Artigo
| LC-HRMS analysis of cafestol isolated from coffee and its metabolites after fermentation with Saccharomyces cerevisiae commercial yeasts |
|
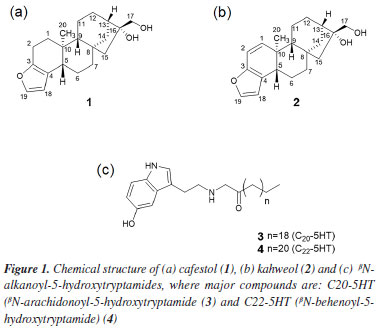
Natália A. B. TinocoI I. Instituto de Química, Universidade Federal do Rio de Janeiro, 21941-909 Rio de Janeiro - RJ, Brasil *e-mail: claudia.rezendeufrj@gmail.com Cafestol (1) is an ent-kaurane furanoditerpene found in the lipid fraction of coffee beans and is known for its hypercholesterolemic effect in humans. This adverse biological effect is particularly concerning when consuming non-filtered coffee beverages, such as Turkish and mocha coffees. In previous research, we investigated a wet postharvest process in coffee using three commercial Saccharomyces cerevisiae starter cultures (from bakery, white, and sparkling wines) capable of reducing lipid compounds. A reduction of cafestol (1) by approximately 54% was observed compared to a wet process without this yeast. The wet postharvest process is usually employed to obtain specialty coffees. In this work, we employed liquid chromatography coupled to high-resolution mass spectrometry (LC-HRMS) to better understand the chemical transformations that cafestol underwent under commercial S. cerevisiae starter cultures fermentation conditions. Three metabolites derived from cafestol (1) oxidation at position C-17 (aldehyde isomers (6 and 7) and one hydroxymethyl furfural derivative (8)) were suggested because of coffee fermentation. This work opens perspectives for future studies since little is known about the biotransformation reactions of endogenous compounds in green coffee beans during wet processing. INTRODUCTION The two types of coffee sold worldwide are from the species Coffea arabica L. and C. canephora P., Rubiaceae, with their different varieties, and Brazil is the world's largest coffee producer. Coffee production involves several stages from planting to postharvest, which strongly affects the flavors that will be formed after roasting the beans. Green (or raw) coffee beans of C. arabica L. are traditionally processed by one of the three postharvest methods, known as dry, semi-dry, and wet. In the dry process, coffee beans are dried under the sun without removing the husk and internal layers, generating the so-called natural coffee. In the wet process (or washed coffee), ripe coffee has its peel and pulp removed by a combination of mechanical and fermentative processes through the action of microbial and/or endogenous coffee enzymes.1-3 In the semi-dry process (pulped natural coffee), only the peel is removed before processing in a similar way as the wet process.4 The evolution of postharvest processes has highlighted the importance of conducting fermentation in a controlled environment, particularly through the application of starter cultures. This approach has been recognized as beneficial for enhancing flavor profiles, generating volatile compounds, and ensuring product consistency.5 "Specialty coffee" usually gives beverages that exceed the quality of those available at commodity prices and is characterized by its distinctive flavors. The wet postharvest processing technique plays a crucial role in enhancing the desirable attributes of these coffees.5 Germination and fermentation are considered critical processes that influence the chemical and sensory aspects obtained by postharvest processes.4-8 Selmar et al.9 proposed that germination may be triggered during the postharvest phase, with its degree and characteristics being influenced by the conditions of the processing.9 However, this phenomenon is predominantly observed in the wet method. Germination can start in the early stage of the postharvest and may occur together with fermentation by a combination of bacteria, yeast, and filamentous fungi, all they present in the diverse microbiota that are observed in coffee production, which is influenced by