (C-26), 14.4 (C-27), 17.0 (C-28), 109.6 (C-29), 19.5 (C-30), 173.9 (C-1'), 35.1 (C-2'), 25.4 (C-3') (Figures 1S and 2S, Supplementary Material). Mixture of acetylated triterpenes (MIST-2)12-20 The 1H NMR data were similar to literature data.12,13,15-18,20 13C NMR (125.0 MHz, CDCl3) α-amyrin acetate: δC 38.2 (C-1), 27.1 (C-2), 81.1 (C-3), 38.5 (C-4), 55.5 (C-5), 18.2 (C-6), 32.8 (C-7), 39.8 (C-8), 47.7 (C-9), 37.0 (C-10), 23.8 (C-11), 124.5 (C-12), 139.8 (C-13), 43.2 (C-14), 28.6 (C-15), 26.6 (C-16), 33.9 (C-17), 59.3 (C-18), 39.8 (C-19), 40.0 (C-20), 32.7 (C-21), 41.7 (C-22), 28.2 (C-23), 15.7 (C-24), 16.2 (C-25), 16.7 (C-26), 23.8 (C-27), 28.3 (C-28), 23.4 (C-29), 21.5 (C-30), 171.1 (C=O), 21.5 (CH3); β-amyrin acetate: δC 38.2 (C-1), 27.1 (C-2), 81.1 (C-3), 38.5 (C-4), 55.5 (C-5), 18.2 (C-6), 32.8 (C-7), 39.8 (C-8), 47.7 (C-9), 37.0 (C-10), 23.8 (C-11), 121.8 (C-12), 145.4 (C-13), 41.7 (C-14), 26.1 (C-15), 26.8 (C-16), 33.9 (C-17), 47.4 (C-18), 47.0 (C-19), 32.7 (C-20), 34.9 (C-21), 37.3 (C-22), 28.9 (C-23), 15.9 (C-24), 16.2 (C-25), 16.7 (C-26), 26.3 (C-27), 28.6 (C-28), 32.8 (C-29), 23.6 (C-30), 171.1 (C=O), 21.5 (CH3); lupeol acetate: δC 38.2 (C-1), 23.7 (C-2), 81.1 (C-3), 37.9 (C-4), 55.5 (C-5), 18.2 (C-6), 34.4 (C-7), 40.8 (C-8), 50.5 (C-9), 37.0 (C-10), 21.1 (C-11), 25.3 (C-12), 38.0 (C-13), 42.3 (C-14), 27.6 (C-15), 35.8 (C-16), 43.0 (C-17), 48.2 (C-18), 47.8 (C-19), 151.1 (C-20), 30.0 (C-21), 39.8 (C-22), 28.1 (C-23), 16.4 (C-24), 16.2 (C-25), 15.9 (C-26), 14.7 (C-27), 17.7 (C-28), 109.6 (C-29), 19.5 (C-30), 171.1 (C=O), 21.6 (CH3) (Figures 3S and 4S, Supplementary Material). Mixture of hydroxylated esters of lupeol (MIST-3)8 1H NMR (500 MHz, CDCl3) δH 4.53 (1H, dd, J 6.0, 10.0 Hz, H-3), 0.83 (3H, s, H-23), 1.02 (3H, s, H-24), 0.87 (3H, s, H-25), 0.85 (3H, s, H-26), 0.94 (3H, s, H-27), 0.78 (3H, s, H-28), 4.68 (1H, d, J 2.0 Hz, H-29), 4.56 (1H, dd, J 1.5, 1.5 Hz, H-29), 1.67 (3H, s, H-30), 3.97 (m, H-3'), 1.25 (CH2)n; 13C NMR (125 MHz, CDCl3) δC 38.3 (C-1), 23.9 (C-2), 81.6 (C-3), 37.3 (C-4), 55.6 (C-5), 18.2 (C-6), 34.4 (C-7), 40.2 (C-8), 50.6 (C-9), 21.2 (C-10), 21.2 (C-11), 25.3 (C-12), 38.0 (C-13), 41.9 (C-14), 27.7 (C-15), 35.8 (C-16), 43.1 (C-17), 48.5 (C-18), 48.2 (C-19), 151.1 (C-20), 29.8 (C-21), 38.6 (C-22), 28.3 (C-23), 16.2 (C-24), 16.4 (C-25), 16.8 (C-26), 14.8 (C-27), 18.2 (C-28), 109.6 (C-29), 19.5 (C-30), 173.0 (C-1'), 41.9 (C-2'), 68.4 (C-3'), 36.8 (C-4'), 25.3 (C-5') (Figures 5S and 6S, Supplementary Material). Lupeol acetate (P. amapa) (1)12,18-20 The 1H NMR data were similar to literature data.12,18,20 13C NMR (125 MHz, CDCl3) δC 38.2 (C-1), 23.9 (C-2), 81.2 (C-3), 38.6 (C-4), 55.6 (C-5), 18.2 (C-6), 34.4 (C-7), 40.8 (C-8), 50.6 (C-9), 37.0 (C-10), 21.2 (C-11), 25.3 (C-12), 38.2 (C-13), 43.2 (C-14), 27.6 (C-15), 35.8 (C-16), 43.0 (C-17), 48.2 (C-18), 48.5 (C-19), 151.2 (C-20), 30.0 (C-21), 39.8 (C-22), 28.1 (C-23), 16.4 (C-24), 16.2 (C-25), 16.7 (C-26), 14.7 (C-27), 18.2 (C-28), 109.6 (C-29), 19.5 (C-30), 171.2 (C-1'), 21.5 (C-2') (Figures 7S and 8S, Supplementary Material); HR-APCI-MS:21-23 m/z 409.3843 [M + H - CH3COOH]+ (calculated value m/z 409.3834) (Figure 9S, Supplementary Material). Lupeol acetate (B. parinarioides) (1)12,18-20 The 1H NMR data were similar to literature data.12,18,20 13C NMR (125 MHz, CDCl3) δC 38.8 (C-1), 23.9 (C-2), 81.2 (C-3), 38.7 (C-4), 55.6 (C-5), 18.2 (C-6), 34.5 (C-7), 41.1 (C-8), 50.6 (C-9), 37.3 (C-10), 21.1 (C-11), 25.3 (C-12), 38.2 (C-13), 43.2 (C-14), 27.6 (C-15), 35.8 (C-16), 43.0 (C-17), 48.2 (C-18), 48.5 (C-19), 151.2 (C-20), 32.2 (C-21), 40.0 (C-22), 28.2 (C-23), 16.4 (C-24), 16.2 (C-25), 16.7 (C-26), 14.7 (C-27), 18.4 (C-28), 109.6 (C-29), 19.6 (C-30), 171.2 (C-1'), 21.2 (C-2') (Figures 10S and 11S, Supplementary Material); HR-APCI-MS:21-23 m/z 409.3809 [M + H - CH3COOH]+ (calculated value m/z 409.3834) (Figure 12S, Supplementary Material). Butyrospermol (B. parinarioides) (2)24,25 1H NMR (500 MHz, CDCl3) δH 3.24 (1H, dd, J 11.5, 4.0 Hz, H-3), 5.25 (1H, dd, J 7.0, 3.0 Hz, H-7), 0.81 (3H, s, H-18), 0.74 (3H, s, H-19), 0.85 (3H, d, J 6.5 Hz, H-21), 5.10 (1H, m, H-24), 1.60 (3H, s, H-26), 1.68 (3H, s, H-27), 0.97 (3H, s, H-28), 0.97 (3H, s, H-29), 0.86 (3H, s, H-30); 13C NMR (125 MHz, CDCl3) δC 37.6 (C-1), 28.1 (C-2), 79,6 (C-3), 39.3 (C-4), 50.9 (C-5), 24.3 (C-6), 118.1 (C-7), 146.2 (C-8), 49.3 (C-9), 35.3 (C-10), 18.5 (C-11), 34.2 (C-12), 43.9 (C-13), 51.6 (C-14), 34.3 (C-15), 28.8 (C-16), 53.6 (C-17), 22.4 (C-18), 13.4 (C-19), 36.1 (C-20), 18.9 (C-21), 35.5 (C-22), 25.7 (C-23), 125.5 (C-24), 131.3 (C-25), 18.0 (C-26), 26.1 (C-27), 27.7 (C-28), 27.9 (C-29), 15.1 (C-30); HMBC: δH 3.24 (H-3) (C-29 and C-30), 5.25 (H7) (C-9), 0.81 (H-18) (C-12, C-13, C-14), 0.74 (H-19) (C-1, C-5, C-10), 0.85 (H-21) (C-17, C-20), 5.10 (H-24) (C-23, C-26, C-27), 1.60 (H-26) (C-24, C-25, C-27), 1.68 (H-27) (C-24, C-25, C-26), 0.97 (H-28) (C-8, C-13, C-15), 0.97 (H-29) (C-3, C-4, C-5, C-30), 0.86 (H-30) (C-3, C-4, C-5, C-29). The 1D and 2D NMR spectra of butyrospermol are in the Supplementary Material (Figures 13S-17S). LC-HR-APCI-MS: retention time (tR):10.6 min. m/z 427.3913 [M + H]+ (calculated value m/z 427.3939); m/z 409.3845 [M + H - H2O]+ (calculated value m/z 409.3834) (Figures 23S and 24S, Supplementary Material). Tirucalla-7,24-dien-3-β-ol (B. parinarioides) (3)26 1H NMR (500 MHz, CDCl3) δH 3.24 (1H, dd, J 11.0, 4.25 Hz, H-3), 5.25 (1H, dd, J 6.5, 3.0 Hz, H-7), 0.81 (3H, s, H-18), 0.75 (3H, s, H-19), 0.88 (3H, d, J 6.5 Hz, H-21), 1.60 (3H, s, H-26), 1.68 (3H, s, H-27), 0.97 (3H, s, H-28), 0.97 (3H, s, H-29), 0.86 (3H, s, H-30); 13C NMR (125 MHz, CDCl3) δC 37.6 (C-1), 28.1 (C-2), 79.6 (C-3), 39.3 (C-4), 50.9 (C-5), 24.3 (C-6), 118.2 (C-7), 146.2 (C-8), 49.3 (C-9), 35.3 (C-10), 18.5 (C-11), 34.2 (C-12), 43.9 (C-13), 51.5 (C-14), 34.4 (C-15), 28.6 (C-16), 53.3 (C-17), 22.3 (C-18), 13.5 (C-19), 36.5 (C-20), 18.7 (C-21), 36.3 (C-22), 25.4 (C-23), 125.6 (C-24), 131.3 (C-25), 17.9 (C-26), 26.1 (C-27), 27.7 (C-28), 27.9 (C-29), 15.1 (C-30); HMBC: δH 3.24 (H-3) (C-29 and C-30), 5.24 (H-7) (C-9), 0.81 (H-18) (C-12, C-13, C-14), 0.75 (H-19) (C-1, C-5, C-10), 0.88 (H-21) (C-20, C-22), 5.09 (H-24) (C-26, C-27), 1.60 (H-26) (C-24, C-25, C-27), 1.68 (H-27) (C-24, C-25, C-26), 0.97 (H-28) (C-8, C-13, C-15), 0.97 (H-29) (C-3, C-4, C-5, C-30), 0.86 (H-30) (C-3, C-4, C-5, C-29). The 1D and 2D NMR spectra of tirucalla-7,24-dien-3-β-ol are in the Supplementary Material (Figures 18S-22S). LC-HR-APCI-MS: tR: 9.8 min. m/z 427.3918 [M + H]+ (calculated value m/z 427.3939); m/z 409.3857 [M + H - H2O]+ (calculated value m/z 409.3834) (Figures 23S and 25S, Supplementary Material). Cycloartenol (B. parinarioides) (4)24,27 1H NMR (500 MHz, CDCl3) δH 3.29 (1H, dd, J 11.5, 4.25 Hz, H-3), 0.89 (3H, s, H-18), 0.56 (1H, d, J 4.0 Hz, H-19), 0.33 (1H, d, J 4.5 Hz, H-19), 0.89 (3H, d, J 7.0 Hz, H-21), 5.10 (1H, m, H-24), 1.61 (3H, s, H-26), 1.69 (3H, s, H-27), 0.97 (3H, s, H-28), 0.97 (3H, s, H-29), 0.81 (3H, s, H-30); 13C NMR (125 MHz, CDCl3) δC 32.3 (C-1), 30.8 (C-2), 79.2 (C-3), 40.8 (C-4), 47.5 (C-5), 21.5 (C-6), 28.5 (C-7), 48.3 (C-8), 20.4 (C-9), 26.4 (C-10), 25.8 (C-11), 33.3 (C-12), 45.7 (C-13), 49.2 (C-14), 35.9 (C-15), 26.9 (C-16), 52.7 (C-17), 19.7 (C-18), 30.2 (C-19), 36.2 (C-20), 18.4 (C-21), 36.7 (C-22), 25.3 (C-23), 125.6 (C-24), 131.2 (C-25), 17.9 (C-26), 26.4 (C-27), 