Revisão
| Brazilian scientific contributions to antimycobacterial natural products: potential of inspiring novel anti-tuberculosis agents development |
|
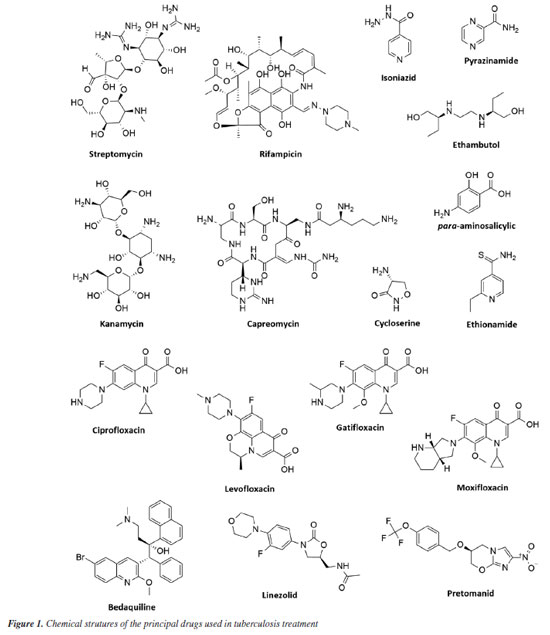
Marlon H. de Araujo; Natalie G. R. Ximenes; João V. R. Reis; Igor F. O. Ramos; Michelle Frazão Muzitano* Laboratório de Produtos Bioativos, Instituto de Ciências Farmacêuticas, Universidade Federal do Rio de Janeiro, 27930-560 Macaé - RJ, Brasil Received: 03/01/2025 *e-mail: mfmuzitano@macae.ufrj.br Tuberculosis, caused by Mycobacterium tuberculosis, is the leading cause of death globally from an infectious agent. The search for new antituberculosis compounds is vital for developing effective drugs that reduce treatment duration and side effects, particularly for multidrug-resistant (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB). Natural products from plants, marine organisms, microorganisms, and animals have demonstrated antimycobacterial properties. Brazilian research on natural products is crucial, especially concerning antituberculosis activity, as Brazil is among the nations with the highest TB burden and possesses immense biodiversity. This study reviewed Brazilian research on antimycobacterial natural products, identifying promising compounds and extracts for antituberculosis studies. In summary, the most promising extracts reported were obtained from the plants Peschiera affinis, Plathymenia foliosa, Pouteria filipes, and Psychotria vellosiana, and the cyanobacterium Microcystis aeruginosa, showing minimum inhibitory concentration (MIC) values ≤ 1 µg mL-1. Among the isolated natural compounds, the alkaloid piperovatine (MIC 7.8 µg mL-1) and the neolignan eupomatenoid-5 (MIC 1.9 µg mL-1) stood out. Considering the results compiled and discussed in the present review, it is important to highlight the need to continue the studies with the most promising extracts, in order to identify the antimycobacterial compounds responsible for their activity. In the case of piperovatin and eupomatenoid-5, it is essential to perform advanced studies to complement the preclinical results, characterizing them as new candidates for antituberculosis drug development. INTRODUCTION Human tuberculosis (TB) is a granulomatous infectious disease that is typically caused by Mycobacterium tuberculosis but may also occasionally be due to other species of Mycobacterium, such as M. bovis and M. africanum.1 They are facultative intracellular and highly aerobic bacteria that multiply within macrophages, dendritic cells, and other cell types, typically establishing infection in the lungs.2,3 These are the main organs affected, but other manifestations may also occur in organs such as the intestines, kidneys, bones, nervous system, and other areas of the body. The inhalation of aerosol droplets containing the infectious bacillus from patients with active pulmonary TB is the main mode of transmission.1 Despite advances in research regarding its characteristics, diagnosis, and treatment, TB affects a significant portion of the population in underdeveloped and developing countries. It continues to pose an important public health challenge in several regions of the world, partly due to the rising incidence rates of multi- and extensively drug-resistant strains. The latest report by the World Health Organization (WHO)4 estimated that approximately 10.8 million cases of TB occurred worldwide in 2023, with 1.25 million people dying from this disease in the same year. In Brazil, the incidence in 2023 was estimated at 80,012 new cases, with a mortality rate of two deaths per 100,000 inhabitants in 2022.5 Brazil remains among the 30 countries with the most TB cases, accounting for over 80% of all TB cases worldwide. In addition, according to the WHO,4 Brazil is among the 48 priority countries, as it is considered one of the countries with the highest number of cases of the disease in the world, as well as one of the countries with the highest number of cases of TB-HIV (human immunodeficiency virus) co-infection. TB is the leading cause of death in patients with HIV and the main cause of deaths due to an infectious agent.5 TB treatment is based on identifying the pathogen and administering antibiotics for a prolonged period. The conventional treatment recommended by the World Health Organization for new cases of susceptible TB is quite adequate, leading to the cure of 85% of patients. However, this cure rate drops to 55% for cases of multidrug-resistant tuberculosis (MDR-TB), which also has a longer duration and more adverse effects.4 The challenges for effective TB control are extensive, highlighting the need to search for and develop new drugs. In this context, many natural products are used in folk medicine and as an approach for biological studies, which have shown significant invitro activity against M. tuberculosis. This activity has been attributed to compounds obtained mainly from plants, but other organisms, such as fungi and marine organisms, have also contributed significantly to this activity.6 In Brazil, a combined approach of ethnobotany and chemotaxonomy has revealed bioactive species of specific genera, resulting in the discovery of new compounds with interesting pharmacological properties.7 Due to the potential of using natural products as an efficient strategy for treating tuberculosis, a bibliographic search was conducted to gather and discuss studies by Brazilian research groups that have presented extracts and/or compounds with antimycobacterial activity, focusing on tuberculosis, whether this activity has been experimentally proven or of ethnopharmacological origin. In summary, this approach is justified by the high incidence of TB in Brazil and its enormous genetic biodiversity, highlighting the interest in systematizing the contribution of the national scientific community in this area of research, which is highly relevant to both Brazilian and global public health. Tuberculosis Mycobacterium sp. Mycobacteria are straight or slightly curved, non-motile, non-sporulating, non-encapsulated bacilli that measure 1 to 10 µm in length by 0.2 to 0.6 µm in width. They are facultative pathogens, with an emphasis on their intracellular life cycle. They are highly aerobic, with an optimum growth temperature range of 35-37 ºC.8 Slow growth and resistance to discoloration by alcohol and acids are characteristics associated with the high levels of lipids, primarily mycolic acids, in the mycobacterial cell wall. These lipids account for approximately 50% of the mycobacterial dry weight and are covalently linked to arabinogalactans. Other cell wall components include mannosylated lipoarabinomannans (Man-LAM), lipomannans (LM), and mannoglycoproteins. The thick layer of mycolic acids forms a hydrophobic barrier and, although it hinders the entry of nutrients, confers cellular antibiotic resistance, as well as resistance to enzymatic degradation and discoloration processes.9,10 The genus Mycobacterium comprises more than 120 species of bacteria, most non-pathogenic and found in the environment. However, some species are highly pathogenic, including M. leprae, the causative agent of leprosy, M. ulcerans, which causes Buruli ulcers, and M. tuberculosis, which, as previously mentioned, causes tuberculosis. The species that comprise the Mycobacterium tuberculosis complex include M. tuberculosis, M. bovis, M. africanum, M. canettii, M. caprae, M. microti, M. orygis, and M. pinnipedii.11,12 M. tuberculosis infection has been present since ancient times. Pathological signs of tuberculosis have been discovered in the spinal cords of Egyptian mummies.13 Initially, tuberculosis was thought to be a hereditary and non-contagious disease.14,15 However, in 1882, Robert Koch discovered the microscopic organism responsible for tuberculosis, revealing that it could be transmitted from person to person. Later, in 1908, French bacteriologists Jean-Marie Camille Guerin and Albert Calmette developed the Bacillus Calmette-Guérin (BCG) vaccine.13 Infection occurs through the airborne route by inhaling droplets that contain the infectious bacillus. Following the physical barriers, the bronchial epithelium serves as the host's line of defense, capable of producing antimicrobial peptides with a broad spectrum of activity.16 Upon inhalation and infection, the bacillus is phagocytosed by antigen-presenting cells (APCs) in the lung, such as alveolar and parenchymal macrophages and dendritic cells (DC). Consequently, the bacilli can be eliminated by immune system cells, enter a latent state, or trigger a productive infection characterized by bacterial multiplication and evasion of the immune system, evolving into active disease.17 Severe pathological lesions in the lungs and other affected tissues can result in death, particularly in cases of immunosuppression or infection by hypervirulent or treatment-resistant strains.18 Challenges Due to the rising occurrence of drug-resistant strains and patients co-infected with HIV, the current TB situation is becoming more alarming. These factors undermine host defenses and enable the reactivation of latent infections or increase the susceptibility of the individual to the disease.19,20 These challenges to effective TB control, combined with the variable protection offered by the BCG vaccine, highlight the urgent need to discover and develop more potent and less toxic compounds. Ideally, these new treatments should shorten the duration needed for a lasting cure.21 There are cases of the disease on all continents. Consequently, the facilitated flow of people around the world, including the movement of human labor between different countries, may contribute to the spread of various strains across regions, creating a challenge for global tuberculosis control. Multidrug-resistant tuberculosis (MDR-TB) poses a significant challenge. This issue typically arises from inadequate or incomplete treatment or non-adherence to treatment by the patients. These factors can also contribute to the emergence of even more resistant strains, known as extensively drug-resistant (XDR-TB).22 These forms of resistant strains continue to pose a public health crisis and can indicate a chronic disease, with insufficient diagnosis and treatment in most cases, making reporting a challenge. In 2023, it was estimated that 400,000 people developed rifampicin-resistant tuberculosis (RR-TB), a primary option in first-line treatment, and of these, 68% had cases of MDR-TB.4 People with risk factors such as malnutrition, diabetes, smoking, and alcohol consumption may also face higher rates of developing tuberculosis. Co-infection cases also present a significant challenge and have been frequently reported. For example, infection with M. tuberculosis in individuals with HIV increases the likelihood of developing active tuberculosis. Among the 1.25 million TB deaths expected in 2023, 161,000 (12%) occurred in HIV-infected individuals.4 Recent years have seen advances in new drugs aimed at overcoming these challenges and improving TB treatment. However, reported strains resistant to these compounds indicate that bacteria are continually been selected and evolving, highlighting the need to develop new drugs for tuberculosis treatment.23 Treatment The first effective antibiotic for tuberculosis was streptomycin (STM), introduced to the market in 1944. Following that, during the 1940s and 1950s, when a cure for tuberculosis was deemed feasible, drugs such as pyrazinamide (PZA), rifampicin (RIF), isoniazid (INH), and ethambutol (EMB) (Figure 1), along with streptomycin, were introduced as front-line drug cocktails for the treatment of tuberculosis.13,24 Today, PZA, RIF, INH, and EMB make up the first-line anti-TB treatment. Although in 2012 the US Food and Drug Administration (FDA)25 approved the drug bedaquiline for tuberculosis treatment (and MDR-TB), treatments are still based on various combinations of the mentioned drugs, adapting them to the new challenges faced by tuberculosis patients.
Although TB patients can be cured with a drug cocktail, treatment is extensive, lasting at least 6 months using first-line drugs (combinations of RIF, INH, EMB, and PZA for 2 months, followed by a continuous phase with RIF and INH for 4 months). In cases of MDR-TB, at least 9 months are required using second-line drug combinations (para-aminosalicylic acid, kanamycin, fluoroquinolones [ciprofloxacin, levofloxacin, moxifloxacin, gatifloxacin], capreomycin, ethionamide, cycloserine) (Figure 1).4,26 Treatment costs also vary considerably; in cases of susceptible TB, the estimated cost is US$ 40 per person. For RR-TB and MDR-TB, it can exceed US$ 1,000, and the drugs are more toxic.4 Two regimens have recently been approved and introduced in Brazil to shorten the treatment of drug-resistant tuberculosis: BPaL (bedaquiline, pretomanid, and linezolid) and BPaLM (bedaquiline, pretomanid, linezolid, and moxifloxacin) (Figure 1). It is worth noting that both are recommended by the WHO.27 Currently, there are 20 anti-tuberculosis drugs in clinical trials and 12 vaccines in phase I, phase II, or phase III trials.4 However, the number of antimycobacterial candidate compounds selected in clinical trials that advance to the registration stage is even smaller, mainly due to toxic effects. This reinforces the need to develop new, more effective drugs with fewer adverse effects. Natural products play an essential role in the search for new antibacterials. The high chemical diversity is one of the reasons for the success of natural products as a strategy in the search for new antibacterial drugs.28 Natural products Natural products, primarily derived from plant species, have formed the basis of traditional medicine, which has existed for thousands of years, and continue to provide new drugs to humanity today. The first records, written on clay tablets in cuneiform script, date back to around 2600 BC (before Christ) and originate from Mesopotamia. The Ebers Papyrus, discovered in 1873 and dated to approximately 1555 BC, is considered one of the oldest pharmacopoeias. In its introduction, it states: "Here begins the book on the preparation of remedies for all parts of the body."29,30 According to the WHO,31 traditional medicine is widely used and part of an economically significant and rapidly growing health system. Approximately 40% of the population of China utilizes traditional medicine as a form of medical care. In Africa, traditional medicine accounts for up to 80% of healthcare. In developed countries such as Australia, Canada, United States, Belgium, and France, this practice has become increasingly popular. In Asia and Latin America, populations continue to use it due to historical circumstances and cultural beliefs. Interest in nature as a source of potential therapeutic agents continues. The contribution of natural products and their derivatives is indispensable in the discovery and development of effective drugs for treating various diseases that afflict humanity. Natural products and their derivatives represent more than 50% of the drugs in clinical use globally, with the contribution of higher plants accounting for at least 25% of the total.29 Furthermore, from 1981 to 2019, 52.7% of small molecules with anti-infective activity (bacterial, fungal, parasitic, and viral) were natural or directly originated from natural products.28 This further underscores the potential of natural products as a strategic source for the discovery of novel antibacterial agents and, potentially, new therapeutics for the treatment of TB. The scientific literature encompasses several studies on compounds of natural origin that are active against Mycobacterium sp. This activity has been attributed to compounds from different chemical classes, among the most promising being alkaloids, terpenoids, and polyphenols. However, the optimization and development stages for a new drug candidate are long and complex.6,32 However, this complexity should not become an obstacle to the continuity of research, as natural products continue to provide prototypes for developing drugs with promising antimycobacterial activity, whether for resistant strains or not.33 Above all, traditional knowledge about medicinal plants is often one of the few therapeutic resources available to specific communities. In less favored regions of Brazil, and even in large cities, medicinal products of natural origin are easily found for personal consumption.34 In this context, the registration of medicinal species from each region of Brazil, their traditional uses, and their therapeutic forms plays a significant role in the advancement of basic and applied studies and may lead to the discovery of new, promising drug candidates.35 The importance of Brazilian biodiversity Natural products continue to contribute significantly to the search for antimycobacterial compounds and, consequently, to the search for new medicines. This makes the biodiversity of Brazil a promising target for research and development of new drugs. The Convention on Biological Diversity (CBD),36 which was opened for signature at the Earth Summit in Rio de Janeiro in 1992, aims to promote the conservation, sustainable use, facilitated access to, and equitable sharing of benefits arising from the utilization of genetic resources. As part of this broad objective, Article 8(j) of the CBD calls explicitly on its members to "respect, preserve and maintain the knowledge, innovations and practices of indigenous and local communities of traditional lifeways relevant to the conservation and sustainable use of biological diversity, and to promote their wide application with the approval and participation of holders of such knowledge, innovations and practices, and to encourage the sharing of benefits arising from the utilization of such knowledge, innovations and practices". More than 187 countries (with the notable exception of the United States) have ratified the CBD.36 In a biologically rich country, with its broad spectrum of species and rich ethnic and cultural heritage, research on medicinal plants is fundamental for preserving the traditional knowledge of the communities involved and developing new therapeutic strategies. Finally, studied consciously, the great Brazilian diversity can be attractive to pharmaceutical companies. Therefore, legal approaches should also be considered to avoid the loss of different resources and the extinction of germplasm. According to Gottlieb and Borin37 this is essential because "any lasting social benefit depends not only on the adequate exploitation of our biodiversity, but also on how it is exploited, with the objective of self-sustainable development, with preservation".
