Artigo
| Remediation of tannery and electroplating wastewaters: adsorption of heavy metals by Chlorella sorokiniana and Heterochlorella luteoviridis |
|
Allan MorcelliI,* I. Departamento de Engenharia Química, Instituto Federal de Educação, Ciência e Tecnologia Sul-rio-grandense, Campus Pelotas, 96015-360 Pelotas - RS, Brasil Received: 03/28/2025 *e-mail: allanmorcelli@ifsul.edu.br The activities of tanneries and electroplating industries are hazardous in the context of water and soil contamination by heavy metals. The use of biological materials for the adsorption of heavy metals presents as an important treatment alternative. This work evaluated the capacity of Chlorella sorokiniana and Heterochlorella luteoviridis biomasses to absorb heavy metals commonly found in these industrial wastewaters. The heavy metal uptakes were 61.4% Cu2+, 90.2% Cr3+, 6.9% Cr6+, 93.7% Fe2+, 90.6%, Fe3+ and 70.3% Zn2+ for H. luteoviridis, and 65.6% Cu2+, 75.7% Cr3+, 5.4% Cr6+, 86.0% Fe2+, 63.5%, Fe3+ and 62.7% Zn2+ for C. sorokiniana. Regression analysis showed that sorption capacity was dependent on the microalga, the heavy metal concentration in solution and the atomic number of the adsorbate. These results indicated that the use of microalgae as biosorbents is a promising alternative technology, and the efficient removal of iron has been highlighted. INTRODUCTION Contamination by heavy metals has become a global problem due to rapid urbanization and increased industrial activity. The heavy metals present in industrial effluents reduce the recovery capacity of bodies of water due to their toxic action on microorganisms; these microorganisms are responsible for the recovery of the waters through the decomposition of the organic matter present in the waters.1 The main aspect that generates interest in the removal of heavy metals from wastewaters is that these metals are non-degradable contaminants and their accumulation in the human body is associated with the incidence of several degenerative diseases, such as Parkinson's and Alzheimer's diseases.2,3 In this context, there is a wide range of conventional treatments available for removing heavy metals from industrial effluents, such as precipitation, evaporation and membrane separation. However, these methods, in addition to being expensive, generate new by-products that either require further treatment or an adequate final disposal of the sludge that is generated.4 In turn, adsorption is a simple mass transfer operation that explores the ability of certain solids to retain substances on their surface in liquid or gaseous fluids, allowing the separation of the components of these fluids. Since the adsorbed components are concentrated on the external surface of the solid, the larger the area available per unit of solid mass in the adsorbent, the more favorable the adsorption will be.5 While the saturated adsorbent is a solid waste that will also require disposal, a wide variety of environmentally-safe materials have been tested, and the possibility of using adsorbents for many cycles by regeneration makes this technology attractive.6 The adsorption phenomena are the result of a combination of the types of forces involved in physical and chemical adsorption. Thus, there are several factors that influence the adsorption process, such as the surface area, the properties of the adsorbent and adsorbate, the temperature of the system, the nature of the solvent and the pH of the medium.7 As for the forces involved, adsorption can also be denominated chemisorption and physisorption. Chemical adsorption is characterized by a greater interaction between the adsorbent and the solute, sharing electrons, and generating a chemical bond. It is considered irreversible, due to the bond formed and because it occurs only in the active sites of the adsorbent. Physical adsorption is characterized by a weak interaction between adsorbent and adsorbate, approaching van der Waals forces and, therefore, is much weaker than chemisorption. It is considered reversible, does not alter the chemical nature of the adsorbent and is non-specific, because it occurs on the entire surface of the adsorbent.8 The use of biomass is called biosorption, whether the absorbent is active or inactive (i.e., presenting or not metabolic activity). It is an alternative to traditional methods for the decontamination of wastewaters with heavy metals by adsorption and has been investigated, with promising results reported in the literature.9 All biosorbents come from some biological form, such as microorganisms, vegetables or animals. When active biomasses are used as biosorbents, metal ion removal systems become more complex, involving metabolic bioaccumulation routes. The removal of metal ions by inactive biomass is achieved through physical and chemical processes, as in the case of the application of agricultural or industrial waste as biosorbents.10 The removal of metals by non-living cells is independent of metabolism and proceeds rapidly by complexation, ion exchange, physical adsorption, etc. Among its advantages, the absence of toxicity limitations and nutrient requirements in the feed solution and the possibility of reuse of the regenerated biomass can be cited.11 Adsorption by biosorbents is a process that can be reversible (with destructive regeneration or even without destruction of the biomass), making it possible to recover the adsorbed metal (by desorption or incineration of the biosorbents, or by the formation of metal oxides, for example). Contrary to what occurs in most conventional processes, the use of biosorbents presents high efficiency for the remediation of effluents containing low concentrations of metal ions.12 Potential alternatives in this area include the application of microalgae, which can be used as biosorbents, allowing the capture of these metals in the effluents by adsorption. Research on the use of microalgae as biosorbents has grown in recent years, although there is still insufficient data on this technology. Different strains of microalgae have been investigated for the adsorption of heavy metals such as Cd2+, Pb2+, Zn2+, Cu2+ and Ni2+ in isolated forms and mixtures.9 In addition, the use of Chlorellasorokiniana immobilized in loofa was tested to remove Cr3+ and Ni2+.13,14 The application of freeze-dried biomass of Chlorella vulgaris as a biosorbent for lanthanides was also tested with varying pH levels in the medium.15 Given the low density of experimental results in the literature and the need to expand knowledge regarding this technology, the objective of this work was to use the in natura biomass of microalgae Chlorella sorokiniana and Heterochlorella luteoviridis as biosorbents in the removal of Cu2+, Cr3+ and Cr6+, Fe2+ and Fe3+, and Zn2+ from monoelementary solutions of these metals with concentrations similar to those found in wastewater from tanneries and electroplating industries.