18.6 (C-28), 26.1 (C-29), 14.4 (C-30); HMBC: δH 3.29 (H-3) (C-30), 0.89 (H-18) (C-13, C-14, C-17), 0.56 (H-19) (C-1, C-9, C-10, C-11), 0.33 (H-19) (C-1, C-9, C-10, C-11), 0.89 (H-21) (C-17, C-20, C-22), 5.10 (H-24) (C-26, C-27), 1.61 (H-26) (C-24, C-25, C-27), 1.69 (H-27) (C-24, C-25, C-26), 0.97 (H-28) (C-8, C-13, C-14), 0.97 (H-29) (C-3, C-4, C-5, C-30), 0.81 (H-30) (C-3, C-4, C-5, C-29) (Figures 26S-29S, Supplementary Material). HR-APCI-MS:22 m/z 427.3925 [M + H]+ (calculated value m/z 427.3939); m/z 409.3824 [M + H - H2O]+ (calculated value m/z 409.3834) (Figure 30S, Supplementary Material). Cycloeucalenol (B. parinarioides) (5)28 1H NMR (500 MHz, CDCl3) δH 3.21 (1H, m, H-3), 0.97 (3H, s, H-18), 0.14 (1H, d, J 3.5 Hz, H-19), 0.39 (1H, d, J 3.5 Hz, H-19), 0.89 (3H, d, J 6.5 Hz, H-21), 1.02 (3H, d, J 2.5 Hz, H-26), 1.03 (3H, d, J 2.5 Hz, H-27), 4.71 (1H, s, H-28), 4.66 (1H, d, J 6.5 Hz, H-28), 0.98 (3H, d, J 6.5 Hz, H-29), 0.89 (3H, s, H-30); 13C NMR (125 MHz, CDCl3) δC 31.1 (C-1), 35.2 (C-2), 76.9 (C-3), 44.9 (C-4), 43.7 (C-5), 25.0 (C-6), 25.5 (C-7), 47.2 (C-8), 23.9 (C-9), 29.9 (C-10), 27.3 (C-11), 33.3 (C-12), 45.7 (C-13), 49.3 (C-14), 35.7 (C-15), 28.5 (C-16), 52.6 (C-17), 18.1 (C-18), 27.6 (C-19), 36.5 (C-20), 18.7 (C-21), 35.4 (C-22), 31.7 (C-23), 157.3 (C-24), 34.2 (C-25), 22.3 (C-26), 22.2 (C-27), 106.3 (C-28), 14.7 (C-29), 19.5 (C-30); HMBC: δH 1.21 (H-11) (C-8, C-9, C-12, C-13, C-19), 1.96 (H-11) (C-9, C-10, C-12, C-19), 1.62 (H-12) (C-9, C-11, C-18), 1.29 (H-15) (C-16), 1.92 (H-16) (C-15), 0.97 (H-18) (C-12, C-13, C-14, C-17), 0.14 (H-19) (C-1, C-5, C-8, C-9, C-11), 0.39 (H-19) (C-1, C-5, C-8), 0.89 (H-21) (C-17, C-20, C-22), 2.12 (H-23) (C-24), 1.89 (H-23) (C-24, C-28), 2.23 (H-25) (C-23, C-24, C-26, C-27, C-28), 1.02 (H-26) (C-24, C-25, C-27), 1.03 (H-27) (C-24, C-25, C-26), 4.71 (H-28) (C-23, C-25), 4.66 (H-28) (C-23, C-25), 0.98 (H-29) (C-3, C-4, C-5), 0.89 (H-30) (C-8, C-13, C-14, C-17) (Figures 31S-35S, Supplementary Material). HR-APCI-MS: m/z 427.3954 [M + H]+ (calculated value m/z 427.3939); m/z 409.3838 [M + H - H2O]+ (calculated value m/z 409.3834) (Figure 36S, Supplementary Material). Obtusifoliol (B. parinarioides) (6)24 1H NMR (500 MHz, CDCl3) δH 3.09 (1H, m, H-3), 0.71 (3H, s, H-18), 0.93 (3H, d, J 6.0 Hz, H-21), 0.97 (3H, s, H-19), 1.03 (3H, d, J 3.0 Hz, H-26), 1.02 (3H, d, J 3.0 Hz, H-27), 4.72 (1H, s, H-28), 4.66 (1H, d, J 1.0 Hz, H-28), 0.99 (3H, d, J 6.5 Hz, H-29), 0.89 (3H, s, H-30); 13C NMR (125 MHz, CDCl3) δC 35.4 (C-1), 31.4 (C-2), 76.9 (C-3), 39.6 (C-4), 47.4 (C-5), 21.0 (C-6), 28.5 (C-7), 135.0 (C-8), 133.9 (C-9), 36.7 (C-10), 21.1 (C-11), 25.9 (C-12), 44.9 (C-13), 50.2 (C-14), 31.1 (C-15), 31.5 (C-16), 50.8 (C-17), 16.1 (C-18), 18.6 (C-19), 36.8 (C-20), 19.1 (C-21), 35.4 (C-22), 31.7 (C-23), 157.3 (C-24), 34.2 (C-25), 22.3 (C-26), 22.2 (C-27), 106.3 (C-28), 15.4 (C-29), 24.8 (C-30); HMBC: δH 0.71 (H-18) (C-13, C-14), 0.97 (H-19) (C-1, C-5, C-9, C-10), 0.93 (H-21) (C-17, C-20, C-22), 2.23 (H-25) (C-23, C-24, C-26, C-28), 1.03 (H-26) (C-24, C-25, C-27), 1.02 (H-27) (C-24, C-25, C-26), 4.72 (H-28) (C-23, C-25), 4.66 (H-28) (C-23, C-25), 0.99 (H-29) (C-3, C-4, C-5), 0.89 (H-30) (C-8, C-13, C-14, C-15) (Figures 37S-40S, Supplementary Material). HR-APCI-MS: m/z 427.3896 [M + H]+ (calculated value m/z 427.3939); m/z 409.3824 [M + H - H2O]+ (calculated value m/z 409.3834) (Figure 41S, Supplementary Material). Transesterification of mixtures (MIST-1 and MIST-3) The mixtures were submitted to transesterification as described by Sobrinho et al.,8 with some modifications. The MIST-1 (5 mg) was refluxed for 6 h with MeOH (5 mL) and HCl 37% (1 mL). After cooling, the solution was