METHODOLOGY Search correlating natural products and tuberculosis in Brazil The search was conducted using the scientific database provided by ScienceDirect38 and the Web of Science39 database provided by Clarivate. Combinations of the most common terms related to tuberculosis were used, including "tuberculosis", "antimycobacterial", "Mycobacterium" and "antitubercular", followed by "natural products". To select natural products studied by Brazilian scientific groups, the affiliation was restricted to Brazil, and a filtering by only Research articles was applied. No restrictions were made regarding the year or the sciences. In a search using ScienceDirect, a total of 1804 articles were identified, of which 1318 were repeated across searches with different terms. In addition, 425 articles were excluded that were related to other Mycobacterium species (not related to tuberculosis) or to sub-themes such as: morphology, immunology, genetics, agriculture, economy, and others, which did not present natural products with biological activity directed at tuberculosis. Articles related to compounds obtained by organic synthesis were also excluded, restricting the review to natural products only. Thus, 58 articles were selected from the search through this database. Four hundred eighty-four articles were found in the Web of Science search, of which 387 were repeated between searches. Forty-one articles were excluded based on the abovementioned criteria, resulting in 56 articles. Of these, 21 were duplicates of articles selected through ScienceDirect, resulting in 35 new articles selected through this second database. Thus, a total of 93 articles were used to prepare this review on natural products studied by Brazilian research groups with antimycobacterial activity, either through proven inhibition in vitro or in vivo (Tables 1, 2, 5, and 6) or through knowledge of ethnopharmacological action (Tables 3 and 4).
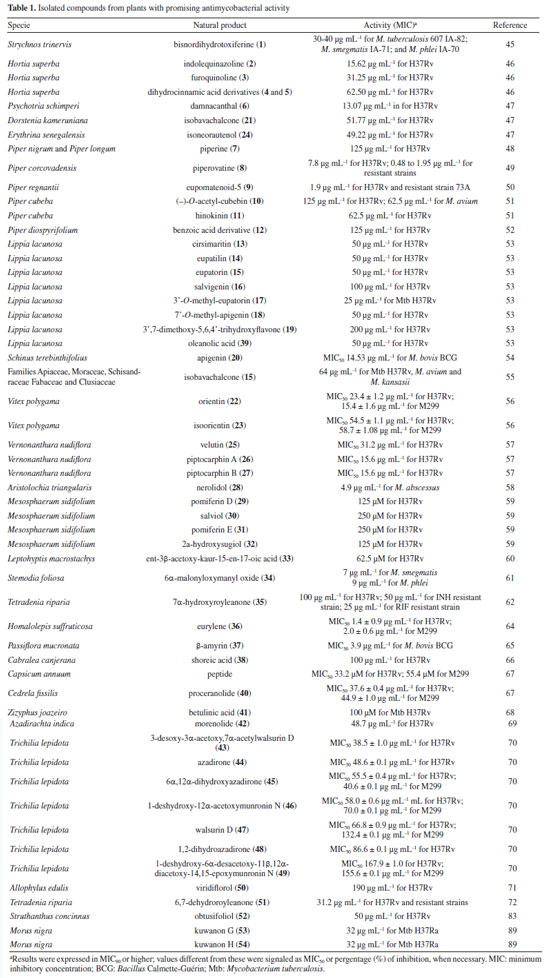
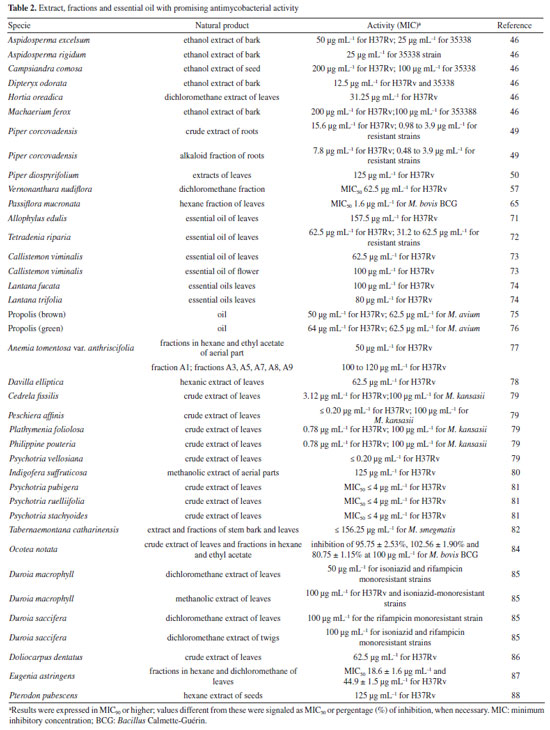
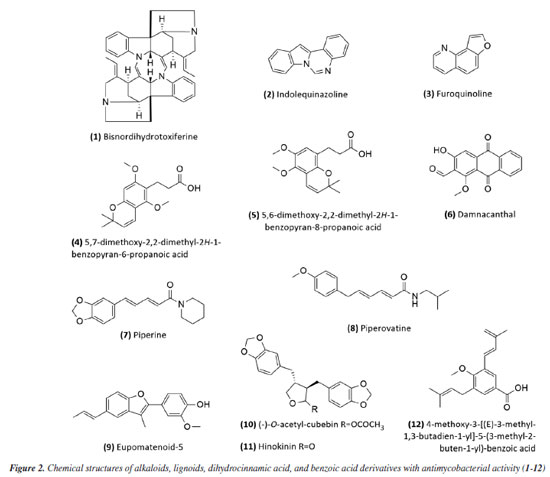
RESULTS Research into natural products in Brazil as a strategy for treating tuberculosis Natural products have gradually had a significant impact on human health, and various classes of natural products have been isolated and characterized. The production of secondary metabolites with distinct biological activities has further established natural products as a source of health products and structural models for discovering new therapeutic compounds actions.40 Natural compounds and extracts from plants, marine organisms, microorganisms, animals, and other natural sources have demonstrated inhibitory activity against M. tuberculosis.6,32,33 This supports the potential of natural products to inhibit mycobacterial growth, as data indicate promising activity from various sources, species, and compounds in experimental studies and research involving traditional knowledge of using natural products for tuberculosis treatment. This establishes a foundation for future experimental research to enhance treatment for tuberculosis patients. Several reviews regarding natural products and tuberculosis published in reputable journals were examined to establish criteria for defining promising antimycobacterial natural products. Among them, only three detailed the requirements adopted. García et al.41 considered compounds with minimum inhibitory concentration (MIC) values ≤ 60 μg mL-1 to be promising, while Copp and Pearce6 used a ≤ 64 μg mL-1 threshold. Okunade et al.32 set a higher limit of ≤ 200 μg mL-1. Defining a universal parameter is challenging for extracts, but some authors suggested a promising MIC of ≤ 100 μg mL-1.37,42,43 To be more inclusive in this study, we will consider samples with MIC values ≤ 200 μg mL-1 as exhibiting antimycobacterial activity. Table 1 summarizes the data for isolated compounds, while Table 2 presents the data for extracts, fractions, and essential oils. Studies based on isolated compounds and extracts from plant species The use of plant species is one of the oldest forms of treatment for health problems. Approximately 60% of the population of the world still depends on medicinal plants for primary health care.44 Although Brazilian biodiversity contributes significantly to the search for new bioactive compounds, few species have been thoroughly investigated for their medicinal properties. This fact highlights the need for research in this area, which will enable the sustainable use of plant species as a resource for treating various diseases. For decades, Brazilian researchers have evaluated the antimycobacterial activity of plant species and the compounds isolated from them. This review presents these studies, organizing them according to the chemical class and natural source. Compounds Alkaloids Initially, Melo et al.45 evaluated the activity of the alkaloid bisnordihydrotoxiferine (1) (Figure 2) extracted from the roots of Strychnos trinervis (Loganiaceae) against various bacteria, including three species of Mycobacterium, among which M. tuberculosis was inhibited in its growth by the present alkaloid, with a MIC of 30-40 µg mL-1.
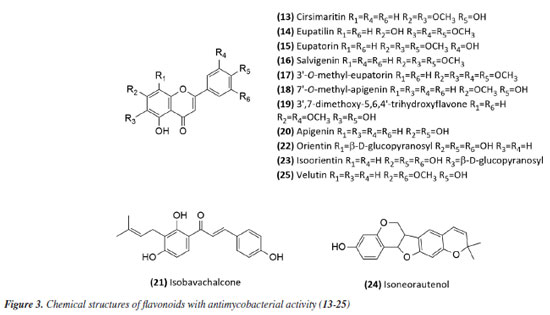
Then, continuing in the alkaloids class, Severino et al.46 investigated the antimycobacterial activity of several natural products extracted from Hortia species. The alkaloids indolequinazoline (2) and furoquinoline (3) (Figure 2) were isolated from leaves and stems of H. oreadica, H. superba, and H. brasiliana (Rutaceae). Compounds 2 and 3 had a MIC of 15.62 and 31.25 µg mL-1, respectively. Isolated from these species, dihydrocinnamic acid derivatives (4 and 5) (Figure 2) had a MIC of 62.50 µg mL-1 against the H37Rv strain of M. tuberculosis. These compounds were kept together with the alkaloids, considering the same plant source and the higher activity observed for the alkaloids. The dichloromethane extract of Hortia oreadica also showed significant activity against M. tuberculosis H37Rv with a MIC of 31.25 μg mL-1.46 The results suggest that the structure of the alkaloids may be crucial for their antimycobacterial activity, especially the indole and quinoline skeletons. These constituents have great potential for developing new antimycobacterial agents, offering a less toxic alternative to synthetic drugs. Pollo et al.47 evaluated 52 compounds against the reference strain H37Rv, and damnacanthal (6), an alkaloid isolated from Psychotria schimperi, was the most active compound in the screening, with a MIC of 13.07 µg mL-1. Piperine (7) (Figure 2), an alkaloid found in Piper nigrum and Piper longum (Piperaceae), was evaluated for its antimycobacterial activity against M. tuberculosis strains, yielding MIC values ranging from 31.2 to 125 μg mL-1. These values were reduced when the piperine alkaloid was combined with rifampicin and streptomycin, and the MIC values of the combinations also suggested a synergistic effect against the susceptible strain H37Rv and clinical isolates evaluated.48 Following studies on the genus Piper and alkaloids, the crude extract of the roots of Piper corcovadensis (Piperaceae), its alkaloid fraction, and an isolated isobutylamide, piperovatine (8) (Figure 2), showed MIC values of 15.6, 7.8, and 7.8 μg mL-1, respectively, against the M. tuberculosis H37Rv strain. These samples were also evaluated against clinical isolates, with MIC values of 0.98 to 3.9 μg mL-1 for the extract, MIC of 0.48 to 3.9 μg mL-1 for the fraction, and MIC of 0.48 to 1.95 μg mL-1 for piperovatine. The samples did not show cytotoxic effects when evaluated in the active concentrations and indicated a synergistic effect when assessed in combination with rifampicin.49 A summary of the MIC values presented in this sub-section is provided in Table 1. Lignoids Alkaloids were not the only class reported for Piper species as promising antimycobacterial compounds. Scodro et al.50 isolated three neolignans from Piper regnellii (Piperaceae) and obtained five derived compounds. Among the neolignans evaluated against M. tuberculosis, the isolated compound eupomatenoid-5 (9) (Figure 2) was the most active, presenting a MIC of 1.9 µg mL-1 against the H37Rv strain and the 73A strain (resistant to isoniazid, rifampicin, and pyrazinamide). Additionally, it exhibited a selectivity index (SI) of 20 and was suggested by the group as a potential candidate for future anti-tuberculosis drugs. The lignan cubebin was isolated from seeds of Piper cubeba L. (Piperaceae), and its derivatives were analyzed against different Mycobacterium species, including M. tuberculosis, M. kansasii, and M. avium. The two compounds (-)-O-acetyl-cubebin (10) and hinokinin (11) (Figure 2) showed activity with a MIC of 62.5 and 125 μg mL-1, respectively, against the M. tuberculosis strain H37Rv.51 The results suggest the potential of these lignans as candidates for the development of new antimycobacterial agents, particularly in the context of increasing drug resistance in tuberculosis treatment.51 Table 1 provides a summary of the MIC values presented in this sub-section. Benzoic acid derivatives Leaf extracts of another Piper species, Piper diospyrifolium, obtained by supercritical fluid extraction, and a novel benzoic acid derivative: 4-methoxy-3-[(E)-3-methyl-1,3-butadien-1-yl]-5-(3-methyl-2-buten-1-yl)-benzoic acid (12) (Figure 2) were evaluated against the M. tuberculosis H37Rv strain and eight other clinical isolates of M. tuberculosis. The benzoic acid derivative showed a MIC of 125 μg mL-1 for the H37Rv strain (Table 1), as well as its extracts (Table 2); for the clinical isolates, the activity was ≥ 250 μg mL-1, suggesting low activity for these strains.52 It can be inferred that the evaluated Piper species contain active compounds and are promising candidates for the development of anti-TB drugs. However, further studies are needed to confirm their in vivo efficacy and possible mechanisms of action. Flavonoids For the flavonoid class, in 2011,53 seven methoxyflavones named cirsimaritin (13), eupatilin (14), eupatorin (15), salvigenin (16), 3'-O-methyl-eupatorin (17), 7'-O-methyl-apigenin (18), and 3',7-dimethoxy-5,6,4'-trihydroxyflavone (19) were isolated from the dichloromethane extract of Lippia lacunosa (Verbenaceae). All compounds were evaluated for their antimycobacterial activity in M. tuberculosis culture and showed MIC values ranging from 50 to 200 µg mL-1. Except for the tetramethoxylated flavone (3'-O-methyl-eupatorin) (17) (Figure 3), which showed better activity with a MIC value of 25 µg mL-1, compared to the other six methoxyflavones.