EXPERIMENTAL Microalgal biomass C. sorokiniana was purchased in dry powder form from a company specialized in the cultivation of microalgae (Taiwan Chlorella Manufacturing Co. Ltd., Taiwan), through a representative in Brazil (Paversul, Flores da Cunha, RS). The biomass had been grown in open lagoons using fresh water, and separated by dewatering and spray-drying. Importantly, this biomass underwent a patented process16 for cell wall disruption. In turn, the H. luteoviridis biomass used in this work was cultivated in the Laboratory of Microalgal Bioprocesses of the Institute of Food Sciences and Technology (ICTA/UFRGS, Porto Alegre, Brazil) using an adapted methodology.17 The strain was donated by the Fluminense Federal University (Niterói, Brazil). H. luteoviridis was grown in aerated flat-plate airlift photobioreactors by adding 1 L min-1 of filtered air (0.22 µm membrane, Midistart 2000, Sartorius Stedim Biotech, Germany) using diffusing stones.18 The airflow rate was controlled using rotameters (Dwyer Instruments, USA) and the cultivation was homogeneously and continuously illuminated by artificial light, using a panel composed of 12 electronic lamps (Taschibra, Brazil, 13 W, white light) at 18 klx. Cultures were performed using modified f1/2 culture medium with NaNO3 concentration adapted to 600 mg L-1. The nutrient concentrations were maintained by daily addition of a solution containing 1 mL L-1 phosphate (5 g L-1 NaH2PO4.H2O) and 1 mL L-1 of a trace-minerals solution (9.8 mg L-1 CuSO2.5H2O, 22 mg L-1 ZnSO4.7H2O, 1 mg L-1 CoCl2.6H2O, 180 mg L-1 MnCl2.H2O, 6.3 mg L-1 Na2MoO4.2H2O, 4.36 g L-1 Na2EDTA and 3.15 g L-1 FeCl3.6H2O). The flasks containing culture media were sterilized in an autoclave at 121 ºC for 15 min. Cultivation was carried out under a semi-continuous regime so that, after the first 7 days of cultivation, half of the photobioreactor volume was removed every 48 h and replaced with fresh medium. These cycles of intermittent feeding were maintained for 14 days, and then new cultivations were started from scratch. At each cycle, the volume removed from the photobioreactor was centrifuged (Hitachi, CR 21GIII, 10,000 g, 10 ºC, 10 min). The recovered biomass was then lyophilized (LIOTOP, model L101, Brazil) and stored frozen (-18 ºC) until it was used in the adsorption experiments. Characterization of the microalgal biomass composition and morphology The proximate composition of the microalgae was carried out according to the methods of analysis approved by the American Association of Cereal Chemists.19 The moisture content was determined by the thermogravimetric method in an oven at 105 ºC (method 44-15.0).19 The protein content, was quantified by the Kjeldahl method (method 46-13.01)19 using % N × 5.95, a nitrogen-to-protein conversion factor suggested for microalgae.20 The total lipid content was determined by the Bligh and Dyer method.21 The analysis of the ash content was carried out in a muffle furnace at 550 ºC (method 08-01.01).19 The surface of the microalgal biomass employed in this work was studied by scanning electron microscopy (SEM) at the Central Microscopy and Microanalysis Laboratory (LabCEMM) of Pontifical Catholic University of Rio Grande do Sul (PUCRS, Porto Alegre, Brazil). Freeze-dried cells were placed on a conductive double layer carbon support, coated with a layer of gold (Oerlikon Balzers, model SCD050, Switzerland), and examined by SEM using a Philips-FEI Quanta 200 SEM-FEG (field emission gun) microscope equipped with a FEG canon delivering a 20 kV beam current, coupled to an energy-dispersive X-ray spectroscopy (EDS) system (Oxford Instruments GmbH, X-act model, Germany). For quantitative element analyses of the microalgal biomass, EDS spectrograms were recorded and analyzed using the software Oxford Aztec (AZtecOne, Oxford Instruments, UK, 2012). Synthetic wastewater preparation Synthetic wastewater samples consisting of monoelementary solutions of each ionic species were prepared by dissolving salts containing the heavy metals of interest in ultrapure water (Milli-Q, Merck, Germany). Each species was tested individually in order to avoid synergistic effects. The initial salt concentration was 500 mg L-1 (M214AIH scale, BEL Engineering, Italy) to generate an ion concentration that was deemed high in these types of wastewater, based on values found in literature for electroplating industries22-24 and tanneries,25,26 and the pH was adjusted to 6.0. Table 1 presents the salts used and the resulting concentration of each respective metal ion in the solution, according to analysis by inductively coupled plasma optical emission spectroscopy (ICP-OES).