extracted with hexane HPLC grade. The hexane phase was washed with H2O, dried with anhydrous Na2SO4, and concentrated in vacuo resulting in lupeol and a mixture of methyl esters (MIST-1-1a'-d'), which were analyzed by GC-MS (single quadrupole). The MIST-3 (5.4 mg) was submitted to reflux over HCl 10% in MeOH (10 mL) with agitation for 6 h. The same procedure was performed with MIST-3-3a'-d' and from the first reaction lupeol and a mixture of methyl esters were identified by GC-MS. Analysis by GC-MS Analysis of acetyl triterpenes (MIST-2) MIST-2 was analyzed by GC-MS using a Shimadzu, QP-2010, equipped with automatic injector AOAC-5000, single quadrupole, and ionization by electron impact at 70 eV; DB-17MS column (30 m × 0.25 mm and film of 0.25 mm of thickness), programmed oven temperature: 120 ºC, rising 20 ºC min-1 to 260 ºC, isotherm for 5 min, up 2 ºC min-1 to 280 ºC, isotherm for 9 min, up 2 ºC min-1 to 290 ºC, isotherm for 20 min with the time of analysis 56 min. The carrier gas was helium at 1.4 mL min-1. The injection temperature was 260 ºC, the transfer line temperature 290 ºC, and the injection mode was split (1:10). The scan range was set to m/z 50.0 to 550.0. The sample was solubilized in CHCl3, with a final concentration of 1 mg mL-1 and an injection volume of 1 μL. Analysis of methyl esters mixture (MIST-1 and MIST-3) After transesterification, the mixture of non-hydroxylated (MIST-1) and hydroxylated methyl esters (MIST-3) were analyzed by GC-MS gas chromatograph, model Ultra Trace coupled to a Thermo Scientific mass spectrometer, DSQ-II model, equipped with automatic injector, AI/AS 3000 model, with single quadrupole analyzer and electron impact ionization at 70 eV. A Zebron ZB-FFAP column from Phenomenex (60 m length, 0.25 mm internal diameter, film thickness 0.25 mM) was used; and the oven temperature started at 210 ºC isothermal for 5 min, then rising 10 ºC min-1 up to 260 ºC, followed by an isothermal for 50 min, 60 min analysis time. Injector in split mode (1:10) to 250 ºC. Helium carrier gas at 1.8 mL min-1. The temperature of the transfer line was 275 ºC. The mass spectrometer operated in the full scan mode (scan range of m/z 40-500), positive ion mode with a source operating at 200 ºC. The samples were solubilized in hexane (1 mg mL-1), and the injection volume was 1.0 μL. The identification of components was based on spectrum analysis, compared with the NIST (2.0) and Adams libraries. Comparison of species profiles by HPLC-UV-DAD The chromatographic profile analyses by HPLC-DAD were performed in a Shimadzu Prominence LC-20 equipment. The separation was obtained using a Phenomenex Luna Si 100A column (250 × 2.0 mm, 5 µm) and as mobile phase a linear gradient system Hex (A)/MTBE (B): 0-5 min (100% A), 5-28 min (0-6% B), 28-95 min (6% B), 95-100 min (94-100% A), 100-120 min (100% A), at the flow rate of 0.2 mL min-1 and injection volume of 10 µL. The chromatograms were monitored at 254 and 200 nm. The extracts were dissolved in hexane at 5.0 mg mL-1 and the isolated compounds at 1.0 mg mL-1, and the mixture of isolated compounds at 1.0 mg mL-1. They were used as standards for identification in each sample. Co-injection standards The isolated compounds and mixtures of triterpenoids were co-injected with extracts of B. parinarioides (dry and rainy season) and extracts of P. amapa. The final concentration was 2.5 mg mL-1 for the extracts and 0.5 mg mL-1 for the standards. Extracts that presented a larger peak area of the standards than the area of the respective isolated standards were diluted by 50% for better visualization, with a final concentration for the extract of 1.25 mg mL-1.