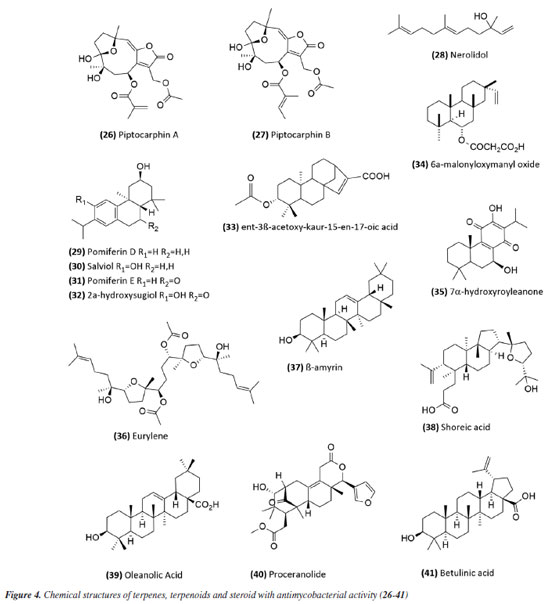
In 2014,54 the extract, fraction, and isolated apigenin (20) (Figure 3) from the fruits of Schinus terebinthifolius (Anacardiaceae) were evaluated against M. bovis BCG. The compound (MIC50 14.53 ± 1.25 μg mL-1) exhibited greater activity than the fraction (MIC50 108.5 ± 1.05 μg mL-1) from which it was derived, and this was more active than the methanolic extract (MIC50 279.5 ± 1.14 μg mL-1). This suggests that although the extract was considered slightly active or inactive, the fractionation process to obtain the active compound was successful. The chalcone isobavachalcone (21) (Figure 3), isolated from the Dorstenia kameruniana had a MIC of 51.77 μg mL-1 against the reference strain H37Rv.47 It was also reported against M. avium, and M. kansasii, with a MIC of 64 µg mL-1.55 Isobavachalcone is a natural prenylated chalcone with a broad spectrum of pharmacological properties, found in plants of the Fabaceae, Clusiaceae, Moraceae, Schisandraceae, and Apiaceae families.55 In a study56 of natural products from Vitexpolygama Cham (Lamiaceae), orientin (22) and isoorientin (23) (Figure 3) were isolated from the dichloromethane fraction, and presented MIC50 values against M. tuberculosis H37Rv of 23.3 ± 1.2 and 54.5 ± 1.2 μg mL-1, and against M299 strain of 15.4 ± 1.6 and 58.7 ± 1.2 μg mL-1, respectively. In addition, the pterocarpan, that is a special type of isoflavonoid, isoneorautenol (24) (Figure 3), isolated from the Erythrina senegalensis, also exhibited antimycobacterial activity, with MIC of 49.22 μg mL-1.47 The MIC values presented in this sub-section are summarized in Table 1. Terpenes and terpenoids One of the most studied plant secondary metabolites regarding antimycobacterial activity is the terpenes and terpenoids. The studies carried out by Brazilian groups involving this class are presented in this topic. The extract of the flowers of Vernonanthura nudiflora (Asteraceae), its fractions in hexane, dichloromethane, ethyl acetate, butanol, and methanol, the subfraction consisting of the dimethoxyflavone velutin (25) (Figure 3), and the sesquiterpene lactones piptocarphin A (26) and B (27) (Figure 4), were evaluated against the H37Rv strain of M. tuberculosis. Among these, the dichloromethane fraction stood out, with a MIC50 of 62.5 µg mL-1, velutin (25) with a MIC50 of 31.2 µg mL-1, and the fraction consisting of piptocarphin A and B (26 and 27) with a MIC50 of 15.6 µg mL-1.57 Despite being a flavonoid, velutin was kept together with the sesquiterpene lactones, considering its contribution to the antimycobacterial activity of the extract.
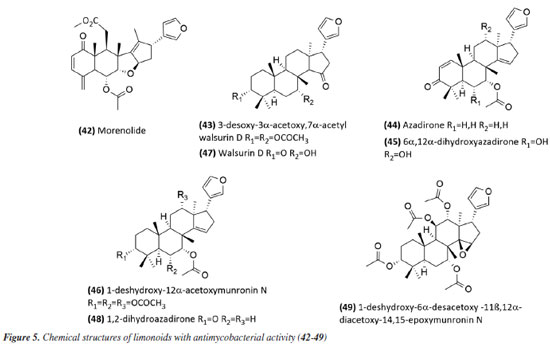
The species Aristolochia triangularis (Aristolochiaceae) collected in the region of Santa Maria, RS, Brazil, was also able to inhibit the mycobacterial growth of three different strains of Mycobacterium abscessus, M. fortuitum, and M. massiliense. Its samples: extract, fractions, and three isolated compounds presented MIC of 1 to 165.6 μg mL-1, with the compound nerolidol (28) (Figure 4) indicated as the most active with a MIC of 4.9 μg mL-1 against M. abscessus.58 In the study59 using the aerial parts of Mesosphaerum sidifolium to obtain extracts, four diterpenes were isolated and initially identified through insilico analysis. The compounds pomiferin D (29), salviol (30), pomiferin E (31), and 2a-hydroxysugiol (32) (Figure 4) demonstrated in vitro antituberculosis activity against M. tuberculosis H37Rv, with MIC values of 125 µM for pomiferin D and 2a-hydroxysugiol, and 250 µM for salviol and pomiferin E. A new labdane diterpenic acid named hyptenol, together with five other compounds: erythroxylol B, oleanolic acid acetate, betulinic acid, tormentic acid, and ent-3β-acetoxy-kaur-15-en-17-oic acid, were identified as chemical constituents of Leptohyptis macrostachys (Lamiaceae). In silico evaluations were performed to predict the antimycobacterial activity, and the in vitro MIC against M. tuberculosis H37Rv was determined. The results demonstrated that ent-3β-acetoxy-kaur-15-en-17-oic acid (33) (Figure 4) showed significant activity, with a MIC of 62.5 μM.60 Another three labdane-type diterpenoids, 6α-acetoxymanyl oxide, 6α-malonyloxymanyl oxide, and 6α-malonyloxy-n-butyl ester manoyl oxide, were isolated from the aerial parts of Stemodia foliosa (Scrophulariaceae) and tested against a panel of bacteria, including Mycobacterium smegmatis and M. phlei. The diterpenoid 6α-malonyloxymanyl oxide (34) was the most promising, showing activity against these strains with MIC values of 7 and 9 µg mL-1 for M. smegmatis and M. phlei, respectively.61 A study62 with Tetradenia riparia (Lamiaceae) isolated the compound 7α-hydroxyroyleanone (35) (Figure 4). This diterpenequinone showed potent inhibition against M. tuberculosis, with a MIC of 100 μg mL-1 for H37Rv, 50 μg mL-1 for isoniazid-resistant strains, and 25 μg mL-1 for rifampicin-resistant strains. This compound was subsequently obtained selectively and efficiently through high-speed countercurrent chromatography.63 A new triterpene, named millemaronol, was isolated from Homalolepis suffruticosa (Simaroubaceae), together with ten known metabolites: chaparrinone, scopoletin, 5-methoxyanthin-6-one, eurylene, hispidol A, hispidol B, nilocytin, α-dihydronylocytin, β-dihydronylocytin, and theurilene. The compounds, except for hispidol B, β-dihydronylocytin, and theurilene, were evaluated for their antimycobacterial activity against two strains of M. tuberculosis (H37Rv and M299). Eurylene (36) (Figure 4) showed the best MIC50 value for both strains, 1.4 ± 0.9 µg mL-1 for H37Rv and 2.0 ± 0.6 µg mL-1 for M299.64 In a study65 conducted with the extract of Passiflora mucronata (Passifloraceae) leaves, collected from the Jurubatiba restinga, the hexane fraction and the isolated triterpene β-amyrin (37) (Figure 4) showed activity against M. bovis BCG, with MIC50 values of 1.6 and 3.9 μg mL-1, respectively. The authors identified this species as promising and suggested further studies, including tests in infected macrophages and in vivo evaluations using a murine model of pulmonary tuberculosis. The same study also evaluated cytotoxicity and nitric oxide (NO) production in macrophages, reporting minimal toxic effects and inhibition of NO production at active antimycobacterial concentrations. In a study conducted by Leitão et al.,66 the triterpene shoreic acid (38) (Figure 4), isolated from the dichloromethane extract of Cabraleacanjerana (Meliaceae) leaves, exhibited a MIC of 100 µg mL-1 against M. tuberculosis H37Rv. In a subsequent study, Leitão and co-authors53 reported that oleanolic acid (39) (Figure 4), a triterpene isolated from the dichloromethane extract of the leaves of Lippia lacunosa (Verbenaceae), demonstrated a MIC of 50 µg mL-1 against the same strain. Four compounds isolated from the seeds of Cedrela fissilis, the triterpene piscidinol A and the limonoids andirolide N, mexicanalide, and proceranolide were able to inhibit the growth of M. tuberculosis, with emphasis on proceranolide (40) (Figure 4) presenting MIC50 of 37.6 ± 0.4 μg mL-1 for H37Rv and 44.9 ± 1.0 μg mL-1 for M299. Andirolide N and piscidinol A also demonstrated antimycobacterial activity: MIC50 of 60.1 ± 0.3 and 49.6 ± 0.5 μg mL-1 for H37Rv, respectively, and MIC50 of 50.4 ± 1.0 and 66.0 ± 1.0 μg mL-1 for M299, respectively. However, cytotoxicity evaluation revealed toxic effects for all compounds, except proceranolide (40) (Figure 4), which presented a less toxic profile.67 Another study68 evaluated the antimicrobial potential of betulinic acid (41) (Figure 4) extracted from the bark of Zizyphus joazeiro Mart. (Rhamnaceae). This pentacyclic triterpene demonstrated significant antimicrobial potential, inhibiting the growth of several microbial species, including M. tuberculosis, with a MIC value of 100 μM against the H37Ra strain. The study also employed molecular modeling to investigate the mechanism of action, indicating that betulinic acid targets essential bacterial resistance factors, such as DNA gyrase and beta-lactamase. Furthermore, predictive models estimated an activity of 63% against M. tuberculosis, confirming the efficacy of the compound and expanding its potential application. Continuing in the terpene class, the anti-inflammatory, antimycobacterial, and cytotoxic activities of several terpenoids isolated from the roots of Azadirachta indica (Meliaceae) were evaluated. All compounds tested showed potent NO inhibition (inhibitory concentration, IC50 < 15 μg mL-1). Antimycobacterial activity was assessed against M. tuberculosis H37Rv and the hypervirulent strain M299, with limonoid morenolide (42) (Figure 5) standing out for inhibiting the growth of H37Rv (MIC50 of 48.7 μg mL-1). Although other compounds showed antimycobacterial activity against both H37Rv and M299 strains, they were highly cytotoxic.69 Ten limonoids isolated from Trichilia lepidota (Meliaceae) seeds demonstrated antimycobacterial, anti-inflammatory, and cytotoxic activity. Compounds 3-desoxy-3α-acetoxy,7α-acetylwalsurin D (43), azadirone (44), 6α,12α-dihydroxyazadirone (45), 1-deshydroxy-12α-acetoxymunronin N (46), walsurin D (47), 1,2-dihydroazadirone (48) and 1-deshydroxy-6α-desacetoxy-11β,12α-diacetoxy-14,15-epoxymunronin N (49) (Figure 5) demonstrated inhibitory effect against H37Rv (MIC50 range of 38-167 μg mL-1), while 45, 46, 47 and 49 were also effective against M299 (MIC50 of 40.6 ± 0.1, 70.0 ± 0.1, 132.4 ± 0.1 and 155.6 ± 0.1 μg mL-1, respectively). In this case, the increased resistance of the M299 strain was evidenced by the six-fold reduction in the potency of rifampicin compared to the H37Rv strain. Furthermore, the most active limonoids (45 and 46) showed low cytotoxicity and good selectivity indices. These findings reinforce the importance of the structure-activity relationship in modulating the efficacy of limonoids as candidates for the treatment of tuberculosis, especially against resistant strains.70
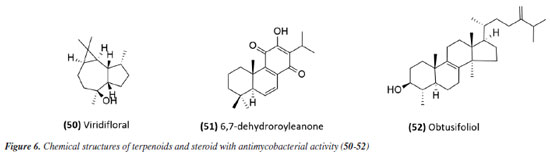
A summary of the MIC values discussed in this sub-section is provided in Table 1. Essential oils Studies using essential oils have also been conducted. The essential oil from Allophylus edulis (Sapindaceae) leaves and the isolated compound viridiflorol (50) (Figure 6) showed promising activity with MIC values of 157.5 and 190.0 μg mL-1, respectively (Tables 1 and 2).71
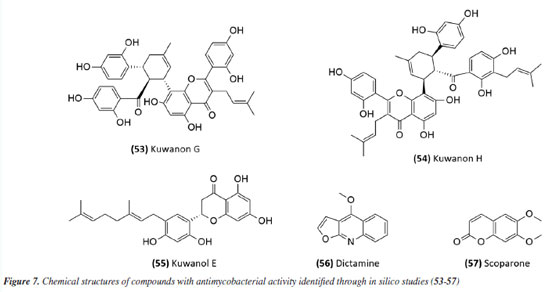
The antimycobacterial activity of the essential oil and the compound 6,7-dehydroroyleanone (51) (Figure 6) from leaves of Tetradenia riparia (Lamiaceae) against M. tuberculosis H37Rv strain and 19 other clinical isolates of M. tuberculosis (seven drug-sensitive, six isoniazid-resistant, and six MDR) suggested promising activity. The essential oil presented MIC values of 31.2 and 62.5 μg mL-1 depending on the strain (Table 2), while the isolated compound presented a MIC of 31.2 μg mL-1 against all strains evaluated (Table 1). Neither the essential oil nor the isolated compound presented significant toxic effects at the effective concentration, indicating SI values of 1.9 and 7.9, respectively.72 The essential oil extracted from the leaves and flowers of Callistemon viminalis presented a MIC of 62.5 and 100 μg mL-1, respectively, against the M. tuberculosis H37Rv strain (Table 2). Chemical analysis revealed that the principal constituent of both essential oils is 1,8-cineole (eucalyptol).73 Julião et al.74 described the antimycobacterial activity of essential oils extracted from the leaves of Lantanatrifolia L. and L. fucata (Verbenaceae), with a MIC of 80 and 100 μg mL-1, respectively, for M. tuberculosis H37Rv strain (Table 2). The composition of these oils is primarily composed of sesquiterpenes, with germacrane D being predominant in L. trifolia and β-elemene in L. fucata. In the study by Lima et al.,75 the potential of brown propolis essential oil was evaluated against the H37Rv strain of M. tuberculosis and the standard strain of M. avium. The oil presented a MIC of 50 and 62.5 μg mL-1 for the respective strains (Table 2), highlighting its potential as a source of antimycobacterial compounds. Another study76 evaluated the antimycobacterial activity of propolis. The essential oil of Brazilian green propolis presented a MIC of 64 μg mL-1 against the H37Rv strain of M.tuberculosis and a MIC of 62.5 μg mL-1 against the standard strain of M. avium (Table 2). The major constituents detected were carvacrol, acetophenone, spathulenol, (E)-nerolidol, and β-caryophyllene. The interaction between their bioactive compounds may result in synergistic effects, enhancing their antibacterial properties. The study highlights the need for in vivo investigations to elucidate the mechanisms of action and validate their therapeutic potential. The study conducted by Pinto et al.77 investigated the antimycobacterial activity of the essential oil from the leaves of Anemia tomentosa var. anthriscifolia (Anemiaceae), as well as its fractions obtained by liquid chromatography. The essential oil was characterized by gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS), where 60 compounds were identified, the main triquinane sesquiterpenes being (-)-epi-presylphiperfolan-1-ol (30.6%), silphiperfol-6-ene (14.7%), and presilphiperfolan-8-ol (8.3%). Among the 18 fractions analyzed, those containing unsaturated sesquiterpenes (A1 and A3) showed activity against M. tuberculosis H37Rv strain. Fraction A1 exhibited greater potency (MIC of 50 μg mL-1) (Table 2), suggesting that lipophilicity influenced the antimycobacterial activity. However, the fractions containing sesquiterpene alcohols (A5-A8) demonstrated MICs similar to the essential oil (MIC 100 μg mL-1) (Table 2). Fraction A18, rich in silphiperfolan-6β-ol (31.2%), was inactive against M. tuberculosis H37Rv at 100 μg mL-1. Extracts In a study78 evaluating immunological and microbiological activity, the hexane extract from the leaves of Davilla elliptica (Dilleniaceae) demonstrated significant antimycobacterial activity, with a MIC of 62.5 μg mL-1 against the H37Rv strain of M.tuberculosis. Furthermore, the extract induced a dose-dependent increase in the production of H2O2 (1.79 ± 0.23 to 20.5 ± 2.1 nmol), NO (2.64 ± 1.02 to 29.4 ± 5.9 µmol), and TNF-α (2.44 ± 1.46 to 41.6 ± 0.9 unit mL-1), suggesting a relevant immunomodulatory activation, as the macrophage-mediated immune response plays a crucial role in containing Mycobacterium tuberculosis infection. Stimulating the production of these inflammatory mediators can enhance the action of the immune system against the bacteria, making the hexane extract of Davilla elliptica a promising candidate for the development of new therapeutic strategies to combat tuberculosis. In a study79 investigating the antimycobacterial effect of leaves from 36 plants from the Atlantic Forest, the species Psychotria vellosiana (Rubiaceae), Cedrela fissilis (Meliaceae), Pouteria filipes (Sapotaceae), Plathymenia foliolosa (Fabaceae), and Peschieraaffinis (Apocynaceae) were active against M. tuberculosis H37Rv with MIC ranging between 3.12 and 20 μg mL-1. Peschiera affinis was the most promising species and did not present a significant toxic effect on mammalian cells. In 2010, the methanolic extract of aerial parts from Indigofera suffruticosa (Fabaceae) showed activity with a MIC of 125 μg mL-1 against M. tuberculosis H37Rv.80 In 2011, Morais et al.81 showed the antimycobacterial activity of ethanolic extract from leaves of 10 different species of Psychotria. All species studied inhibited the growth of the Mycobacterium bovis BCG strain. Among these, the extracts of P. pubigera, P. ruelliifolia, and P. stachyoides were the most active, presenting a MIC50 ≤ 4 μg mL-1. In this same study, the toxic effects and nitric oxide production in mammalian cells were evaluated. When analyzed together with the inhibition of M. bovis BCG, the species P.stachyoides stood out. The authors81 suggested that this activity is due to alkaloids, but further studies are needed. In 2015, the antimycobacterial activity described by Boligon etal.82 demonstrated the activity of extracts and fractions of the bark and leaves of Tabernaemontanacatharinensis (Apocynaceae) against strains of M. smegmatis, M. avium, and M. tuberculosis. The fractions obtained from the leaves were the most promising, with a greater sensitivity being observed for the M. smegmatis strain (MIC between 19.53 and 156.25 μg mL-1). Furthermore, the authors suggested that this activity may be related to the alkaloids ibogamine and voacangine, which had previously been described and identified in T.catharinensis. A study examining medicinal plants sold at fairs in 20 cities across Rio de Janeiro collected data on 36 plants commonly used for tuberculosis and related diseases. Among the active and inactive species, Chenopodium ambrosioides (Amaranthaceae), Bidens pilosa (Asteraceae), Mentha piperita L. (Lamiaceae), and Vernonia polyanthes (Asteraceae) had already been described in the literature83 for antimycobacterial activity. The ethanolic extract of aerial parts and leaves of Struthanthus concinnus (Loranthaceae) and Struthanthus marginatus were evaluated and showed moderate to good activity against the H37Rv and 35338 strains of M. tuberculosis. The compound obtusifoliol (52) (Figure 6) (Table 1), isolated from S. concinnus, showed a MIC of 50 μg mL-1.83 In another study,84 extract, fractions and compounds from the leaves of Ocoteanotata (Lauraceae), collected in the Jurubatiba restinga, were evaluated against the M. bovis BCG strain and although the extract (95.75 ± 2.53%), hexane fraction (102.56 ± 1.90%) and ethyl acetate fraction (80.75 ± 1.15%) showed promising inhibition of mycobacterial growth at 100 μg mL-1, the isolated compounds, from the flavonoid class, did not maintain this activity. However, these results suggest that the species O. notata may be a promising source in the search for compounds capable of inhibiting mycobacterial growth. Further studies should be carried out to isolate and identify the compounds responsible for the activity. In 2016, Reis et al.85 evaluated antifungal, antimycobacterial, and larvicide activity of the Duroiamacrophylla and Duroia saccifera (Rubiaceae). The dichloromethane and methanolic extracts of the leaves of D. macrophylla presented MICs between 50 and 200 μg mL-1 against the H37Rv strain and monoresistant strains of M. tuberculosis to isoniazid and rifampicin, standing out as the most active. For D. saccifera, the antimycobacterial activity was observed mainly in the dichloromethane extracts of the leaves and branches, with MICs between 100 and 200 μg mL-1 against the three strains studied. These results suggest that both plants have promising antimicrobial properties. The extract obtained from the leaves of Doliocarpus dentatus (Dilleniaceae) was evaluated for its antimycobacterial activity and showed activity with a MIC of 62.5 μg mL-1. Since these results were obtained from the extracts, it is possible to identify promising compounds with lower MIC values during fractionation, suggesting D. dentatus as a promising species. An interesting point encouraging further studies with D. dentalus is that its extract did not induce genotoxic effects, even at a concentration 10 times greater than the effective one.86 The ethanol extract and fractions from leaves of Eugenia astringens (Myrtaceae), also collected in the Jurubatiba restinga, were evaluated for their antimycobacterial potential. Among the samples evaluated, the hexane and dichloromethane fractions showed the most significant activity, with MIC50 of 15.9 ± 1.2 and 28.7 ± 1.4 μg mL-1 for M. bovis and 18.6 ± 1.6 and 44.9 ± 1.5 μg mL-1 for the H37Rv strain of M. tuberculosis. The hexane fraction also reduced the bacillary load in macrophages infected with M.tuberculosis H37Rv (IC50 20 μg mL-1). The chemical profile of the hexane fraction obtained by GC-MS demonstrated the presence of sesquiterpenes.87 Additionally, in 2023, a study88 investigated the potential of Pterodonpubescens seeds against different bacterial strains. The hexane extract demonstrated antimycobacterial activity, with a MIC of 125 μg mL-1 against the M. tuberculosis H37Rv strain, and β-caryophyllene (29.9%), trans-caryophyllene (24.6%), and epi-cubebol (15.7%) were identified as major constituents. The MIC values from extracts, fractions and essential oils obtained in these studies have been compiled in Table 2. In silico-based studies Studies aimed at therapeutic targets have also been a strategy in the search for active compounds; in this sense, protein tyrosine phosphatase A and B (PtpA and PtpB) have been recognized as potential molecular targets for developing new drugs against TB. In this context, compounds from Morus nigra (Moraceae) were evaluated as potential inhibitors of PtpB, and kuwanon G (53) and kuwanon H (54) (Figure 7), presented inhibition values (Ki) of 0.39 ± 0.27 and 0.20 ± 0.01 μM, respectively, interacting with the active site of the enzyme. The MIC value against Mycobacterium tuberculosis H37Ra was determined to be 32 μg mL-1 for both kuwanon G and kuwanon H (Table 1). Kuwanon G was also evaluated in infected macrophages, inhibiting 61.3% of mycobacterial growth, suggesting that this compound is relevant for developing PtpB inhibitors in anti-TB therapy.89
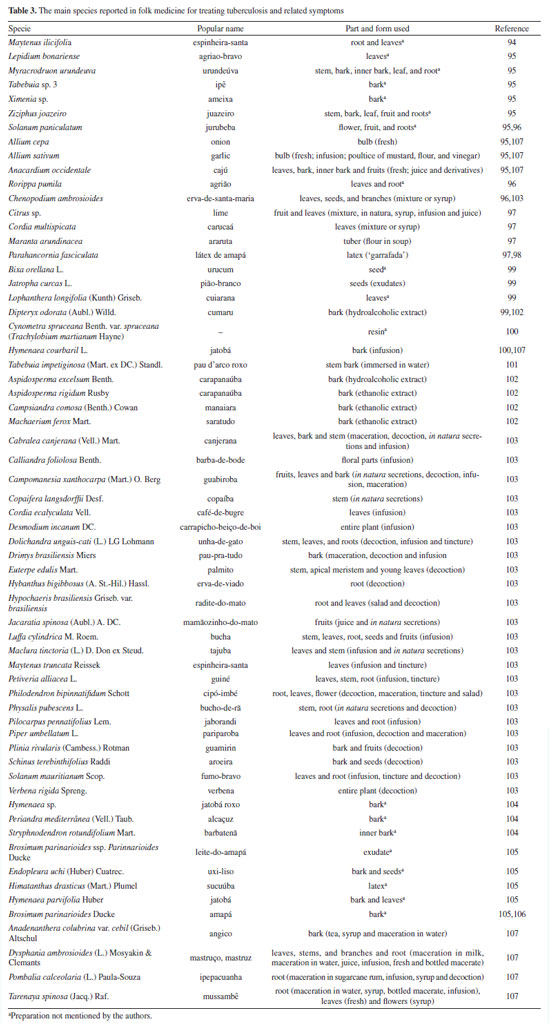
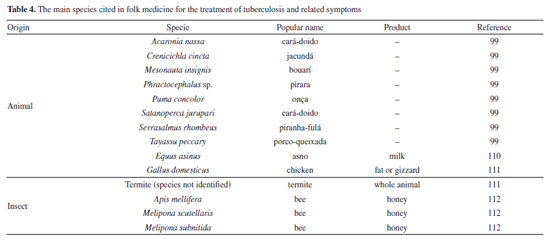
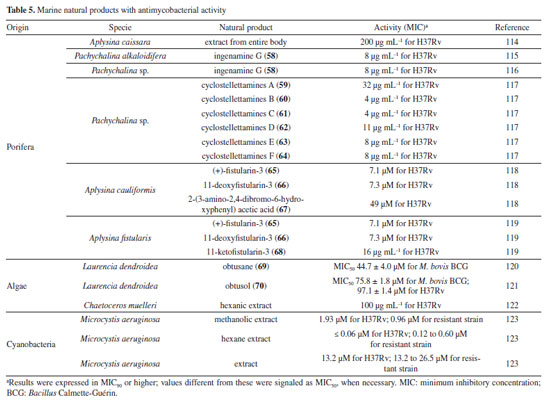
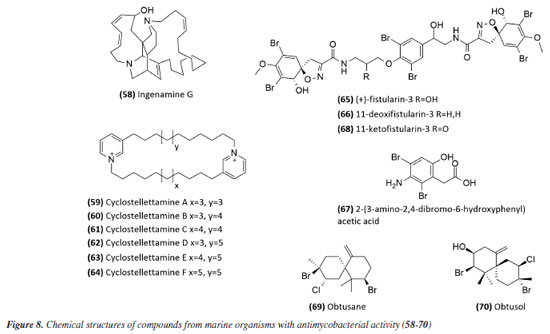
Mascarello et al.90 also conducted an in silico study with more than 800 natural compounds. Six compounds were shown to inhibit PtpB at micromolar concentrations, with kuwanol E (55) (Figure 7) exhibiting the highest inhibition value (Ki) of 1.6 ± 0.1 µM. In a study by Antunes et al.,91 molecular modeling was used to identify candidates for ligands of protein kinase serine/threonine (PknB) among constituents present in a Brazilian biodiversity database (NuBBE) through chemogenomics. Among the thirteen promising ligands, six had descriptions in the literature that supported this activity and were further studied for docking and molecular dynamics. The observed results demonstrated the potential of the alkaloid dictamine (56) and the coumarin scoparone (57) (Figure 7) as inhibitors of PknB and PknA. In addition, these compounds already have antimycobacterial activity described in the literature. The study of Taveira et al.92 addresses the bioinspired peptide CadeF2.1 G27-K44, derived from the defensins present in the fruits of Capsicum annuum (Solanaceae), which are natural antimicrobial peptides. Instead of being isolated directly from the plant, the peptide was synthesized based on the structure of the defensins found in C. annuum. The peptide presented a MIC50 of 33.2 μM for the standard strain H37Rv of M. tuberculosis and 55.4 μM for the hypervirulent strain M299 (Table 1). Even at high concentrations, cytotoxicity was low, suggesting that the peptide has a good safety profile for therapeutic use. Ethnobotanical approach to tuberculosis treatment Investigating antibacterials based on ethnobotanical knowledge and enabling the discovery of new natural products is also an interesting strategy, given the cultural and natural wealth of Brazil. Before the advent of antibiotic therapy, plants were widely used as antiseptic agents materials with antibacterial activity.93 Ethnopharmacological research contributes substantially to the medicinal knowledge of potentially essential species, and the reported species can be used to help guide chemical and biological studies aimed at proving therapeutic properties and isolating biologically active compounds, hopefully leading to a new drug candidate.93 The following ethnobotanical studies, conducted in Brazil, described the widespread use of plant species for treating tuberculosis and/or a group of diseases or symptoms related to the respiratory tract. The reported species, the popular names, and the parts used were listed in Table 3. In a study94 on the antimicrobial evaluation of the most commonly used species in Rio Grande do Sul (RS), the roots and leaves of Maytenus ilicifolia (Celastraceae) were cited for use in cases of tuberculosis, as well as for the treatment of conditions associated with microorganisms. In a review by Albuquerque et al.,95 the Caatinga (semiarid vegetation) was the target of study. The available published information on medicinal plants used by traditional communities was analyzed to contribute to future phytochemical and pharmacological investigations. In this study, eight species were reported for use in the treatment of tuberculosis, namely: Anacardiumoccidentale (Anacardiaceae), Myracrodruon urundeuva (Anacardiaceae), Tabebuia sp. (Bignoniaceae), Lepidium bonariense (Brassicaceae), Chenopodium ambrosioides (Amaranthaceae), Ximenia sp. (Olacaceae); Ziziphus joazeiro (Rhamnaceae), and Solanum paniculatum (Solanaceae), thus, highlighting the significant biodiversity of this Brazilian biome. In Brazil, another field of study is popular markets, which are public spaces where various types of products are sold and serve as venues for exchanging cultural information. Considering the lack of ethnobotanical studies in these markets and the significant methodological difficulties in obtaining access to this type of information, Albuquerque et al.96 conducted interviews with vendors in an important traditional market in the city of Recife (Pernambuco, northeastern Brazil) in two different years, 1995 and 2002. During this study, three species were indicated for the treatment of tuberculosis, namely: Rorippa pumila (Brassicaceae), Solanum paniculatum (Solanaceae), and Chenopodium ambrosioides. In interviews about widespread use, whether local, regional, or national, for the treatment of tuberculosis, coughs, colds, asthma, pneumonia, and respiratory diseases, the following species