Batch adsorption experiments The contact between the biosorbent and the synthetic wastewater was promoted in 250 mL flasks with 100 mL of mixture containing 10 g L-1 of lyophilized biomass. The microalgae were treated separately, so that each strain was paired with each ion individually, and the experiments were performed in duplicates. Agitation was carried out in an orbital shaker (Nova Ética, model 109, Brazil) at 60 rpm for 24 h at room temperature (23 ± 2 ºC) with no pH control. After contact, the treated solution was separated from the microalgal biomass by centrifugation at 4000 rpm for 10 min at 23 ± 2 ºC (FANEM, 206 R, Brazil). The precipitate was discarded and the centrifugate was filtered with a nitrocellulose membrane (5 μm, Merck, Ireland) and analyzed by ICP-OES. ICP-OES Optical emission spectrometry with inductively coupled plasma (ICP-OES) was employed to analyze the concentration of metal ions in the samples before and after the adsorption process (Thermo Scientific, iCAP 6200, USA). The analytical parameters of the equipment were 1150 W radio frequency power, 15.0 L min-1 plasma flow rate, 0.5 L min-1 auxiliary gas flow rate, 0.5 L min-1 nebulization gas flow rate, with the use of a concentric nebulizer and a cyclonic nebulizer chamber. The wavelengths were adjusted to each metal: 324.7 nm for copper, 283.5 nm for chromium, 238.3 nm for iron and 213.8 nm for zinc. 5-point standard curves were prepared by the dilution of a high purity multi-element standard solution containing the metals studied in this work, with coefficient of determination (R2) > 0.995 for all curves. The dilution of samples was performed using ultrapure water in order to obtain readings within the working range of the calibration curves. All analyses were performed in duplicates. Adsorbate uptake and sorption capacity calculations Heavy metal uptake by biomass was calculated according to Equation 1: 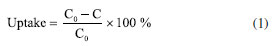 where: C0 is the initial metal concentration in solution (mg L-1) and C is the final metal concentration in solution (mg L-1). Sorption capacity of biomass was calculated according to Equation 2: 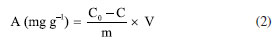 where: V is the volume of the sample (L) and m is the mass of microalgal biomass (g). Regression analysis The effects of different variables on the sorption capacities found in this work were analyzed using software R for Windows, version 4.0.3 (R Core Team, Austria, 2023), a free software environment for statistical computing and graphics.
RESULTS AND DISCUSSION Microalgal biomass characterization and morphology The main composition and the elemental analysis of the microalgae H. luteoviridis and C. sorokiniana are shown in Table 2. In general, the biomass compositions found in this work were compatible with data previously reported in the literature.27,28 The compositions of the two species presented substantial differences, as H. luteoviridis showed a higher carbohydrate content (55.2%), and C. sorokiniana presented as a protein-rich microalgal biomass (62.1%). Also, while H. luteoviridis presented a high lipid content (11.8%), an attribute for which this species has been highlighted, C. sorokiniana showed a notably low lipid content (3.4%).
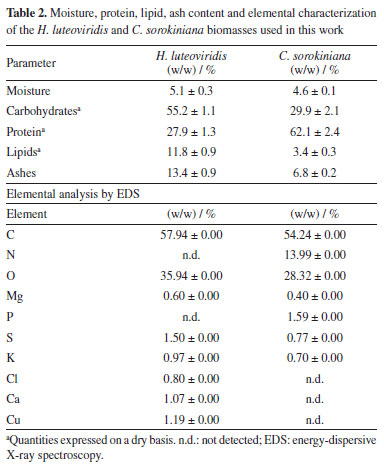
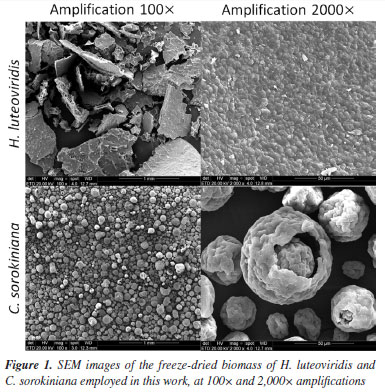
The freeze-dried biomasses of the microalgae H. luveoviridis and C. sorokiniana used in this work were also analyzed by SEM with the purpose of assessing the effects of particle structure on adsorption results, and the images generated are shown in Figure 1 at different amplifications. The two microalgae formed differently structured particle clusters while presenting similar particle sizes (ca. 5 µm). The average cell size had been previously reported29,30 by studies describing the morphology of these microalgae species. C. sorokiniana cells formed well-defined spherical clusters with rough surfaces, ranging on average between 50-200 µm. In contrast, H. luteoviridis forms clusters presented as wide plaques, suggesting strong aggregation. This observed phenomenon has been widely reported,31 as microalgae assume the form of polymicrobial aggregates at the interface due to excretions known as extracellular polymeric substances (EPS), composed of a complex mixture of biopolymers comprising polysaccharides, proteins, nucleic acids, uronic acids, humic substances, lipids, etc. These EPS significantly alter the physicochemical properties of algae aggregates, such as surface charge, viscosity, flocculation, structure, and settling properties. Furthermore, while cell integrity was verified for H. luteoviridis, the count in the generated images indicated that approximately 10% of the C. sorokiniana cells were ruptured, an effect attributed to the pre-treatment that this biomass had undergone during processing.