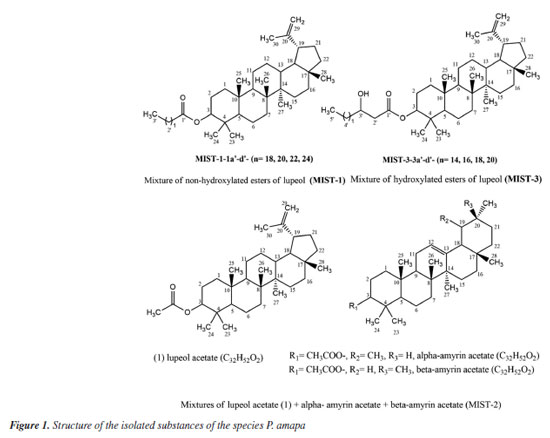
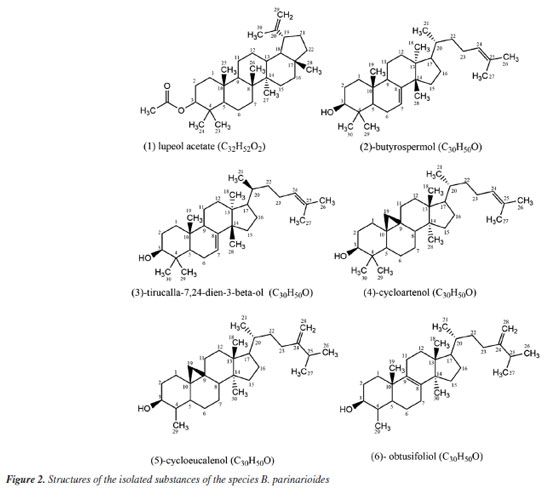
RESULTS AND DISCUSSION The phytochemical study of dichloromethane extract from latex of P. amapa yielded the isolation of a mixture of non-hydroxylated lupeol esters (MIST-1-1a'-d') (315.4 mg), a mixture of the acetyl triterpenes α-amyrin acetate, β-amyrin acetate and lupeol acetate (MIST-2) (118.2 mg), the lupeol acetate (1) (13.0 mg) and a mixture of hydroxylated esters of lupeol (MIST-3-3a'-d') (Figure 1). From B. parinarioides, the compounds 1 (12.3 mg), 2 (6.5 mg), 3 (12.5 mg), 4 (3.5 mg), 5 (31.1 mg) and 6 (26.0 mg) were isolated and identified by 1D, 2D NMR and analysis by LC-MS and DI-MS (direct infusion) (Figure 2).
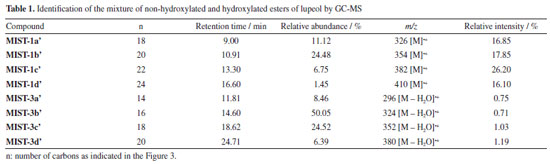
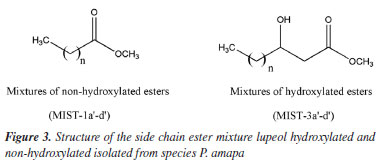
The NMR spectra of MIST-1 and MIST-3 (Figure 3) confirmed these mixtures as 3-O-acil-lupeol esters and compared with the literature.8,9 These mixtures of esters of acyl-lupeol were analyzed by GC-MS,8 after acid transesterification to determine the side chain of these compounds (Table 1) (Figures 42S-51S, Supplementary Material).