were cited: Parahancornia fasciculata (Apocynaceae),97,98 Bixa orellana, Dipteryx odorata (Fabaceae), Jatrophacurcas (Euphorbiaceae), Lophanthera longifolia (Malpighiaceae), Brosimum parinarioides ssp. Parinnarioides (Moraceae),99 Cynometra spruceana Benth. var. spruceana (Trachylobium martianum) (Fabaceae), Hymenaea courbaril (Fabaceae)100 and Tabebuia impetiginosa.101 In an ethnobotanical study102 conducted in the Amazon region with the quilombola community of Oriximiná, Pará, published in 2011, 43 plant species used by the community to treat symptoms related to tuberculosis were listed. Of these species, 10 were evaluated for their antimycobacterial activity against M. tuberculosis H37Rv. Five plant extracts, Dipteryx odorata, Campsiandra comosa (Fabaceae), Machaerium ferox (Fabaceae), Aspidosperma excelsum, and A. rigidium (Apocynaceae), were active with MIC ranging from 12.5-200 μg mL-1 (Table 2). The most active species was the ethanolic extract of the bark of Dipteryx odorata with a MIC of 12.5 μg mL-1 (Table 2). Only the five species listed above were included in Table 3; the complete list of 43 species can be accessed in the original publication. In 2015, a study103 on the use of medicinal plants in the region of the Parque Estadual da Cabeça do Cachorro in Paraná, Brazil, grouped the reported traditional knowledge into ten categories, one of which was infectious diseases, which includes symptoms such as fever, organs such as the lungs and different infectious diseases, including tuberculosis. Although it is not possible to state the specific use for tuberculosis, the species cited in this category were: Chenopodium ambrosioides; Schinus terebinthifolius; Philodendron bipinnatifidum; Euterpe edulis (Arecaceae); Hypochaeris brasiliensis var. brasiliensis (Asteraceae); Dolichandra unguis-cati (Bignoniaceae); Cordia ecalyculata (Boraginaceae); Jacaratia spinosa (Caricaceae); Maytenus truncata; Luffa cylindrica (Cucurbitaceae); Calliandra foliolosa (Fabaceae); Copaifera langsdorffii (Fabaceae); Desmodium incanum (Fabaceae); Cabralea canjerana (Meliaceae); Maclura tinctoria (Moraceae); Campomanesia xanthocarpa (Myrtaceae); Plinia rivularis (Myrtaceae); Petiveria alliacea (Phytolaccaceae); Piper umbellatum; Pilocarpus pennatifolius (Rutaceae); Physalis pubescens (Solanaceae); Solanum mauritianum; Verbena rigida; Hybanthus bigibbosus (Violaceae); Drimys brasiliensis (Winteraceae). Some of these species were also cited in the category of respiratory system diseases, including general lung problems, chronic cough, pneumonia, and others. These symptoms can sometimes be related to tuberculosis. The species cited were: Chenopodium ambrosioides, Dolichandra unguis-cati, Piper umbellatum, Pilocarpus pennatifolius, Drimys brasiliensis.103 In another ethnopharmacological study,104 conducted in the Cerrado region of the state of Pernambuco, northeastern Brazil, 78 plants were identified as therapeutic resources. Among these, three were mentioned explicitly for the treatment of tuberculosis: Hymenaea sp., Periandra mediterranea (Fabaceae), and Stryphnodendron rotundifolium (Fabaceae). In 2016, two studies in the Amazon region reported the use of medicinal plants to treat tuberculosis. In communities near the Jauaperi River, 119 species were identified, and among these, four were cited for their tuberculosis treatment properties: Himatanthus drasticus, Hymenaea parvifolia, Endopleura uchi (Humiriaceae), and Brosimum parinarioides.105 This last species was also noted for the same use in the study by Lago et al.,106 carried out in the riverside community of the Unini River, Amazonas, Brazil. In 2019, a review107 based on plants from the Caatinga reported five species with therapeutic use for tuberculosis, namely: Dysphania ambrosioides (Amaranthaceae); Tarenaya spinosa (Asteraceae); Hymenaea courbaril; Anadenanthera colubrina var. cebil (Fabaceae); Pombalia calceolaria (Violaceae). This demonstrates that the Caatinga is a region rich in knowledge of medicinal plants, particularly in addressing diseases related to inadequate sanitation, difficulties in medical care, and cultural issues such as preventive health care for women. The possibilities of resources from animals and insects Various animal parts (skin, nails, feathers, penis, bone and meat) and products (wax, lard and bee propolis) as well as their derivatives (nests and cocoons) were mentioned in the preparation of animal-derived medicines. Fat was the most commonly used animal product (25%), followed by meat (15%).99 Regarding insects as a source, natural products are based on antagonistic relationships, prokaryotic and eukaryotic organisms have developed hundreds of different cytolytic peptides and proteins during their evolution in different phyla of the plant108 and animal kingdoms. Many cytolytic peptides are specific peptides with antimicrobial action that are part of the innate immune system of invertebrates and vertebrates. They serve as primary defense weapons against invading prokaryotic and eukaryotic microorganisms.109 Notably, the number of plant species used is greater than that of animal species. This is probably because there is a greater evaluation of products of plant origin, and animals are more unilaterally used for food purposes. Although plants are widely targeted as a medicinal resource, with the examples presented in this review, it was possible to demonstrate that both flora and fauna can be sources of studies. The data obtained in field research are summarized in Table 4 and, to date, products from animals and insects have only been reported for popular use in the treatment of diseases of the respiratory system (pneumonia, tuberculosis, asthma, nasal congestion and cough);110-112 there is no evidence of studies proving antimycobacterial activity. Studies using natural products obtained from marine organisms The oceans are a unique and underexplored resource offering diverse natural products, mainly from invertebrates such as sponges, tunicates, bryozoans, mollusks, and marine bacteria and cyanobacteria. As infectious diseases evolve and resist existing drugs, the aquatic environment provides new compounds against fungal, parasitic, bacterial, and viral diseases.113 Although a few researchers are searching for marine products with potential activity against M. tuberculosis, the results have been promising. The antimycobacterial activity of extracts obtained from the marine sponge Aplysinacaissara inhibited the growth of M. tuberculosis with MICs starting at 200 μg mL-1, and this activity was correlated with the presence of the alkaloids fistularin-3 and 11-hydroxyaerothionine.114 Another alkaloid, ingenamine G (58) (Figure 8), isolated from the marine sponge Pachychalina alkaloidifera, also had its activity evaluated and showed a MIC of 8 μg mL-1 against M. tuberculosis (Table 5).115
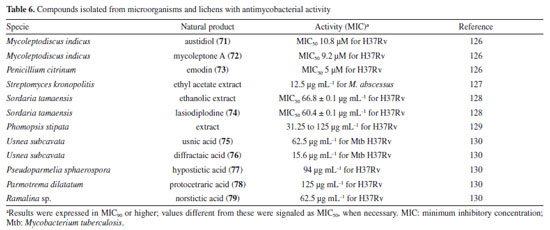
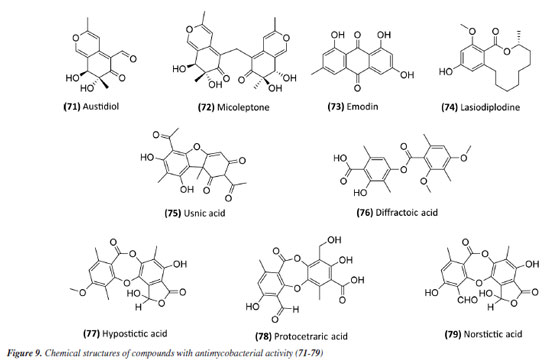
Following studies with marine sponges, ingenamine G (58) (Figure 8), isolated from the methanolic extract obtained from Pachychalina sp., was evaluated for its antimicrobial potential, and its activity against the H37RV strain also showed a MIC value of 8 μg mL-1 (Table 5).116 In another study117 carried out with Pachychalina sp., it was possible to isolate cyclostellettamines A-F (59-64) (Figure 8) from the butanolic extract. These alkaloids were tested against the H37Rv strain and presented MIC values of 32, 4, 4, 8, 11, and 8 μg mL-1, respectively (Table 5). The study by Oliveira et al.118 carried out from a screening of 500 crude extracts of marine invertebrates, involved the isolation of four compounds containing bromine from the extracts with the most significant antimycobacterial potential. From the methanolic extract of Aplysina cauliformis, the compounds (+)-fistularin-3 (65), 11-deoxyfistularin-3 (66), and the 2-(3-amino-2,4-dibromo-6-hydroxyphenyl) acetic acid (67) (Figure 8) were isolated, which presented MIC values of 7.1, 7.3, and 49 μM for the H37Rv strain of M. tuberculosis (Table 5). Another species of the genus Aplysina had its anti-TB activity investigated. Three metabolites with antimycobacterial potential were isolated from the methanolic extract of Aplysina fistularis: fistularin-3 and 11-deoxyfistularin-3 with MIC values of 7.1 and 7.3 μg mL-1, respectively, and 11-ketofistularin-3 (68) (Figure 8) with a MIC of 16 μg mL-1 (Table 5). This was the first report of the antimycobacterial activity of the latter.119 Studies with the red alga Laurenciadendroidea have shown that extracts and isolated compounds may be promising sources for antimycobacterial activity. The active extracts yielded two active compounds: obtusane (69) (Figure 8) with a MIC50 of 44.7 ± 4.0 µM120 against M. bovis BCG and obtusol (70) (Figure 8) with a MIC50 of 75.8 ± 1.8 μM and 97.1 ± 1.4 µM against M. bovis BCG and M. tuberculosis H37RV, respectively121 (Table 5). This suggests that these samples are promising and can be used in macrophage infection models and animal models. Another study122 demonstrated the antimycobacterial activity of polyunsaturated fatty acids in the hexane extract of the marine diatom Chaetoceros muelleri. The hexane extract was the most active and presented a MIC of 100 μg mL-1 against the H37Rv strain of M. tuberculosis. 1H NMR (1H nuclear magnetic resonance spectroscopy) analysis revealed the presence of linoleic acid (LA), α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), suggesting that these lipid constituents may be responsible for the observed antimicrobial activity. These findings reinforce the potential of marine algae as sources of new candidates for drug development against bacterial infections, especially M. tuberculosis. Ramos et al.123 investigated the antimycobacterial activity of extracts of the RST 9501 strain of the cyanobacterium Microcystis aeruginosa. The methanolic extract presented a MIC of 1.93 μM against the H37Rv strain and 0.96 μM against resistant strains of M. tuberculosis (Table 5). The hexane extract demonstrated the highest activity, with MIC of ≤ 0.06 μM against H37Rv and between 0.12 and 0.60 μM against resistant strains (Table 5). 1H NMR analysis identified fatty acids such as linoleic acid, α-linolenic acid, eicosapentaenoic acid, and docosahexaenoic acid, suggesting its antimicrobial potential. Furthermore, the study evaluated a variant of the extract M. aeruginosa, the [D-Leu 1] MC-LR variant, which presented a MIC of 13.2 μM for susceptible strains and between 13.2 and 26.5 μM for resistant strains (Table 5). Regarding cytotoxicity, exposure of rat hepatoma cells (HTC) to the M. aeruginosa extract variant resulted in a significant decrease in cell viability at concentrations higher than 192.71 μM. These results indicate that the M. aeruginosa 9501 strain and its variant are promising candidates for developing new antimycobacterial agents. Other living organisms as a source of antimycobacterial activity Microorganisms have been a rich source of medicines. However, the range of microorganisms used as precursor sources is minimal compared to the diversity that exists worldwide, which is largely unknown. Only about 6,000 species of bacteria have been named (compared to over a million plant and animal species), and estimates suggest that there are approximately 1.5 million species of fungi, 1.5 million species of algae, and 1.5 million species of prokaryotes.124 Although streptomycin, obtained from cultures of the bacterium Streptomyces griseus, was the first effective antibiotic for treating tuberculosis and a milestone in the history of antibiotics, the first antibiotic, penicillin, had been discovered a few years earlier from fungi of the genus Penicillium.125 Currently, advances in research have provided new approaches to microorganisms in Brazil in search of small bioactive molecules to obtain potential antibiotics. The approach from fungi of different origins has also been investigated for antimycobacterial properties. Andrioli et al.126 evaluated the inhibition of mycobacterial growth of six compounds isolated from fungi against M. bovis BCG, three strains of M. tuberculosis, and three strains of M. kansasii. The compounds were isolated from the endophytic fungus Mycoleptodiscus indicus: austdiol, austdiol diacetate, mycoleptone A, and eugenitin. The compound emodin was isolated from the endophytic fungus Penicillium citrinum, and δ-lactam from the soil fungus Humicolagrisea. The compounds austidiol (71), mycoleptone A (72), and emodin (73) (Figure 9) showed promising activity against susceptible M. tuberculosis and clinical isolates, including a hypervirulent strain. Respectively, these compounds presented MIC50 values of 10.8, 9.2, and 5 µM against M. tuberculosis H37Rv (Table 6). Furthermore, the intracellular efficacy to reduce the growth of the H37Rv strain in infected macrophages was also observed with the respective MIC50 of 31.2, 17.9, and 6.5 µM (Table 6).