Heavy metal uptake and sorption capacity of biomass Batch adsorption experiments were carried out by allowing the contact of the microalgal biomasses of H. luteoviridis and C. sorokiniana with each monoelementary solution individually. ICP-OES analysis of the treated solutions was carried out to determine the residual heavy metal concentration (Table 3). Adsorbate uptake and the sorption capacity of each microalgal biomass were calculated by Equations 1 and 2, respectively.
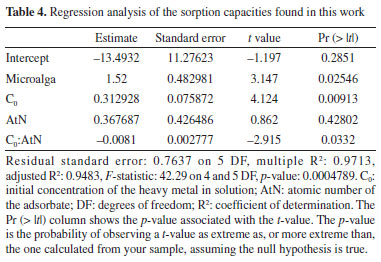
H. luteoviridis and C. sorokiniana were both able to naturally adsorb all metal species studied in this work, although to different extents. This was shown by the changes in the metal concentrations in solution after contact with untreated microalgal biomass (i.e, without the application of any pre-treatments aiming at activation). Through the analysis of the adsorbate uptakes achieved, it was observed that the microalgae presented qualitatively similar behaviors as adsorbents. High removal efficiencies were observed for Cu2+, Cr3+, Fe2+, Fe3+ and Zn2+. However, notably low removal efficiency was found for Cr6+, a highly toxic and carcinogenic species that is commonly found in the wastewaters generated in tanneries and in electroplating industries. It should be noted that hexavalent chromium usually exists in wastewater as oxyanions such as chromate CrO42- and dichromate Cr2O72- and is known to not precipitate easily using conventional precipitation methods.32 Given that the pH corresponding to the point of zero charge (PZC) of Chlorella sorokiniana is around 2.5,33 its biomass surface carries a net negative charge at the typical pH values encountered in industrial wastewaters (above 2.5). Chlorella species generally exhibit acidic to slightly acidic PZCs as well.34 This negatively charged surface readily attracts positively charged metal cations, however, a strong electrostatic repulsion is found between the negatively charged biomass and the negatively charged chromate CrO42- and dichromate Cr2O72-. This fundamental charge mismatch is considered to be the primary reason for the poor adsorption efficiency observed for Cr6+. While minor adsorption might occur through other mechanisms, the dominant electrostatic repulsion significantly limits Cr6+ uptake compared to cationic heavy metals. The high adsorbate uptakes achieved by both microalgae with most metal species are highlighted. The best uptake results for in naturaH. luteoviridis, all above 90.0%, were observed for Fe2+, Fe3+ and Cr3+ (93.7, 90.6 and 90.2%, respectively). For this microalga, lower uptakes were achieved for Zn2+ and Cu2+ (70.3 and 61.4%, respectively), while its capacity to adsorb Cr6+ was remarkably the lowest (6.9%). In turn, the best results for in naturaC. sorokiniana were found for Fe2+ and Cr3+ (86.0 and 75.7%, respectively). These species were again seen as the most easily adsorbed in the process, although lower uptakes were found when compared to those achieved by H. luteoviridis. C. sorokiniana was also able to adsorb Cu2+, Fe3+ and Zn2+ with similar efficiency (with uptakes of 65.6, 63.5, and 62.7%, respectively). Again, the adsorption of Cr6+ achieved by this microalga was very low (5.4%). Regarding the analysis of sorption capacity, the best results of this work were achieved in the removal of Fe2+ by H. luteoviridis, at 16.6 mg g-1. This would signify a lower demand in the quantity of biosorbent necessary to treat wastewater contaminated by this metal. It is also important to note that this satisfactory result for sorption capacity for Fe2+ by H. luteoviridis also corresponded to a high uptake (93.7%). Good results were also obtained regarding the ability of this microalga to adsorb Zn2+ and Fe3+, with sorption capacities of 13.2 and 10.4 mg g-1, respectively. Lower sorption capacities were achieved for Cu2+, Cr3+ and Cr6+ (7.8, 8.7 and 1.2 mg g-1, respectively). It should be noted the aforementioned high Cr3+ uptake by H. luteoviridis (90.2%) was associated with a higher demand of the biosorbent (approximately twice the quantity nedeed to adsorb Fe2+, the best result achieved in this work, as denoted by the respective sorption capacities). Comparatively, a much lower sorption capacity was found for Cr6+ (1.2 mg g-1). Moreover, when C. sorokiniana was tested, the sorption capacity for Fe2+ was also the highest (14.7 mg g-1). For the other metals, this microalga showed lower sorption capacities, with 11.8 mg g-1 for Zn2+, 8.2 mg g-1 for Cu2+, 7.5 mg g-1 for Fe3+ and 6.9 mg g-1 for Cr3+. Again, much lower sorption capacity was found for Cr6+ (0.9 mg g-1). The effects of different variables on sorption capacities were tested by regression analysis to find a mathematical model that better represented the experimental results found in this work for all species, except Cr6+, as it is found as an oxyanion in solution, as previously discussed. Regression models considering the effects of the microalga, the cation charge (i.e., oxidation number), the initial concentration of the cation in the synthetic wastewater, the atomic number of the species, and the 2-way interactions of these factors were reduced by incrementally removing the least significative effects, in such a way to obtain a simple model with a high regression coefficient composed of statistically significant effects. Through this approach, a regression model presenting the microalga, the initial concentration of the metal in solution and the 2-way interaction of the latter effect with the atomic number presented a high correlation, with R2 = 0.97 (Table 4).