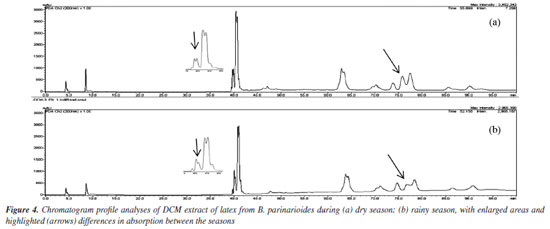
The 1H NMR data of the mixture of acetyl triterpenes (MIST-2) were compared with literature12,13 and confirmed by GC-MS (Figure 52S and Table 1S, Supplementary Material). The three compounds in the mixture showed MS spectra with the same fragmentation profile (m/z 189, 203, 218) to β- and α-amyrin acetate and m/z 189 to lupeol acetate (Figures 53S-55S, Supplementary Material). The ion m/z 218 is a key fragment observed in ursane and oleanane skeletons, which resulted from a Retro-Diels-Alder rearrangement on ring C.29,30 The analysis of the mass spectra obtained for MIST-2, and the comparison with data from the spectral libraries available in the equipment such as NIST, Wiley and the library created by the Research Laboratory (NPPS, Research Center for Natural Products and Synthesis), showed that it was the expected mixture of triterpenes, with similarity index (SI) 95% for β-amyrin acetate, SI 96% for α-amyrin acetate and SI 88% for lupeol acetate. The compound 1 isolated from P. amapa had its acetyl unit determined by 1H and 13C NMR, identified as lupeol acetate (Figures 7S and 8S, Supplementary Material).9 The MS spectra presented the ion m/z 409, resulting from the loss of the acetyl group (Figure 9S, Supplementary Material). The 1H and 13C NMR spectra and MS spectrum of compound 1, isolated from B. parinarioides, presented signals similar to those published for lupeol acetate (Figures 10S-12S, Supplementary Material).12,18-20 The 1H NMR, 13C NMR, DEPT 135, HSQC, and HMBC data of 2 (Figures 13S-17S, Supplementary Material), have helped to identify this compound as being the triterpene butyrospermol.24,25 But based on the HMBC observed correlations of hydrogen at δH 0.805 (3H, s, H-18) to the carbon signals δC 34.179 (C-12), 43.891(C-13) and 51.633 (C-14); along with the hydrogen at δH 0.744 (3H, s, H-19) that presented correlations to the carbon signals at δC 37.559 (C-1), 50.992 (C-5) and 35.296 (C-10), the methyl groups C-18 and C-19 were reassigned. The MS obtained by LC-MS at positive mode and APCI ionization showed the m/z 427.3913 (calculated m/z 427.3939) and the base peak with m/z on 409.3845 [M + H - H2O]+ (calculated m/z 409.3834) (Figure 23S-24S, Supplementary Material). The chemical shifts from 1H and 13C NMR, and correlations on HSQC and HMBC observed from compounds 2-6, confirmed the identification of tirucalla-7,24-dien-β-ol (3) (Figures 18S-22S, Supplementary Material), an epimer of butyrospermol (2) (Figures 13S-17S, Supplementary Material), cycloartenol (4) (Figures 26S-29S, Supplementary Material), cycloeucalenol (5) (Figures 31S-35S, Supplementary Material) and obtusifoliol (6) (Figures 37S-40S, Supplementary Material) when compared to literature.23,26,27 Reassignments of 13C NMR chemical shifts for obtusifoliol (6) were conducted based on literature comparisons for C-8 and C-9, based on the HSQC and HMBC experiments performed, as previously mentioned for butyrospermol. Determination of the side chain of methyl esters by GC-MS After an acid transesterification of the mixture of lupeol esters, the determination of the side chains from these compounds was performed by GC-MS and is presented in Table 1. The acid methanolysis of MIST-1 yielded lupeol and a mixture of four methyl of non-hydroxylated esters (MIST-1-1a'-d') (Figures 42S-46S, Supplementary Material). MIST-1 presents side chains containing 18 to 24 carbons as observed on MS spectra and described as follows. EIMS: m/z (rel. int): MIST-1a' 326 (M.+, 17), 283 (17), 241 (9), 199 (15), 143 (33), 129 (17), 87 (99), 75 (32), 74 (100); MIST-1b' 354 (M•+, 18), 311 (16), 213 (6), 199 (21), 143 (40), 87 (78), 75 (32), 74 (100); MIST-1c' 382 (M.+, 26), 339 (15), 283 (9), 241 (2), 199 (21), 143 (44), 87 (80), 75 (41), 74 (100); MIST-1d' 410 (M•+, 16), 367 (10), 311 (5), 199 (13), 143 (51), 87 (69), 75 (37), 74 (100). The data obtained in this work for MIST-1 showed variation in comparison to the data obtained by Sobrinho et al.8 The four methyl esters presented a base peak of m/z 74, which has high intensity due to McLafferty rearrangement. On the other hand, the identification of MIST-3-3a'-d' was performed by the characteristic ion m/z 103, which shows the presence of hydroxyl esters on the side chain, as confirmed by NMR spectra. To some MS spectra of these esters that present hydroxyl group on C-3' and that did not present molecular ion, the ion [M - H2O]+ has been used to identify these characteristics once this ion corresponds to the loss of an H2O molecule. The esters of MIST-3 presented five peaks on chromatogram, where four are from the side chain of lupeol esters hydroxylated and one to lupeol. MIST-3 is a mixture of lupeol esters with the side chains of 14 to 20 carbons (MIST-3-3a'-d'). The data obtained in this work for MIST-3 showed variation in comparison to the data obtained by Sobrinho et al.8 EIMS: m/z (rel. int): MIST-3a' 296 (0.7, M-H2O), 103 (100); MIST-3b' 324 (0.7, M-H2O), 103 (100); MIST-3c' 352 (1.0, M-H2O), 103 (100); MIST-3d' 380 (1.0, M-H2O), 103 (100) (Figures 47S-51S, Supplementary Material). The retention time and relative percent (based on the area of each peak) of each identified methyl esters on the mixture are presented on the Table 1. HPLC profile analysis of latex from P. amapa and B. parinarioides The HPLC profile of dichloromethane extracts from B. parinarioides, obtained during both the dry and rainy seasons, shows a similar composition (Figure 4). However, the intensity of some peaks varies between the two extracts. Based on these findings, the dichloromethane extract of B. parinarioides from the dry season was selected for comparison with the dichloromethane extract of P. amapa.