environmental conditions and the management of processes on the farm.10,11 Green Arabica coffee bean is mainly composed of carbohydrates (around 60%), up to 17% of lipids, 15% of protein, 4% of minerals besides caffeine (up to 1.3%), trigonelline (up to 2.0%) and chlorogenic acid (up to 7.9%). Coffee lipids are composed of triacylglycerols (75%), ent-kaurane diterpene esters (up to 18%), free diterpenes (0.4%, cafestol (1) and kahweol (2)), sterol esters (2.2%), free sterols (3.2%), tocopherols (up to 0.06%), phosphatides (0.1-0.5%) and βN-alkanoyl-5-hydroxytryptamides (up to 1.0%).12 Aspects about the impact of fermentative processes on coffee have been helping to explain the chemical transformations that occur in postharvest and their influence on roasted coffee flavor. Metabolites typically present in coffee fermentation processes are simple carbohydrates such as glucose, sucrose, and fructose; organic acids including lactic, acetic, and malic acids; sugar alcohols like glycerol, mannitol, and sorbitol; as well as acetaldehyde, ethanol, ethyl acetate, and ethyl lactate.13-17 Related to studies with starter cultures, Saccharomyces cerevisiae and Pichia fermentans are the most used.5 In a different approach, our group evaluated how S. cerevisiae commercial yeasts (from bakery, white, and sparkling wines), fermentation time, and pH influence the chemical composition of coffee bioactives and beverage quality of Arabica coffee beans.18 Fifty-six conditions were evaluated using fractional factorial and mixture experimental designs. No difference was observed in caffeine and chlorogenic acid contents, but a substantial impact on the lipid fraction was observed. βN-Alkanoyl-5-hydroxytryptamides were reduced by up to 38% for βN-arachydoyl-5-hydroxytryptamine (3) (C20-5HT) and 26% for βN-behenoyl-5-hydroxytryptamide (4) (C22-5HT), the major compounds of this class (Figure 1). Besides, an impressive reduction was observed for the pentacyclic diterpene alcohols of ent-kaurane skeleton present in the coffee lipid fraction, 54% for cafestol (1) and 53% for kahweol (2) (Figure 1). This reduction occurred after treating the Arabica green coffee beans with 0.6 g of a 1:1:1 mixture of the three yeasts (bakery, white, and sparkling wines) for 12 h at 15 ºC and pH 4, the condition that most affected these compounds among the 56 experiments studied.18
Cafestol (1) shows different beneficial biological activities, including antioxidant, anti-carcinogenic, anti-inflammatory and anti-diabetic.19 However, it has a significant hypercholesterolemic activity,20 which is especially worrying when ingesting hot, unfiltered coffee beverages, such as Turkish and mocha coffees. In this context, the development of postharvest processes that favor the reduction of chemical compounds such as caffeine (as in decaffeinated coffees) and, in this context, compound 1, without affecting the quality of the drink, are interesting for the market. Every day our concern about the foods we consume grows and consequently the need to understand their chemical composition in terms of how they affect our health. Aspects relating to the composition of the food itself, those formed in fermentative processes and the possible microbiome present have been explored due to advances in spectrometric techniques and computational methodologies, as is the case of metabolomics carried out by mass spectrometry (MS). Mass spectrometry is a robust technique that can be coupled to different types of chromatography applied to investigate different metabolites in food matrices.21,22 In the present study, the metabolism of cafestol (1), possibly induced by S. cerevisiae commercial yeasts, was investigated using liquid chromatography coupled to high-resolution mass spectrometry (LC-HRMS). As far as we know, this is the first investigation related to coffee diterpenes metabolites induced by a fermentative process with S. cerevisiae.