The capacity of biomaterials to adsorb metals depends on the composition of their cellular surface, and some of the differences in elemental composition and morphology between C. sorokiniana and H. luteoviridis have been discussed in this work, being correlated to cell disruption. In addition, as the proximal composition of the biomasses differed in many aspects, this factor has yet to be further studied in order to correlate it to the effective mechanisms of biosorption. Generally, the mechanisms of adsorption by inactive microalgal biomass is based on a number of metal-binding processes taking place with components of the cell wall, and is therefore dependent on its composition. The cell walls of microalgal can reversibly biosorb metals, and thus function in a similar way to an ion-exchange resin.11 The microalgal cell wall generally has many functional groups, such as, hydroxyl (-OH), phosphoryl (-PO3O2), amino (-NH2), carboxyl (-COOH), sulfhydryl (-SH), among others, which confer negative charge to the cell surface, and concomitantly a high binding affinity for metal cations via counterion interactions.35 The biosorption mechanism has been described as not involving van der Waals forces at the cellulose network of the cell walls, thus both ionic charge and covalent bonding are involved in the metal biosorption process. A covalent bond with amino and carboxyl groups and an ionic bond with carboxyl and sulfate groups associated with these components can be expected.36 Adsorption via ion exchange appears to contribute to about 90% of the total amount of metal ions uptaken by microalgal cells.37 In addition, the aforementioned exopolymers formed by microalgae are known to play a crucial role in the biosorption of heavy metals, as they are polyanionic in nature and form complexes with metal cations resulting in metal immobilization within the exopolymeric matrix. These complexes generally result from electrostatic interactions between the metal ligands and negatively charged components of biopolymers.38 A combination of a high initial concentration of heavy metals in solution and a low atomic number correlated with increased sorption capacities (Figure 2). The efficiency on the removal of metals by microalgae is known to depend on the original concentration of the metals in solution. The maximum sorption level initially increases with an increase in metal concentration and eventually reaches saturation after a certain metal concentration threshold is achieved,37 and this behavior is predicted by classical adsorption isotherms.39 In addition, the sorption capacity decreases with increasing atomic number, and the effect of the concentration on the sorption capacity is stronger at low atomic numbers. As the atomic number increases along the same row of the periodic table (Zn > Cu > Fe > Cr), the additional electrons go into the same outermost shell, causing the atomic radius to decrease due to the increasing nuclear charge.40 It is speculated that the increase in adsorbate size at lower atomic numbers may have facilitated adsorption, rendering the aforementioned effect that resulted in higher sorption capacities.
The results obtained in this work are further discussed in the light of studies currently available in literature using aquatic plant biomass and the reports addressing the application of microalgae as biosorbents. Dry biomass of the aquatic plants Potamogeton lucens, Salvinia herzogii and Eichhornia crassipes were tested to remove Zn2+, Cu2+ and Cr3+ from synthetic industrial wastewaters.41 This study pointed out that the removal of metals was controlled by the pH equilibrium of the medium. This has been corroborated when Chlorella vulgaris and Scenedesmus armatus were modified with 1 M HNO3 in order to determine the influence of acid-treatment on the removal of Zn2+, Pb2+ and Cd2+ from zinc smelting wastewater.4 The aforementioned study showed that the sorption process of heavy metals was highly pH-dependent and the differences between uptake by non-modified and acid-modified biomass were relatively small, a result that was attributed to the fact that the non-modified algal biomass already presented high sorption capacity. A review9 on the state of the art of biosorption processes with brown, red and green algae also highlighted the importance of working at optimal pH and assessed the effect of pre-treatments in adsorbate uptake. The differences found in literature indicated that the pre-treatment of the biomass generally improves metal uptake, but the increase has occasionally not been very significant in the case of algae; therefore, its application should be evaluated considering the economic impact it may represent to the overall process. Although the use of H. luteoviridis as a biosorbent is virtually absent in the literature to date, C. sorokiniana has been tested as both a living and a lifeless adsorbent. The greater adsorption capacity of lifeless cells was observed when compared to the use of living C. sorokiniana,42 and uptakes ranging from 53.1-74.7% were found in the adsorption of Pb. Also, the potential of alginate-immobilized C. sorokiniana for removing Cu2+, Ni2+ and Cd2+ ions from drinking water solutions has also been investigated, and the highest experimental heavy metal uptakes achieved were 97.1, 50.9 and 64.6%, respectively.43 In other studies,13,14 the adsorption of Ni2+ and Cr3+ from aqueous solution by loofa sponge-immobilized biomass of Chlorella sorokiniana were tested. Maximum uptakes of ca. 75.0% Ni+2 and 50.0% Cr3+, and sorption capacities of 48.08 mg g-1 Ni+2 and 58.8 mg g-1 Cr3+ were found for the free biomass of C. sorokiniana, while increases in sorption capacities were noted when the biomass was immobilized in loofa (25.3 and 17.8%, for Ni+2 and Cr3+, respectively). Importantly, a significant decrease in the uptake of Ni+2 by free biomass was noted after the biosorbent concentration was increased from 1.0 to 2.5 g L-1, an effect that has been attributed to the occurrence of cell aggregation. Overall, the quality of the best results in this work was comparable to that of works found in literature using other biosorbents to remove heavy metals under optimized