A study127 carried out with an ethyl acetate extract obtained from the culture of the bacterium Streptomyceskronopolitis in potato dextrose broth showed that this extract presented a MIC of 12.5 μg mL-1 against M. abscessus (Table 6). The bacterial sample used in this study was obtained from rhizosphere soil collected in São Luís, MA, Brazil. The compounds responsible for the observed effect were not identified. Ethanolic extracts of sixteen endophytic fungi obtained from the plant species Tocoyenabullata (Rubiaceae) and Humiria balsamifera (Humiriaceae) were evaluated for antimycobacterial activity against M. tuberculosis H37Rv. The most active extract was from the fungus Sordaria tamaensis, with a MIC50 of 66.8 ± 0.1 μg mL-1 (Table 6). The macrolide lasiodiplodine (74) (Figure 9) was isolated from this extract, presenting a MIC50 of 60.4 ± 0.1 μg mL-1 (Table 6). Furthermore, lasiodiplodine inhibited the intracellular growth of M. tuberculosis and was not cytotoxic to macrophages. C57BL/6 animals infected with M. tuberculosis treated with lasiodiplodine had reduced bacillary load and lung pathology. Fructose 1,6-bisphosphate aldolase class IIa was predicted to be the hypothetical target for lasiodiplodine.128 Prince et al.129 isolated the fungus Phomopsis stipata B. Sutton (Diaporthaceae) from the leaves of the plant species Styrax camporum Pohl (Styracaceae). They evaluated the antimycobacterial efficacy against M. tuberculosis H37Rv of its extracts grown in different culture media: yeast medium, potato dextrose broth, Czapek medium, nutrient broth, and a homemade corn medium. Among these, the extracts grown in homemade corn medium (MIC of 31.25 μg mL-1) and in potato dextrose broth medium (MIC of 62.5 μg mL-1) stood out (Table 6), which are satisfactory values considering that they are crude extracts. Notably, these extracts yielded the highest amount. For the other samples, the MIC values were: 125 μg mL-1 for the extract obtained in Czapek medium and 250 μg mL-1 for the extracts obtained in yeast medium and nutrient broth. In a study130 evaluating the antimycobacterial activity of compounds isolated from lichens, five compounds stood out with MIC lower than 250 μg mL-1. Usnic acid (75) and diffractaic acid (76) (Figure 9) isolated from Usnea subcavata presented MIC of 62.5 and 15.6 μg mL-1, respectively (Table 6). Hypostictic acid (77) (Figure 9) isolated from Pseudoparmelia sphaerospora presented a MIC of 94 μg mL-1 (Table 6). Protocetraric acid (78) (Figure 9) isolated from Parmotrema dilatatum presented a MIC of 125 μg mL-1, and norstictic acid (79) (Figure 9) isolated from Ramalina sp. presented a MIC of 62.5 (Table 6). Thus, this demonstrates the capacity of other natural sources also to be a source of compounds with antimycobacterial activity.
DISCUSSION Currently, several screening strategies are employed for the development of new anti-TB drugs, which can be broadly categorized into phenotypic screens and target-based screens. The second strategy has grown greatly since the complete sequencing of the Mycobacterium tuberculosis genome in 1998. However, its major problem remains translating in vitro biochemical activity into potent and selective antimycobacterial activity in whole cells.131 On the other hand, screening assays using the mycobacterial cell as a whole (phenotypic screening) have proven to be a promising strategy for the discovery of new anti-TB drugs. This approach has several advantages, allowing the identification of molecules active against mycobacteria and overcoming problems such as absorption and efflux. Additionally, assays in macrophage cultures will enable us to gain knowledge about the selectivity of a given compound. A study132 that analyzed 176 drugs approved between 1999 and 2008 revealed that most of the so-called first-in-class molecules were discovered through phenotypic approaches using whole cells and focusing on molecular targets. When analyzing the results in this review, we can observe that most studies with natural products and TB carried out by Brazilian research groups employed the phenotypic approach, except for the studies involving the protein tyrosine phosphatases PtpA and PtpB, and the study involving the protein serine/threonine kinase PKnB.91 Despite the success in identifying active chemical compounds through antimycobacterial phenotypic screenings, the conversion of such results into chemical series for hit-to-lead (H2L) optimization and, ultimately, the acquisition of clinical candidates is hampered by the lack of knowledge about the specific biochemical target(s) that such compounds inhibit and/or upregulate. However, advances in the field of Mtb genomics/proteomics have provided tools that allow the elucidation of Mtb targets involved in the action of a promising compound identified by the phenotypic approach. Target identification enables the performance of H2L optimization studies, such as in silico studies, development and synthesis of analogues for structure-activity relationship (SAR) studies, and collaboration in identifying new pharmacological, essential, and druggable targets. Therefore, this highlights the importance of continuing the most promising studies. The bacteria used in most studies conducted by the Brazilian groups were Mycobacterium tuberculosis H37Rv (ATCC 27294), M. bovis (BCG), and M. smegmatis. The latter two overcame the slow growth and/or safety issues encountered when treating M. tuberculosis.133 The efficiency and efficacy of using M. smegmatis and M. bovis BCG as surrogate in vitro models have been previously studied. A parallel screen using both strains and approximately 5,000 samples revealed that 48-50% of M. tuberculosis inhibitors could not be detected during the screen against M. smegmatis, and 21% were missed using M.bovis. Additionally, genomic comparisons indicated that 30% of M. tuberculosis proteins lack conserved orthologs in M. smegmatis and 3% in M. bovis.134 Although M. bovis represents a more sensitive model, M. smegmatis is often favored because of its faster growth. For example, bedaquiline, the first FDA-approved TB drug in 40 years, was identified in a screen using M. smegmatis as a surrogate.135 According to the data collected in this review, some plant species and/or isolated compounds studied by Brazilian research groups regarding their potential to inhibit the growth of M. tuberculosis deserve to be highlighted. Below are those whose extract or isolated compound has a MIC90 or MIC99 less than or equal to 16 μg mL-1 against M. tuberculosis H37Rv. For plant species, Cedrela fissilis (extract), Hortia superba (rutaecarpina), Peschiera affinis (extract), Piper regnelli (extract and neolignana eupomatenoid-5), Plathymenia foliosa (extract), Pouteria filipes (extract), Psycotria schimperi (damnacanthal), and P. vellosiana (extract) were listed according to the criteria mentioned above and are listed together with their MIC values in Tables 1 and 2. For the other sources, Microcystis aeruginosa (extract and D-Leu1 microcystin), Aplysina cauliformis (fistularin-3), Pachychalina alkaloidifera (ingenamine G), and Pachychalina sp. (cyclostellettamines B and C) stood out with the lowest MIC values, considering the criteria mentioned above (Table 5). Among all the samples highlighted in this topic, only three have additional information in the literature that contributes to advancing knowledge about their anti-TB potential. For all the others, we emphasize the importance of continuing the studies, given their promising potential for the search for new candidates for anti-TB prototypes. Below, we present the additional results found for the three samples mentioned. Regarding Cedrela fissilis extract, in a second study, isolated compounds were evaluated for their potential against M. tuberculosis H37Rv.67 Still, none stood out, compared to the MIC of 3.12 μg mL-1 previously described for the promising extract.79 As for the neolignan eupomatenoid-5, new studies showed that it can act synergistically in combination with rifampicin and ethambutol.136 And the proteomic profile of M. tuberculosis after treatment with eupomatenoid-5 revealed its potential targets: citrate synthase (Rv0896), phosphoglycerate kinase (Rv1437), ketol-acid reductoisomerase (Rv3001c), and ATP alpha synthase (Rv1308).137 The third continued sample came from Pipercorcovadensis, whose crude extract in dichloromethane from its roots presented a MIC of 15.6 μg mL-1. The alkaloid piperovatine was isolated from this extract with a MIC of 7.8 μg mL-1.49 The other alkaloids identified in the extract, piperlonguminine, isopiperlonguminine, and chingchengenamide A, were not isolated and evaluated.49 Data in the literature138 indicate that the alkaloid piperlonguminine inhibited the growth of M. tuberculosis H37Rv in infected macrophages; further studies are still needed for the other alkaloids identified. No subsequent studies have been published continuing the study of the antimycobacterial effect of piperovatine. Studies conducted by Brazilian and foreign researchers indicate progress regarding the pesticide, anti-Trypanosoma,139 and anesthetic potential of this alkaloid.140 However, complete toxicological studies for this alkaloid are still lacking to encourage future studies aiming at a new drug prototype. Moreover, its anti-tuberculosis potential is still lacking, with the evaluation of its effect on infected macrophages, in vivo effect, and mechanism of action. If proven promising, these studies should be followed by further assessments. Data from the literature indicate only its cytotoxic effect on mammalian cells of the LLCMK2 lineage, with a cytotoxic concentration (CC50) of 332.0 ± 2.2 μM,136 and for the VERO lineage with a CC50 of 192 μg mL-1.49 However, regarding the extract of Pipercorcovadensis, the same authors reported the development and evaluation of nanoformulations containing this extract.141 Solid lipid nanoparticles containing the crude extract of Piper corcovadensis roots (SLN-CEPc) and chitosan-coated solid lipid nanoparticles containing the crude extract of P. corcovadensis roots (C-SLN-CEPc) showed MIC of 12.5 μg mL-1 against M. tuberculosis H37Rv, similar to that observed for the extract in the initial study of the group. However, CC50 in VERO cells of 60 and 70 μg mL-1 demonstrated greater cytotoxicity when compared to the previously described extract.49 Finally, we highlight the importance of encouraging new studies with the plant species Peschiera affinis, Plathymenia foliosa, Pouteria filipes and Psychotriavellosiana and with the cyanobacterium Microcystes aeruginosa due to the MIC of its extracts being quite promising (≤ 1 μg mL-1). Additionally, natural compounds such as piperovatine, eupomatenoid-5, fistularin-3, ingenamine G, cyclostellettamines B and C; advancing to complement the preclinical results with in vitro more complex studies, such as in the infection of macrophages, in vivo models of tuberculosis,142,143 with the study of the target in the mycobacteria, and on its toxicological and pharmacokinetic parameters. Although the species and compounds described here have shown promising antimycobacterial potential, some studies have not yielded such results. The data for these species, their extracts and fractions, as well as their isolated compounds, are summarized in Table 1S (Supplementary Material).