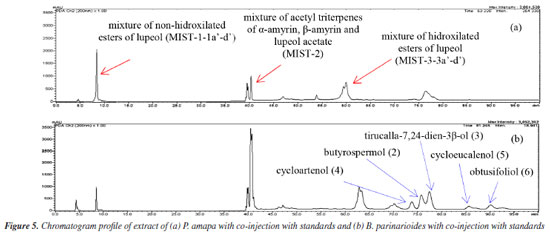
The chromatographic profiles for PAL-D (dichloromethane extract of P. amapa) and BPL-D (dichloromethane extract of B. parinarioides) by normal phase HPLC showed similar and different compounds when compared with the standards analysis under the same conditions. The profile of extracts, followed by co-injection of authentic standards isolated of both species (Figure 5), identified the presence or absence of these compounds in extracts by the increased intensity of the peaks and time retention.
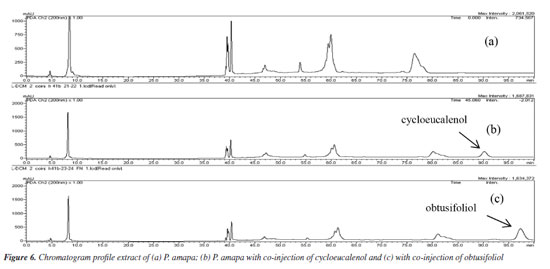
The chromatographic profiles of PAL-D, followed by co-injection with each isolated standard 1, 2, 3, 4, 5, and 6, showed increased intensity peaks at 75.25, 81.27, and 83.27 min and similar UV spectra to standards 4, 2 and 3, and these compounds were identified as cycloartenol, butyrospermol and its epimer tirucalla-7,24-dien-3-β-ol. The HPLC profile analysis of PAL-D with co-injection of compounds 5 and 6 confirmed that these compounds are not present in this extract. The chromatogram presented a different profile of the original extract, with peaks of 5 and 6 (Figure 6).
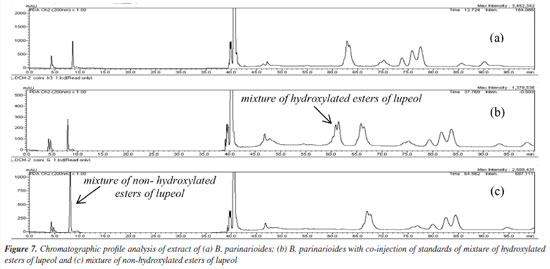
The HPLC profile analysis of BPL-D with co-injection of isolated standards of P. amapa (MIST-1, MIST-2 and MIST-3) and lupeol acetate (1) indicated solely the presence of the mixture of acetyl triterpenes (MIST-2) and lupeol acetate (1). Clearly the presence of mixture of hydroxylated esters of lupeol (MIST-3) could not be detected, while the presence of mixture of non-hydroxylated esters of lupeol (MIST-1) was inconclusive (Figure 7). The standard MIST-3 is clearly chemotypes from the latex of P. amapa and not from B. parinarioides. Besides, some minor triperpenes, namely cycloeucalenol (5), and obtusifoliol (6), could only be isolated and detected from the dichloromethane extract from the latex of B. parinarioides and not from P. amapa. All other compounds could be either isolated or detected in both species.
CONCLUSIONS The present work reported the phytochemical study of amapa latex from two amazon species, P. amapa and B. parinarioides, known to produce the medicinal amapa latex. The isolation of the triterpenes lupeol acetate, butyrospermol, tirucalla-7,24-dien-3β-ol, cycloartenol, cycloeucalenol, and obtusifoliol is reported for the first time to occur in B. parinarioides. From P. amapa, it was possible to isolate a mixture of acetates of α-amyrin, β-amyrin, and lupeol. Besides, two mixtures of lupeol esters (hydroxylated and non-hydroxylated) were identified and fully characterized. The normal phase HPLC analyses clearly showed that the chromatographic profiles of P. amapa (PAL-D) and B. parinarioides (BPL-D) present significant differences, due to the presence of hydroxylated and non-hydroxylated esters of lupeol, solely in the latex of P. amapa. These results can be the basis of a quality control method to identify the botanical species that produced the latex.
SUPPLEMENTARY MATERIAL Supplementary material for this work is available at http://quimicanova.sbq.org.br/, as a PDF file, with free access.
DATA AVAILABILITY STATEMENT All data are available in the text and supplementary material.
ACKNOWLEDGMENTS The authors thank the Amazonas State Research Support Foundation (FAPEAM) for funding research (Ed 013/2022, FAPEAM Productivity Program in ST&I), the Postgraduate Program in Chemistry of the Universidade Federal do Amazonas for their support and the Centro de Bionegócios da Amazônia (CBA) for some analyses performed. We also thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for fellowships.