conditions. Also, the gain in process efficiency achieved in some of the aforementioned studies indicated that there is opportunity to improve the results here presented for the microalgae H. luteoviridis and C. sorokiniana, especially for the metal species that presented low uptakes and sorption capacities when treated with in natura biomass. Particularly, it is believed that the process could be substantially improved under controlled pH conditions. The significantly limited removal of Cr6+ by C. sorokiniana and H. luteoviridis biomass demands further discussion, particularly given its high toxicity. While the primary issue is the electrostatic repulsion between the negatively charged biomass surface (at typical pH values above its PZC) and the anionic Cr6+ oxyanions, several strategies could enhance its adsorption. One approach involves adjusting the pH of wastewater to highly acidic conditions (e.g., pH 1-3) to induce a positive charge on the biomass, thereby attracting Cr6+. However, this comes with practical challenges like increased chemical costs and potential corrosion. Another promising strategy is biomass functionalization, which means chemically modifying the algal surface to introduce or enhance specific binding sites. This could involve adding amine-rich groups to increase positive charges or introducing reducing capabilities to convert Cr6+ to the more easily adsorbed cationic Cr3+. Finally, pre-treatments, such as immobilizing the biomass onto different materials, might alter its surface properties. While these strategies hold promise, their economic viability, scalability, and environmental impact must be carefully considered for sustainable implementation.44-46 The successful application of C. sorokiniana and H.luteoviridis non-living biomass for the remediation of industrial wastewater contaminated with heavy metals necessitates a comprehensive consideration of the fate of the resultant metal-laden algal biomass. The efficient removal of these hazardous elements shifts the challenge from an aqueous phase to a solid waste management problem. Therefore, sustainable and environmentally sound strategies for handling this biomass are crucial to ensure the overall efficacy and ecological benefit of microalgae-based biosorption technologies. One primary approach involves the desorption and recovery of the adsorbed metals. This strategy offers a dual advantage: regenerating the biosorbent for reuse and recovering valuable or hazardous metals. Acidic eluents, such as dilute HCl or H2SO4, are commonly employed for metal desorption from algal biomass.47 The efficiency of desorption can be optimized by adjusting pH, contact time, and eluent concentration. Following desorption, the metals can be precipitated as hydroxides or sulfides, or recovered through electrochemical methods. The regenerated algal biomass can then be subjected to further adsorption cycles, significantly reducing the operational costs and waste volume associated with the biosorption process. The economic viability of metal recovery largely depends on the market value of the recovered metals and the scale of the operation. Given the high iron uptake observed in this study, the potential for iron recovery, especially for industrial applications, presents a particularly attractive avenue.48 If metal recovery is not a viable option, the metal-laden algal biomass demands safe disposal through methods like solidification/stabilization, where the biomass is embedded in inert materials such as cement to prevent metal leaching, allowing for safe landfilling or use in construction. Alternatively, thermal treatments like pyrolysis or incineration can reduce biomass volume, though they require careful management to prevent the release of volatile metals and hazardous ash. Emerging techniques such as bioleaching or bioremediation, which use microbial processes to mobilize or transform metals, offer an environmentally friendly avenue, but these approaches are still in the research and development phase.49,50
CONCLUSIONS The ability of H. luteoviridis and C. sorokiniana biomasses to adsorb Cu2+, Cr3+, Cr6+, Fe2+, Fe3+ and Zn2+ has been demonstrated. Altogether, this work indicated the large potential of the application of both microalgal biomasses as biosorbents to remediate tannery and electroplating wastewaters, as both microalgae were able to efficiently adsorb heavy metals typically contained in them, with the noted exception of Cr6+. Sorption capacity was proven to be dependent on the microalgal biomass, the initial metal concentration in solution and the atomic number of the adsorbent. Although the process was conducted without optimization, high uptakes were achieved, and the biosorption of iron has been highlighted. The disruption of particle aggregates has been deemed detrimental to the quality of adsorption, and one of the causes for the better performance of H. luteoviridis. To develop the full potential of this technology, it is suggested that the synergy of coexisting metals in solution should be investigated by performing experiments employing multielement synthetic wastewaters. Finally, the use of residual microalgal biomass from extraction processes should be studied in future works, aiming at the valorization of this residue to help develop an economically advantageous large-scale microalgal refinery. Beyond laboratory-scale efficacy, the practical application of this biosorption technology holds significant promise. Implementing these microalgal biosorbents in fixed-bed columns or through immobilized systems could facilitate continuous wastewater treatment, allowing for easier separation of the biomass from the treated effluent and potentially enabling biosorbent regeneration. The inherent resilience of microalgal biomass, coupled with the high metal uptakes achieved, suggests the feasibility of large-scale implementation. Utilizing residual biomass from existing microalgal cultivation or extraction processes, as proposed, further enhances the economic and environmental attractiveness, aligning with circular economy principles and moving towards a more sustainable and economically viable solution for heavy metal pollution.