CONCLUSIONS The possibility of treating tuberculosis using natural products is remarkable, and continuing to search for new bioactive molecules that make this treatment more efficient is essential, given the immense biodiversity present in Brazil. This research has made it possible to find several studies in this line of research, carried out by Brazilian researchers. Still, the number of strategies that move forward is low, highlighting the importance of continuing these studies. Another point that deserves to be highlighted is the investment in infrastructure for scientific research in a high-level biological containment environment, essential for working in vitro and in vivo with Mycobacterium tuberculosis (biosafety level 3). Given the existence of Brazilian groups committed to this search, receiving greater attention and government incentives will make the continuation of these studies more viable. Furthermore, this review contributed to highlight promising studies to encourage future investigations to identify highly active antimycobacterial natural products from extracts, as well as to deepen the knowledge of natural products already identified as promising, characterizing them as new candidates for antituberculosis prototypes, including the synthesis and optimization of new analogues of natural products with antimycobacterial potential.
SUPPLEMENTARY MATERIAL Complementary material for this work is available at http://quimicanova.sbq.org.br/, as a PDF file, with free access.
DATA AVAILABILITY STATEMENT All data underlying the conclusions are available in the cited literature.
REFERENCES 1. Guerrant, R. L. In Essentials of Tropical Infectious Diseases, 1st ed.; Walker, D. H.; Weller, P. F., eds.; Churchill Livingstone: Philadelphia, 2001. 2. Silva, W. D.; Mota, I.; Imunologia Básica e Aplicada, 5th ed.; Guanabara Koogan: Rio de Janeiro, 2003. 3. Abbas, A. K.; Lichtman, A. H.; Pillai, S.; Cellular and Molecular Immunology, 6th ed.; Elsevier: Philadelphia, 2008. 4. World Health Organization (WHO); Global Tuberculosis Report 2024; WHO: Geneva, Switzerland, 2024. [Link] accessed in July 2025 5. Ministério da Saúde, Secretária da Vigilância em Saúde; Programa Nacional de Controle da Tuberculose, 2011. [Link] accessed in July 2025 6. Copp, B. R.; Pearce, A. N.; Nat. Prod. Rep. 2007, 24, 278. [Crossref] 7. Gregianini, T. S.; Silveira, V. C.; Porto, D. D.; Kerber, V. A.; Henriques, A. T.; Fett-Neto, A. G.; Photochem. Photobiol. 2007, 78, 470. [Crossref] 8. Coelho, F. S.; Marques, E. A.; Revista do Hospital Universitário Pedro Ernesto 2006, 5, 24. [Link] accessed in July 2025 9. Crevel, T. R.; Kleinnijenhuis, J.; Oosting, M.; Joosten, L. A. B.; Netea, M. G.; J. Immunol. Res. 2011, 2011, 405310. [Crossref] 10. Korkegian, A.; Roberts, D. M.; Blair, R.; Parish, T.; J. Biol. Chem. 2014, 289, 35172. [Crossref] 11. Tortoli, E.; FEMS Immunol. Med. Microbiol. 2006, 48, 159. [Crossref] 12. Cosma, C. L.; Sherman, D. R.; Ramakrishnan, L.; Annu. Rev. Microbiol. 2003, 57, 641. [Crossref] 13. Negi, A. S.; Kumar, J. K.; Luqman, S.; Saikia, D.; Khanuja, S. P. S.; Med. Res. Rev. 2010, 30, 603. [Crossref] 14. Karakousis, P. C.; Mooney, G.; J. Infect. Dis. 2024, 1, 231. [Crossref] 15. Zink, A. R.; Sola, C.; Reischl, U.; Grabner, W.; Rastogi, N.; Wolf, H.; Nerlich, A. G.; J. Clin. Microbiol. 2003, 41, 359. [Crossref] 16. Schluger, N. W.; Rom, W. N.; Am. J. Respir. Crit. Care Med. 1998, 157, 679. [Crossref] 17. Thaiss, C. A.; Kaufmann, S. H. E.; Yale J. Biol. Med. 2010, 83, 209. [Link] acessed in July 2025 18. Dheda, K.; Schwander, S. K.; Zhu, B.; Van Zyl-Smit, R. N.; Zhang, Y.; Respirology 2010, 15, 433. [Crossref] 19. Corbett, E. L.; Watt, C. J.; Walker, N.; Maher, D.; Williams, B. G.; Raviglione, M. C.; Dye, C.; Arch. Intern. Med. 2003, 163, 1009. [Crossref] 20. Espinal, M. A.; Laszlo, A.; Simonsen, L.; Boulahbal, F.; Kim, S. J.; Reniero, A.; Hoffner, S.; Rieder, H. L.; Binkin, N.; Dye, C.; Williams, R.; Raviglione, M. C.; N. Engl. J. Med. 2001, 344, 1294. [Crossref] 21. Nunn, P.; Williams, B.; Floyd, K.; Dye, C.; Elzinga, G.; Raviglione, M.; Nat. Rev. Immunol. 2005, 10, 819. [Crossref] 22. Jain, A.; Dixit, P.; J. Biosci. 2008, 33, 605. [Crossref] 23. Bloemberg, G. V.; Keller, P. M.; Stucki, D.; Trauner, A.; Borrell, S.; Latshang, T.; Coscolla, M.; Rothe, T.; Hömke, R.; Ritter, C.; Feldmann, J.; Schulthess, B.; Gagneux, S.; Böttger, E. C.; N. Engl. J. Med. 2015, 373, 1986. [Crossref] 24. Segala, E.; Sougakoff, W.; Nevejans-Chauffour, A.; Jarlier, V.; Petrella, S.; Antimicrob. Agents Chemother. 2012, 56, 2326. [Crossref] 25. Food and Drug Administration (FDA), Sirturo (bedaquiline), https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/204384s000lbl.pdf, accessed in July 2025. 26. Zhang, Y.; Post-Martens, K.; Denkin, S.; Drug Discovery Today 2006, 11, 21. [Crossref] 27. World Health Organization (WHO); WHO Consolidated Guidelines on Tuberculosis. Module 4: Treatment: Drug-Resistant Tuberculosis Treatment, 2022 Update; WHO: Geneva, Switzerland, 2022. [Link] accessed in July 2025 28. Newman, D. J.; Cragg, G. M.; J. Nat. Prod. 2020, 83, 770. [Crossref] 29. Gurib-Fakim, A.; Mol. Aspects Med. 2006, 27, 1. [Crossref] 30. Sampaio-Santos, M. I.; Kaplan, M. A. C.; Quim. Nova 1997, 20, 599. [Crossref] 31. World Health Organization (WHO); WHO Traditional Medicine Strategy, 2014-2023; WHO: Geneva, 2013. [Link] accessed in July 2025 32. Okunade, A. L.; Elvin-Lewis, M. P. F.; Lewis, W. H.; Phytochemistry 2004, 65, 1017. [Crossref] 33. Salomon, C. E.; Schmidt, L. E.; Curr. Top. Med. Chem. 2012, 12, 735. [Crossref] 34. Maciel, M. A. M.; Pinto, A. C.; Veiga Junior, V. F.; Grynberg, N. F.; Echevarria, A.; Quim. Nova 2002, 25, 429. [Crossref] 35. dos Santos, M. R. A.; de Lima, M. R.; Ferreira, M. G. R.; Hortic. Bras. 2008, 26, 244. [Crossref] 36. McMannis, C. In Biodiversity and the Law, 1st ed.; McMannis C., ed.; Earthscan: London, 2007. 37. Gottlieb, O. R.; Borin, M. B. M. R.; Cienc. Cult. 1997, 49, 315. [Link] accessed in July 2025 38. ScienceDirect, http://www.sciencedirect.com/, accessed in July 2025. 39. Web of Science, https://www.webofscience.com/wos/woscc/smart-search, accessed in July 2025. 40. Basso, L. A.; Silva, L. H. P.; Fett-Neto, A. G.; Azevedo Junior, W. F.; Moreira, I. S.; Palma, M. S.; Calixto, J. B.; Astolfi Filho, S.; Santos, R. R.; Soares, M. B.; Santos, D. S.; Mem. Inst. Oswaldo Cruz 2005, 100, 475. [Crossref] 41. García, A.; Bocanegra-García, V.; Palma-Nicolás, J. P.; Rivera, G.; Eur. J. Med. Chem. 2012, 49, 1. [Crossref] 42. Gu, J. Q.; Wang, Y.; Franzblau, S. G.; Montenegro, G.; Yang, D.; Timmermann, B. N.; Planta Med. 2004, 70, 509. [Crossref] 43. Kuete, V.; Planta Med. 2010, 76, 1479. [Crossref] 44. Heinrich, M.; Gibbons, S.; J. Pharm. Pharmacol. 2001, 53, 425. [Crossref] 45. Melo, F. F.; Santos, M.; Chiappetta, A.; de Mello, J. F.; Mukherjee, R.; J. Ethnopharmacol. 1987, 19, 319. [Crossref] 46. Severino, V. G. P.; Monteiro, A. F.; da Silva, M. F. G. F.; Lucarini, R.; Martins, C. H. G.; Quim. Nova 2014, 38, 42. [Crossref] 47. Pollo, L. A. E.; Martin, E. F.; Machado, V. R.; Cantillon, D.; Wildner, L. M.; Bazzo, M. L.; Waddell, S. J.; Biavatti, M. W.; Sandjo, L. P.; Front. Microbiol. 2021, 11, 622629. [Crossref] 48. Hegeto, L. A.; Caleffi-Ferracioli, K. R.; Nakamura-Vasconcelos, S. S.; de Almeida, A. L.; Baldin, V. P.; Nakamura, C. V.; Siqueira, V. L. D.; Scodro, R. B. L.; Cardoso, R. F.; Tuberculosis 2018, 111, 35. [Crossref] 49. Fernandez, C. M. M.; Baldin, V. P.; Ieque, A. L.; Bernuci, K. Z.; Almeida, R. T.; Valone, L. M.; Fonseca, D. P.; Makimori, R. Y.; Andrade, J. P. P.; Pilau, E. J.; Romagnolo, M. B.; Nakamura, T. U.; Cardoso, R. F.; Cortez, D. A. G.; Gazim, Z. C.; Scodro, R. B. L.; Dias Filho, B. P.; Ind. Crops Prod. 2019, 131, 341. [Crossref] 50. Scodro, R. B. L.; Pires, C. T. A.; Carrara, V. S.; Lemos, C. O. T.; Cardozo-Filho, L.; Souza, V. A.; Corrêa, A. G.; Siqueira, V. L. D.; Lonardoni, M. V. C.; Cardoso, R. F.; Cortez, D. A. G.; Phytomedicine 2013, 20, 600. [Crossref] 51. Silva, M. L. A.; Martins, C. H. G.; Lucarini, R.; Sato, D. N.; Pavan, F. R.; Freitas, N. H. A.; Andrade, L. N.; Pereira, A. C.; Bianco, T. N. C.; Vinholis, A. H. C.; Cunha, W. R.; Bastos, J. K.; Silva, R.; da Silva Filho, A. A.; Z. Naturforsch., C: J. Biosci. 2009, 64, 779. [Crossref] 52. Scodro, R. B. L.; Mirror, S. C.; Pires, C. T. A; Garcia, V. A.; Cardozo-Filho, L.; Cortez, R. E.; Pilau, E. J.; Ferracioli, K. R. C.; Siqueira, V. L. D.; Cardoso, R. F.; Cortez, D. A. G.; Phytochem. Lett. 2015, 11, 18. [Crossref] 53. Castellar, A.; Coelho, T. S.; Silva, P. E. A.; Ramos, D. F.; Lourenço, M. C. S.; Lage, C. L.; Julião, L. S.; Barbosa, Y. G.; Leitão, S. G.; Rev. Bras. Farmacogn. 2011, 21, 835. [Crossref] 54. Bernardes, N. R.; Heggdorne-Araújo, M.; Borges, I. F. J. C.; Almeida, F. M.; Amaral, E. P.; Lasunskaia, E. B.; Muzitano, M. F.; Oliveira, D. B.; Rev. Bras. Farmacogn. 2014, 24, 644. [Crossref] 55. de Assis, L. R.; Theodoro, R. S.; Costa, M. B. S.; Nascentes, J. A. S.; Rocha, M. D.; Bessa, M. A. S.; Menezes, R. P.; Dilarri, G.; Hypolito, G. B.; Santos, V.; Duque, C.; Ferreira, H.; Martins, C. H. G.; Regasini, L. O.; Membranes 2022, 12, 269. [Crossref] 56. de Jesus, C. C. M.; de Araújo, M. H.; Simão, T. L. B. V.; Lasunskaia, E. B.; Barth, T.; Muzitano, M. F.; Pinto, S. C.; Nat. Prod. Res. 2022, 36, 1337. [Crossref] 57. Ramos, A. V. G.; de Sá, N.; Araújo, D. L. O.; Cabral, M. R. P.; Costacurta, G. F.; de Freitas, B. C.; Vilegas, L. V.; Scodro, R. B. L.; Siqueira, V. L. D.; Cotica, E. S. K.; do Carmo, M. R. B.; Sarragiotto, M. H.; Baldoqui, D. C.; Nat. Prod. Res. 2023, 37, 502. [Crossref] 58. Pereira, A. O.; Avila, J. M.; do Carmo, G.; Siqueira, F. S.; Campos, M. M. A.; Back, D. F.; Morel, A. F.; Dalcol, I. I.; Ind. Crops Prod. 2018, 121, 461. [Crossref] 59. Cavalcanti, A. B. S.; Maia, M. D.; de Figueiredo, P. T. R.; de Menezes, R. P. B.; Monteiro, A. F. M.; Meireles, R. A. R.; Rodrigues, G. C. S.; Silva, A.; Lins, J. D.; Cordeiro, L. V.; Rodrigues, V. S.; Branco, A.; Agra, M. D.; Sessions, Z. L.; Muratov, E. N.; Scotti, L.; da Silva, M. S.; Costa, V. C. D.; Tavares, J. F.; Scotti, M. T.; Nat. Prod. Res. 2023, 37, 903. [Crossref] 60. Cavalcanti, A. B. S.; Figueiredo, P. T. R.; Veloso, C. A. G.; Rodrigues, G. C. S.; Maia, M.; Monteiro, A. F. M.; Rodrigues, V. S.; Castelo-Branco, A. P. O. T.; Agra, M.; Braz-Filho, R.; Silva, M. S.; Tavares, J. F.; Scotti, L.; Scotti, M. T.; Phytochem. Lett. 2021, 43, 117. [Crossref] 61. da Silva, L. L. D.; Nascimento, M. S.; Cavalheiro, A. J.; Silva, D. H. S.; Castro-Gamboa, I.; Furlan, M.; Bolzani, V. S.; J. Nat. Prod. 2008, 71, 1291. [Crossref] 62. Leitão, G.; Figueiredo, F.; Dantas, S.; von Groll, A.; Silva, R.; da Silva, P. A.; Planta Med. 2012, 78, PJ87. [Crossref] 63. Milato, J. V.; Silva, R. S. F.; Figueiredo, F. S.; Azevedo, D. A.; Ribeiro, C. A. B.; Leitão, G. G.; J. Chromatogr. A 2018, 1537, 135. [Crossref] 64. Boeno, S. I. S.; Passos, M. D.; Felix, M.; Calixto, S. D.; Almir, R. C.; Siqueira, L. F. B.; Muzitano, M. F.; Braz-Filho, R.; Vieira, I. J. C.; Nat. Prod. Commun. 2020, 15, 5. [Crossref] 65. de Araújo, M. H.; da Silva, I. C. V.; de Oliveira, P. F.; Barreto, A. R. R.; Konno, T. U. P.; Esteves, F. A.; Barth, T.; Aguiar, F. A.; Lopes, N. P.; Dermenjian, R. K.; Guimarães, D. O.; Leal, I. C. R.; Lasunskaia, E. B.; Muzitano, M. F.; Rev. Bras. Farmacogn. 2017, 27, 702. [Crossref] 66. Leitão, G. G.; Abreu, L. F.; Costa, F. N.; Brum, T. B.; Ramos, D. F.; Silva, P. E. A.; Lourenço, M. C. S.; Leitão, S. G.; Nat. Prod. Commun. 2008, 3, 1995. [Crossref] 67. Nogueira, T. S. R.; Passos, M. S.; Calixto, S. D.; Ventura, T. L. B.; Lassounskaia, E.; Muzitano, M. F.; Braz-Filho, R.; Vieira, I. J. C.; Rev. Virtual Quim. 2021, 13, 1116. [Crossref] 68. Rodrigues, G. C. S.; Scotti, L.; Scotti, M. T.; Pathogens 2023, 12, 449. [Crossref] 69. Passos, M. S.; de Carvalho, A. R.; Boeno, S. I.; das Virgens, L. L. G.; Calixto, S. D.; Ventura, T. L. B.; Lassounskaia, E.; Braz-Filho, R.; Vieira, I. J. C.; Rev. Bras. Farmacogn. 2019, 29, 40. [Crossref] 70. Passos, M. S.; Nogueira, T. S. R.; Robaina, R. R. S.; Calixto, S. D.; Simão, T. L. B. V.; Muzitano, M. F.; Lassounskaia, E.; Braz-Filho, R.; Vieira, I. J. V.; Phytochemistry 2023, 214, 113818. [Crossref] 71. Trevizan, L. N. F.; do Nascimento, K. F.; Santos, J. A.; Kassuya, C. A. L.; Cardoso, C. A. L.; Vieira, M. C.; Moreira, F. M. F.; Croda, J.; Formagio, A. S. N.; J. Ethnopharmacol. 