REFERENCES 1. Cavalcante, P. B.; Frutos Comestíveis da Amazônia, Coleção Adolpho Ducke, 6ª ed.; CNPQ, Museu Paraense Emílio Goeldi: Belém, 1996. 2. Matta, A.; Flora Médica Brasiliense, 3ª ed.; Editora Valer: Manaus, 2003. 3. Borrás, M. R. L.; Plantas da Amazônia: Medicinais ou Mágicas - Plantas Comercializadas no Mercado Municipal Adolpho Lisboa; Governo do Estado do Amazonas, Editora Valer: Manaus, 2003. 4. Mors, W. B.; Rizzini, C. T.; Pereira, N. A.; Medicinal Plants of Brazil, vol. 246; Reference Publications, Inc.: Algonac-Michigan, 2000, p. 27. 5. Shanley, P.; Medina, G.; Cordeiro, S.; Frutíferas e Plantas Úteis na Vida Amazônica, 2ª ed.; CIFOR: Belém, 2005. 6. Sá, I. S. C.; da Silva, F. M. A.; Nunomura, R. C. S.; Phytochem. Lett. 2022, 50, 25. [Crossref] 7. Sá, I. S. C.; Neves, K. O. G.; Mesquita, R. S.; Bastos, L. M.; Sales, M. L. F.; da Silva, F. M. A.; Nunomura, R. C. S.; Biochem. Syst. Ecol. 2020, 91, 104075. [Crossref] 8. Sobrinho, D. C.; Hauptli, M. B.; Appolinário, E. V.; Kollenz, C. L. M.; de Carvalho, M. G.; Braz-Filho, R.; J. Braz. Chem. Soc. 1991, 2, 15. [Crossref] 9. de Carvalho, M. G.; Velloso, C. R. X.; Braz-Filho, R.; da Costa, W. F.; J. Braz. Chem. Soc. 2001, 12, 556. [Crossref] 10. de Carvalho, M. G.; de Albuquerque, L. A.; Alves, C. C. F.; Cascon, V.; Rev. Bras. Farmacogn. 2008, 18, 667. [Crossref] 11. Henrique, M. C.; Nunomura, R. C. S.; Nunomura, S. M.; Silva, S. G.; Acta Amazonica 2014, 44, 533. [Crossref] 12. Javed, S.; Mahmood, Z.; Khan, K. M.; Sarker, S. D.; Javaid, A.; Khan, I. H.; Shoaib, A.; Sci. Rep. 2021, 11, 8417. [Crossref] 13. Bandeira, P. N.; Lemos, T. L. G.; Costa, S. M. O.; dos Santos, H. S.; Rev. Bras. Farmacogn. 2007, 17, 204. [Crossref] 14. Feleke, S.; Brehane, A.; Bull. Chem. Soc. Ethiop. 2005, 19, 307. [Crossref] 15. Okoye, N. N.; Ajaghaku, D. L.; Okeke, H. N.; Ilodigwe, E. E.; Nworu, C. S.; Okoye, F. B. C.; Pharm. Biol. 2014, 52, 1478. [Crossref] 16. Dias, M. O.; Hamerski, L.; Pinto, A. C.; Quim. Nova 2011, 34, 704. [Crossref] 17. Balestrin, L.; Dias, J. F. G.; Miguel, O. G.; Dall'Stella, D. S. G.; Miguel, M. D.; Rev. Bras. Farmacogn. 2008, 18, 230. [Crossref] 18. Ben nejma, A.; Besbes, M.; Guérineau, V.; Touboul, D.; Ben jannet, H.; Hamza, M. A.; Arabian J. Chem. 2017, 10, S2767. [Crossref] 19. Lucetti, D. L.; Lucetti, E. C. P.; Bandeira, M. A. M.; Veras, H. N. H.; Silva, A. H.; Leal, L. K. A. M.; Lopes, A. A.; Alves, V. C. C.; Silva, G. S.; Brito, G. A.; Viana, G. B.; J. Inflammation 2010, 7, 60. [Crossref] 20. Silva, J. R. A.; Rezende, C. M.; Pinto, Â. C.; Pinheiro, M. L. B.; Cordeiro, M. C.; Tamborini, E.; Young, C. M.; Bolzani, V. S.; Quim. Nova 1998, 21, 702. [Crossref] 21. Rhourri‐Frih, B.; Chaimbault, P.; Claude, B.; Lamy, C.; André, P.; Lafosse, M.; J. Mass Spectrom. 2009, 44, 71. [Crossref] 22. Martelanc, M.; Vovk, I.; Simonovska, B.; J. Chromatogr. A 2009, 1216, 6662. [Crossref] 23. Martelanc, M.; Vovk, I.; Simonovska, B.; J. Chromatogr. A 2007, 1164, 145. [Crossref] 24. Teresa, D. P.; Urones, J. G.; Marcos, I. S.; Basabe, P.; Cuadrado, M. J. S.; Moro, R. F.; Phytochemistry 1987, 26, 1767. [Crossref] 25. Akihisa, T.; Kojima, N.; Kikuchi, T.; Yasukawa, K.; Tokuda, H.; Masters, E. T.; Manosroi, A.; Manosroi, J.; J. Oleo Sci. 2010, 59, 273. [Crossref] 26. Pandreka, A.; Chaya, P. S.; Kumar, A.; Aarthy, T.; Mulani, F. A.; Bhagyashree, D. D.; B, S. H.; Jennifer, C.; Ponnusamy, S.; Nagegowda, D.; Thulasiram, H. V.; Phytochemistry 2021, 184, 112669. [Crossref] 27. Anjaneyulu, V.; Ravi, K.; Prasad, K. H.; Connolly, J. D.; Phytochemistry 1989, 28, 1471. [Crossref] 28. Kikuchi, T.; Kadota, S.; Tsubono, K.; Chem. Pharm. Bull. 1986, 34, 2479. [Crossref] 29. Budzikiewics, H.; Wilson, J. M.; Djerassi, C.; J. Am. Chem. Soc. 1963, 85, 3688. [Crossref] 30. Ogunkoya, I.; Phytochemistry 1981, 20, 121. [Crossref]
Guest Editor handled this article: Lucas S. Abreu |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access