DATA AVAILABILITY STATEMENT All data necessary to support the conclusions of this study are presented in the main text of this paper.
REFERENCES 1. Igberase, E.; Augustine, O.; Osifo, P.; Int. J. Biol. Macromol. 2019, 123, 664. [Crossref] 2. Zendron, R.; Revista Brasileira de Nutrição Funcional 2015, 64, 45. [Link] accessed in July 2025 3. Molazadeh, P.; Khanjani, N.; Rahimi, M.; Nasiri, A.; Journal of Community Health Research 2015, 4, 114. [Link] accessed in July 2025 4. Zabochnicka-Świątek, M.; Rygał, A.; Inz. Ochr. Srodowiska 2017, 20, 211. [Crossref] 5. Ruthven, D. M.; Principles of Adsorption and Adsorption Processes; Wiley: New York, 1984. 6. Lata, S.; Singh, P. K.; Samadder, S. R.; Int. J. Environ. Sci. Technol. 2015, 12, 1461. [Crossref] 7. Soliman, N. K.; Moustafa, A. F.; J. Mater. Res. Technol. 2020, 9, 10235. [Crossref] 8. Vidal, C.; do Nascimento, R.; Raulino, G.; Lima, A. C. A.; Melo, D.; Adsorção: Aspectos Teóricos e Aplicações Ambientais; Imprensa Universitária: Fortaleza, 2014. [Link] accessed in July 2025 9. Romera, E.; González, F.; Ballester, A.; Blázquez, M. L.; Muñoz, J. A.; Crit. Rev. Biotechnol. 2006, 26, 223. [Crossref] 10. Brooks, R. R. In Plants and the Chemical Elements; Farago, M. E., ed.; Wiley-VCH: Weinheim, 1994, ch. 4. [Crossref] 11. Ismail, I.; Moustafa, T. In Heavy Metals - Sources, Toxicity and Remediation Techniques; Pathania, D., ed.; Nova Science Publishers Inc.: New York, 2016, p. 131. [Link] accessed in July 2025 12. Dixit, R.; Wasiullah; Malaviya, D.; Pandiyan, K.; Singh, U. B.; Sahu, A.; Shukla, R.; Singh, B. P.; Rai, J. P.; Sharma, P. K.; Lade, H.; Paul, D.; Sustainability 2015, 7, 2189. [Crossref] 13. Akhtar, N.; Iqbal, J.; Iqbal, M.; J. Hazard. Mater. 2004, 108, 85. [Crossref] 14. Akhtar, N.; Iqbal, M.; Zafar, S. I.; Iqbal, J.; J. Environ. Sci. 2008, 20, 231. [Crossref] 15. Heidelmann, G.; Roldão, T.; Egler, S.; Nascimento, M.; Giese, E.; HOLOS 2017, 6, 170. [Crossref] 16. Taiwan Patent No. 183428. 17. Jaeschke, D. P.; Merlo, E. A.; Mercali, G. D.; Rech, R.; Marczak, L. D. F.; Int. J. Food Sci. Technol. 2019, 54, 396. [Crossref] 18. Kochem, L.; Fré, N.; Redaelli, C.; Rech, R.; Marcilio, N.; Chem. Eng. Technol. 2014, 37, 59. [Crossref] 19. American Association of Cereal Chemists; Approved Methods of the American Association of Cereal Chemists, 9th ed.; Cereals & Grains Assn: Minessota, 1995. [Link] accessed in July 2025 20. González López, C. V.; García, M. C. C.; Fernández, F. G. A.; Bustos, C. S.; Chisti, Y.; Sevilla, J. M. F.; Bioresour. Technol. 2010, 101, 7587. [Crossref] 21. Bligh, E. G.; Dyer, W. J.; Can. J. Biochem. Physiol. 1959, 37, 911. [Crossref] 22. Pereira Neto, A.; Bretz, J. S.; Magalhães, F. S.; Mansur, M. B.; Rocha, S. D. F.; Eng. Sanit. Ambiental 2008, 13, 263. [Crossref] 23. Husain, A.; Javed, I.; Khan, N. A.; J. Chem. Pharm. Res. 2014, 6, 622. [Link] accessed in July 2025 24. Boukhlifi, F. In Recent Advancements in the Metallurgical Engineering and Electrodeposition; Al-Naib, U. B.; Vikraman, D.; Karuppasamy, K., eds.; IntechOpen: London, 2020, ch. 7. [Crossref] 25. Jahan, M. A. A.; Akhtar, N.; Khan, N. M. S.; Roy, C. K.; Islam, R.; Nurunnabi, M.; Bangladesh J. Sci. Ind. Res. 2014, 49, 233. [Link] accessed in July 2025 26. Companhia Ambiental do Estado de São Paulo (CETESB); Guia Técnico Ambiental de Curtumes, 2a ed.; CETESB: São Paulo, 2015. [Link] accessed in July 2025 27. Diprat, A. B.; Thys, R. C. S.; Rodrigues, E.; Rech, R.; LWT 2020, 134, 109974. [Crossref] 28. Diprat, A. B.; Menegol, T.; Boelter, J. F.; Zmozinski, A.; Vale, M. G. R.; Rodrigues, E.; Rech, R.; J. Sci. Food Agric. 