2016, 192, 510. [Crossref] 72. Baldin, V. P.; Scodro, R. B. L.; Lopes-Ortiz, M. A.; de Almeida, A. L.; Gazim, Z. C.; Ferarrese, L.; Faões, V. S.; Torres-Santos, E. C.; Pires, C. T. A.; Caleffi-Ferracioli, K. R.; Siqueira, V. L. D.; Cortez, D. A. G.; Cardoso, R. F.; Phytomedicine 2018, 47, 34. [Crossref] 73. Silva, I. J. M. M.; Fernandes, C. C.; Silva, N. B. S.; Calefi, G. G.; Martins, C. H. G.; Silva, J. B. A.; Crotti, A. E. M.; Miranda, M. L. D.; Nat. Prod. Res. 2024, 30, 1. [Crossref] 74. Julião, L. S.; Bizzo, H. R.; Souza, A. M.; Lourenço, M. C. S.; Silva, P. E. A.; Tavares, E. S.; Rastrelli, L.; Leitão, S. G.; Nat. Prod. Commun. 2009, 4, 1733. [Crossref] 75. de Lima, V. H. M.; Almeida, K. C. R.; Alves, C. C. F.; Rodrigues, M. L.; Crotti, A. E. M.; de Souza, J. M.; Ribeiro, A. B.; Squarisi, I. S.; Tavares, D. C.; Martins, C. H. G.; Miranda, M. L. D.; Rev. Bras. Farmacogn. 2019, 29, 807. [Crossref] 76. Quintino, R. L.; Reis, A. C.; Fernandes, C. C.; Martins, C. H. G.; Colli, A. C.; Crotti, A. E. M.; Squarisi, I. S.; Ribeiro, A. B.; Tavares, D. C.; Miranda, M. L. D.; Braz. Arch. Biol. Technol. 2020, 63, 1. [Crossref] 77. Pinto, S. C.; Leitão, G. G.; Oliveira, D. R.; Bizzo, H. R.; Ramos, D. F.; Coelho, T. S.; Silva, P. E. A.; Lourenço, M. C. S.; Leitão, S. G.; Nat. Prod. Commun. 2009, 4, 1675. [Link] accessed in July 2025 78. Lopes, F. C. M.; Placeres, M. C. P.; Jordão Junior, C. M.; Higuchi, C. T.; Rinaldo, D.; Vilegas, W.; Leite, C. Q. F.; Carlos, I. Z.; Mem. Inst. Oswaldo Cruz 2007, 102, 769. [Crossref] 79. Ramos, D. F.; Leitão, G. G.; Costa, F. D.; Abreu, L.; Villarreal, J. V.; Leitão, S. G. Fernandez, S.; da Silva, P. E. A.; Braz. J. Pharm. Sci. 2008, 44, 669. [Crossref] 80. Carli, A. C. B.; Quilles, M. B.; Maia, D. C. G.; Lopes, F. C. M.; Santos, R.; Pavan, F. R.; Leite, C. Q. F.; Calvo, T. R.; Vilegas, W.; Carlos, I. Z.; Pharm. Biol. 2010, 48, 878. [Crossref] 81. Moraes, T.; Araújo, M.; Bernardes, N.; Oliveira, D.; Lasunskaia, E.; Muzitano, M.; da Cunha, M.; Planta Med. 2011, 77, 964. [Crossref] 82. Boligon, A. A.; Piana, M.; Kubiça, T. F.; Mario, D. N.; Dalmolin, T. V.; Bonez, P. C.; Weiblen, R.; Lovato, L.; Alves, S. H.; Campos, M. M. A.; Athayde, M. L.; J. Appl. Biomed. 2015, 13, 7. [Crossref] 83. Leitão, F.; Leitão, S. G.; Cantos, J.; Coelho, T.; da Silva, P. E. A.; J. Ethnopharmacol. 2013, 149, 513. [Crossref] 84. Costa, I. F. B.; Calixto, S. D.; de Araujo, M. H.; Konno, T. U. P.; Tinoco, L. W.; Guimarães, D. O.; Lasunskaia, E. B.; Leal, I. C. R.; Muzitano, M. F.; Sci. World J. 2015, 2015, 1. [Crossref] 85. Reis, A. J.; Carrion, L. L.; Rodrigues, K.; Fenalti, J. M.; Mata-Santos, T.; Scaini, C. J.; Martins, D.; Mesquita, D. W. D.; Mesquita, A. S. S.; Nunez, C. V.; da Silva, P. E. A.; Ramos, D. F.; Revista de Epidemiologia e Controle de Infecção 2016, 6, 108. [Crossref] 86. Ishikawa, R. B.; Leitão, M. M.; Kassuya, R. M.; Macorini, L. F.; Moreira, F. M. F.; Cardoso, C. A. L.; Coelho, R. G.; Pott, A.; Gelfuso, G. M.; Croda, J.; Oliveira, R. J.; Kassuya, C. A. L.; J. Ethnopharmacol. 2017, 204, 18. [Crossref] 87. Santiago-Carvalho, I.; Simão, T. L. B. V.; Calixto, S. D.; de Albuquerque, T. C.; Konno, T. U. P.; Salgado, R. M.; Muzitano, M. F.; Pinto, S. C.; Lasunskaia, E.; Phytomedicine Plus 2022, 2, 100236. [Crossref] 88. dos Santos, J. G.; Fernandes, C. C.; Silva, N. B. S.; Calefi, G. G.; Martins, C. H. G.; Volpini, G. A.; Crotti, A. E. M.; Ribeiro, A. B.; Esperandim, T. R.; Tavares, D. C.; Batalini, C.; Miranda, M. L. D.; Nat. Prod. Res. 2024, 39, 1428 [Crossref] 89. Mascarello, A.; Orbem Menegatti, A. C.; Calcaterra, A.; Martins, P. G. A.; Chiaradia-Delatorre, L. D.; D’Acquarica, I.; Ferrari, F.; Pau, V.; Sanna, A.; De Logu, A.; Botta, M.; Botta, B.; Terenzi, H.; Mori, M.; Eur. J. Med. Chem. 2018, 144, 277. [Crossref] 90. Mascarello, A.; Mori, M.; Chiaradia-Delatorre, L. D.; Menegatti, A. C. O.; Monache, F. D.; Ferrari, F.; Yunes, R. A.; Nunes, R. J.; Terenzi, H.; Botta, B.; Botta, M.; PLoS One 2013, 8, e77081. [Crossref] 91. Antunes, S. S.; Rabelo, V. W. H.; Romeiro, N. C.; Comput. Biol. Med. 2021, 136, 104694. [Crossref] 92. Taveira, G. B.; Simão, T. L. B. V.; Cherene, M. B.; Muzitano, M. F.; Lassounskaia, E.; Pireda, S.; Basso, L. G. M.; da Cunha, M.; Motta, O. V.; Gomes, V. M.; Biochim. Biophys. Acta, Gen. Subj. 2022, 1866, 130218. [Crossref] 93. Gibbons, S.; Planta Med. 2008, 74, 594. [Crossref] 94. de Souza, C. G.; Haas, A. P. S.; von Poser, G. L.; Schapoval, E. E. S.; Elisabetsky, E.; J. Ethnopharmacol. 2004, 90, 135. [Crossref] 95. Albuquerque, U. P.; Medeiros, P. M.; Almeida, A. L. S.; Monteiro, J. M.; Melo, J. G.; Santos, J. P.; J. Ethnopharmacol. 2007, 114, 325. [Crossref] 96. Albuquerque, U. P.; Monteiro, J. M.; Ramos, M. A.; de Amorim, E. L. C.; J. Ethnopharmacol. 2007, 110, 76. [Crossref] 97. Ferreira, C. M.; J. Ethnopharmacol. 2009, 126, 159. [Crossref] 98. Shanley, P.; Silva, M. S.; Melo, T.; Carmenta, R.; Nasi, R.; For. Ecol. Manage. 2012, 268, 70. [Crossref] 99. Santos, J. F. L.; Pagani, E.; Ramos, J.; Rodrigues, E.; J. Ethnopharmacol. 2012, 142, 503. [Crossref] 100. Breitbach, U. B.; Niehues, M.; Lopes, N. P.; Faria, J. E. Q.; Brandão, M. G. L.; J. Ethnopharmacol. 2013, 147, 180. [Crossref] 101. Ribeiro, D. A.; de Oliveira, L. G. S.; de Macêdo, D. G.; de Menezes, I. R. A.; da Costa, J. G. M.; da Silva, M. A. P.; Lacerda, S. R.; Souza, M. M. A.; J. Ethnopharmacol. 2014, 155, 1522. [Crossref] 102. Oliveira, D. R.; Leitão, G. G.; Coelho, T. S.; da Silva, P. E. A.; Lourenço, M. C. S.; Leitão, S. G.; Rev. Bras. Farmacogn. 2011, 21, 793. [Crossref] 103. Bolson, M.; Hefler, S. R.; Chaves, E. I. D.; Gasparotto Junior, A.; Cardozo Junior, E. L.; J. Ethnopharmacol. 2015, 161, 1. [Crossref] 104. Saraiva, M. E.; Ulisses, A. V. R.; Ribeiro, D. A.; Oliveira, L. G. S.; Sousa, F. F. S.; Menezes, I. R.; Souza, M. M. A.; J. Ethnopharmacol. 2015, 171, 141. [Crossref] 105. Pedrollo, C. T.; Kinupp, V. F.; Shepard, G.; Heinrich, M.; J. Ethnopharmacol. 2016, 186, 111. [Crossref] 106. Lago, J. H. G.; Tezoto, J.; Yazbek, P. B.; Cassas, F.; Santos, J. F. L.; Rodrigues, E.; Rev. Bras. Farmacogn. 2016, 26, 379. [Crossref] 107. Magalhães, K. N.; Guarniz, W. A. S.; Sá, K. M.; Freire, A. B.; Monteiro, M. P.; Disgusting, R. T.; Bieski, I. G. C.; Custodio, J. B.; Balogun, S. O.; Flag, M. A. M.; J. Ethnopharmacol. 2019, 237, 314. [Crossref] 108. Broekaert, W. F.; Terras, F. R. G.; Cammue, B. P. A.; Osborn, R. W.; Plant Physiol. 1995, 108, 1353. [Crossref] 109. Andreu, D.; Rivas, L.; Biopolymers 1998, 47, 415. [Crossref] 110. Alves, R. R. N.; Léo Neto, N. A.; Brooks, S. E.; Albuquerque, U. P.; J. Ethnopharmacol. 2009, 124, 600. [Crossref] 111. Alves, R. R. N.; Rosa, I. L.; J. Ethnopharmacol. 2007, 111, 82. [Crossref] 112. Alves, R. R. N.; Rosa, I. L.; J. Ethnopharmacol. 2007, 113, 541. [Crossref] 113. Donia, M.; Hamann, M. T.; Lancet Infect. Dis. 2003, 3, 338. [Crossref] 114. Azevedo, L. G.; Muccillo-Baisch, A. L.; Filgueira, D. M. V. B.; Boyle, R. T.; Ramos, D. F.; Soares, A. D.; Lerner, C.; Silva, P. A.; Trindade, G. S.; Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2008, 147, 36. [Crossref] 115. Cavalcanti, B. C.; Sombra, C. M. L.; de Oliveira, J. H. H. L.; Berlinck, R. G. S.; de Moraes, M. O.; Pessoa, C.; Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2008, 147, 409. [Crossref] 116. de Oliveira, J. H. H. L.; Grube, A.; Köck, M.; Berlinck, R. G. S.; Macedo, M. L.; Ferreira, A. G.; Hajdu, E.; J. Nat. Prod. 2004, 67, 1685. [Crossref] 117. de Oliveira, J. H. H. L.; Seleghim, M. H. R.; Timm, C.; Grube, A.; Köck, M.; Nascimento, G. G. F.; Martins, A. C. T.; Silva, E. G. O.; de Souza, A. O.; Minarini, P. R. R.; Galetti, F. C. S.; Silva, C. L.; Hajdu, E.; Berlinck, R. G. S.; Mar. Drugs 2006, 4, 1. [Crossref] 118. Oliveira, M. F.; Oliveira, J. H.; Galetti, F. C.; Souza, A. O.; Silva, C. L.; Hajdu, E.; Peixinho, S.; Berlinck, R. G.; Planta Med. 2006, 72, 437. [Crossref] 119. Gandolfi, R. C.; Medina, M. B.; Berlinck, R. G. S.; Lira, S. P.; Galetti, F. C. S.; Silva, C. L.; Veloso, K.; Ferreira, A. G.; Hajdu, E.; Peixinho, S.; Quim. Nova 2010, 33, 1853. [Crossref] 120. Machado, F. S.; Ventura, T.; Gestinari, L.; Cassano, V.; Resende, J.; Kaiser, C.; Lasunskaia, E.; Muzitano, M.; Soares, A.; Molecules 2014, 19, 3181. [Crossref] 121. Ventura, B. L. T.; Machado, F. S.; Araujo, M.; Kaiser, C.; Esteves, F. A.; Lasunskaia, E.; Soares, A.; Muzitano, M.; Pharmacogn. Mag. 2015, 11, 611. [Crossref] 122. Ramos, D. F.; Halicki, P. C. B.; Borges, P.; D’Oca, C. R. M.; Santos, M.; D’Oca, M. G. M.; Roselet, F.; da Silva, P. E. A.; Abreu, P. C.; Chem. Biodiversity 2022, 19, e202100846. [Crossref] 123. Ramos, D.; Matthiensen, A.; Colvara, W.; de Votto, A.; Trindade, G.; da Silva, P.; Yunes, J.; J. Venomous Anim. Toxins Incl. Trop. Dis. 2015, 21, 9. [Link] accessed in July 2025 124. Harvey, A.; Drug Discovery Today 2000, 5, 294. [Crossref] 125. Davies, J. In Aminoglycoside Antibiotics: From Chemical Biology to Drug Discovery; Arya, D. P., ed.; Wiley: Hoboken, 2007, p. 1. 126. Andrioli, W. J.; Simão, T. L. B. V.; Ferreira, D. P.; Araújo, M. H.; Calixto, S. D.; Bastos, J. K.; Seldin, L.; Lasunskaia, E.; Muzitano, M. F.; Phytomedicine Plus 2022, 2, 100312. [Crossref] 127. Azevedo, V. L. S.; Rosa, F. C.; Dias, L. R. L.; Batista, L. A.; Melo, M. C.; Sales, L. A. T.; Branco, A. J. M.; Araújo, T. R. R.; Miranda, R. C. M.; Aliança, A. S. S.; Microorganisms 2024, 12, 476. [Crossref] 128. Calixto, S. D.; Simão, T. L. B. V.; Almeida, F. M.; Antunes, S. S.; Romeiro, N. C.; Borges, W. S.; Andrioli, W. J.; Guimarães, D. O.; Lasunskaia, E.; Muzitano, M. F.; J. Pharm. Pharmacol. 2022, 74, 446. [Crossref] 129. Prince, K. A.; Sordi, R.; Pavan, F. R.; Santos, A. C. B.; Araújo, A. R.; Leite, S. R. A.; Leite, C. Q. F.; Braz. J. Microbiol. 2012, 43, 224. [Link] accessed in July 2025 130. Honda, N. K.; Pavan, F. R.; Coelho, R. G.; Leite, S. R. A. A.; Micheletti, A. C.; Lopes, T. I. B.; Misutsu, M. Y.; Beatriz, A.; Brum, R. L.; Leite, C. Q. F.; Phytomedicine 2010, 17, 328. [Crossref] 131. Cooper, C. B.; J. Med. Chem. 2013, 56, 7755. [Crossref] 132. Swinney, D. C.; Anthony, J.; Nat. Rev. Drug Discovery 2011, 10, 507. [Crossref] 133. Grzelak, E. M.; Choules, M. P.; Gao, W.; Cai, G.; Wan, B.; Wang, Y.; McAlpine, J. B.; Cheng, J.; Jin, Y.; Lee, H.; Suh, J. W.; Pauli, G. F.; Franzblau, S. G.; Jaki, B. U.; Cho, S.; J. Antibiot. 2019, 72, 719. [Crossref] 134. Altaf, M.; Miller, C. H.; Bellows, D. S.; O’Toole, R.; Tuberculosis 2010, 90, 333. [Crossref] 135. Andries, K.; Verhasselt, P.; Guillemont, J.; Göhlmann, H. W. H.; Neefs, J. M.; Winkler, H.; Van Gestel, J.; Timmerman, P.; Zhu, M.; Lee, E.; Williams, P.; de Chaffoy, D.; Huitric, E.; Hoffner, S.; Cambau, E.; Truffot-Pernot, C.; Lounis, N.; Jarlier, V.; Science 2005, 307, 223. [Crossref] 136. Lopes, M. A.; Ferracioli, K. R. C.; Siqueira, V. L. D.; Scodro, R. B. L.; Cortez, D. A. G.; da Silva, R. Z.; Cardoso, R. F.; International Union Against Tuberculosis and Lung Disease 2014, 18, 1513. [Link] accessed in July 2025 137. Ghiraldi-Lopes, L. D.; Campanerut-Sá, P. A.; Meneguello, J. E.; Seixas, F. A.; Lopes-Ortiz, M. A.; Scodro, R. B.; Pires, C. T.; da Silva, R. Z.; Siqueira, V. L.; Nakamura, C. V.; Cardoso, R. F.; Future Microbiol. 2017, 12, 867. [Crossref] 138. Lu, N.; Yang, Y.; Li, X.; Li, J.; Cheng, J.; Lv, Z.; Du, Y.; J. Vet. Res. 2021, 65, 431. [Crossref] 139. Veiga-Santos, P.; Desoti, V. C.; Miranda, N.; Ueda-Nakamura, T.; Dias-Filho, B. P.; Silva, S. O.; Cortez, D. A. G.; de Mello, J. C. P.; Nakamura, C. V.; Acta Trop. 2013, 125, 349. [Crossref] 140. McFerren, M. A.; Cordova, D.; Rodriguez, E.; Rauh, J. J.; J. Ethnopharmacol. 2002, 83, 201. [Crossref] 141. Fernandez, C. M. M.; Kleinubing, S. A.; Karam, T. K.; Lorenzetti, F. B.; Palomo, C. T.; de Oliveira, B. P.; Gonçalves, O. H.; Gazim, Z. C.; Romagnolo, M. B.; Caleffi-Ferracioli, K. R.; Scodro, R. B.; Lima, M. M.; Nakamura, T. U.; Nakamura, C. V.; Dias Filho, B. P.; Bol. Latinoam. Caribe Plant Med. Aromat. 2023, 22, 821. [Link] accessed in July 2025 142. Antunes, S. S.; Forn-Cuní, G.; Romeiro, N. C.; Spaink, H. P.; Verbeek, F. J.; Muzitano, M. F.; Drug Discovery Today 2024, 29, 104163. [Crossref] 143. Yang, H. J.; Wang, D.; Wen, X.; Weiner, D. M.; Via, L. E.; Front. Cell Infect. Microbiol. 2021, 11, 1. [Crossref]
Editor handled this article: Jorge M. David |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access