2017, 97, 3463. [Crossref] 29. Azaman, S. N. A.; Nagao, N.; Yusoff, F. M.; Tan, S. W.; Yeap, S. K.; PeerJ 2017, 5, e3473. [Crossref] 30. Kim, K. M.; Kang, N. S.; Jang, H. S.; Park, J. S.; Jeon, B. H.; Hong, J. W.; Journal of Marine Bioscience and Biotechnology 2017, 9, 22. [Crossref] 31. Xiao, R.; Zheng, Y.; Biotechnol. Adv. 2016, 34, 1225. [Crossref] 32. Wanees, S.; Ahmed, A.; Adam, M.; Mohamed, M.; Asian J. Chem. 2013, 25, 8245. [Crossref] 33. Embaby, M. A.; Haggag, E. A.; El-Sheikh, A. S.; Marrez, D. A.; Environ. Sci. Pollut. Res. 2022, 29, 58388. [Crossref] 34. Tattibayeva, Z.; Tazhibayeva, S.; Kujawski, W.; Zayadan, B.; Musabekov, K.; Heliyon 2022, 8, e10468. [Crossref] 35. Mehta, S. K.; Gaur, J. P.; Crit. Rev. Biotechnol. 2005, 25, 113. [Crossref] 36. Petersen, F.; Aldrich, C.; Esau, A.; Qi, B. C.; Report to the Water Research Commission on the Project "Removal of Heavy Metals from Water by Use of Biomaterials", 2005. [Link] accessed in July 2025 37. Monteiro, C. M.; Castro, P. M. L.; Malcata, F. X.; Biotechnol. Prog. 2012, 28, 299. [Crossref] 38. Pal, A.; Paul, A. K.; Indian J. Microbiol. 2008, 48, 49. [Crossref] 39. Omar, H. H.; Int. Biodeterior. Biodegrad. 2002, 50, 95. [Crossref] 40. Kohout, M.; Savin, A.; Int. J. Quantum Chem. 1996, 60, 875. [Crossref] 41. Schneider, I. A. H.: Biossorção de Metais Pesados com a Biomassa de Macrófitos Aquáticos; Tese de Doutorado, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brasil, 1995. [Link] accessed in July 2025 42. Liang, S.; Kang, Y.; Zeng, L.; Qin, Y.; Wang, L.; Zhang, Q.; Luo, J.; Pol. J. Environ. Stud. 2017, 26, 1139. [Crossref] 43. Petrovič, A.; Simonič, M.; Int. J. Environ. Sci. Technol. 2016, 13, 1761. [Crossref] 44. Michalak, I.; Chojnacka, K.; Witek-Krowiak, A.; Appl. Biochem. Biotechnol. 2013, 170, 1389. [Crossref] 45. Michalak, I.; Dmytryk, A.; Wieczorek, P. P.; Rój, E.; Łęska, B.; Górka, B.; Messyasz, B.; Lipok, J.; Mikulewicz, M.; Wilk, R.; Schroeder, G.; Chojnacka, K.; J. Chem. 2015, 2015, 597140. [Crossref] 46. Hegde, S. M.; Babu, R. L.; Vijayalakshmi, E.; Patil, R. H.; Kumar, M. N.; Kumar, K. M. K.; Nagesh, R.; Kavya, K.; Sharma, S. C.; Desalin. Water Treat. 2016, 57, 8504. [Crossref] 47. Bayuo, J.; Rwiza, M. J.; Choi, J. W.; Mtei, K. M.; Hosseini-Bandegharaei, A.; Sillanpää, M.; Adv. Colloid Interface Sci. 2024, 329, 103196. [Crossref] 48. Paranjape, P.; Sadgir, P.; Water Conservation Science and Engineering 2023, 8, 9. [Crossref] 49. Baskar, A. V; Bolan, N.; Hoang, S. A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L. P.; Singh, G.; Vinu, A.; Sci. Total Environ. 2022, 822, 153555. [Crossref] 50. Faheem, M.; Hassan, M. A.; Du, J.; Wang, B.; Sep. Purif. Technol. 2024, 354, 128907. [Crossref]
Associate Editor handled this article: Cassiana C. Montagner |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access