EXPERIMENTAL Chemicals and reagents Cafestol (1), with a purity above 98%, was isolated from green Arabica coffee beans as described elsewhere.23,24 Formic acid, acetic acid, ammonium formate, sodium phosphate, acetonitrile, and methanol were analytical or HPLC grade (Tedia, Fairfield, OH, USA). Ultrapure water (18 MΩ cm-1) was obtained from a Millipore Milli-Q water system (Burlington, MA, USA). Strata-X-CW weak cation mixed-mode polymeric sorbent (30 mg, 3 mL) SPE cartridges were from Phenomenex (Torrance, CA, USA). Biotransformation of cafestol by S. cerevisiae commercial strains In a 50 mL amber flask, 30 mL of YPD medium (yeast peptone dextrose, 1% yeast extract, 2% peptone, and 2% glucose, pH 4, Sigma-Aldrich, USA) was enriched with cafestol (25 µg mL-1), which was previously solubilized in 1% DMSO (dimethyl sulfoxide). For the inoculum, a mixture of 30 mg of S. cerevisiae strains (Fleischmann, FL; Fermol Elegance, FE; and Perlage BB, PB, 1:1:1 AEB Biochemical, USA) was used. This condition was based on the findings of Tinoco et al.,18 which identified the optimal conditions for reducing cafestol in coffee beans using commercial S. cerevisiae. Two control experiments were performed. The first used YPD medium (30 mL) enriched with cafestol (25 µg mL-1) without yeast. The second used YPD medium (30 mL) without cafestol but inoculated with 30 mg of the S. cerevisiae strains. The cultures were incubated for 12 h at 15 ºC (Nova Ética Incubator, model BOD 411-D, Brazil). The experiments were carried out in triplicate. After the fermentation time, all samples were centrifuged for 5 min at 6800 g and the supernatants were collected and stored in a freezer (-16 ºC) until analysis. Detailed conditions are described elsewhere.18 Sample preparation for liquid chromatography-high resolution mass spectrometry (LC-HRMS) analysis Aliquots of 10 mL of the culture medium were collected in triplicates and centrifugated at 7056 g for 15 min. The liquid upper layer was collected and processed via solid-phase extraction (SPE) using a Strata-X-CW column (30 mg, Phenomenex, USA) preconditioned with 2 mL of methanol, followed by 2 mL of ultrapure water, as described by Andriolo et al.25 Samples were washed with 2 mL of ultrapure water and 1 mL of methanol:water (1:1 v/v). The elution was performed with 3 mL of methanol:formic acid (95:5, v/v) and the eluent dried under a flow of N2 at room temperature, followed by resuspension with 50 µL aqueous acetic acid 2% (v/v), which was then analyzed by LC-HRMS. LC-HRMS analysis The LC separation was performed on a Thermo Scientific Dionex Ultimate 3000 equipment (Thermo Fisher Scientific, USA) using a reversed-phase column (Syncronis C18, 1.7 μm, 50 mm × 2.1 mm, Thermo Fisher Scientific, USA). The mobile phase consisted of Milli-Q water with 0.1% formic acid and 5 mM ammonium formate (mobile phase A) and methanol containing 0.1% formic acid (mobile phase B) in a gradient of 5% B (0-4 min), 50% B (4.1-10 min), 98% B (10.1-17 min), and 5% B (17.1-22 min). The flow rate was 0.35 mL min-1, the injection volume was 5 µL, and the column oven temperature was 40 ºC. The mass spectrometry analysis was performed using a Q-Exactive™ Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific, USA) equipped with an electrospray ion source (ESI). The mass spectrometer conditions were: spray voltage +3.9 kV, ion transfer capillary 400 ºC, sheath, and auxiliary gases 50 and 15 arbitrary units, respectively. Data acquisition was performed in positive ion mode using full scan (Full-MS) over the m/z range 100-1000 at 70,000 resolution, followed by ddMS2 Top 3 experiment at a resolution of 17,500. The collision energy (CE) ramp was 20 to 60 eV. In addition, parallel reaction monitoring (PRM) for the cafestol ion [M + H]+ 317.2111 was used to monitor this substance in the samples. A mass tolerance of 6 ppm was used for compound identification. The equipment was calibrated in positive mode with the manufacturer's calibration solution (Thermo Fisher Scientific, Germany). Data analysis was conducted using Thermo Scientific™ Xcalibur™ 4.3 software (Thermo Fisher Scientific, USA). In silico analysis To investigate the possible metabolites from the biotransformation of cafestol by S. cerevisiae commercial cultures, prediction in silico analyses were performed on Compound Discoverer 3.3 software (Thermo Scientific™, USA, 2018) and Way2Drug (PASS, Russia, 2018)26 a free web platform for metabolism prediction. The analysis was conducted by entering cafestol's SMILES structure, as previously described by Andriolo et al.25
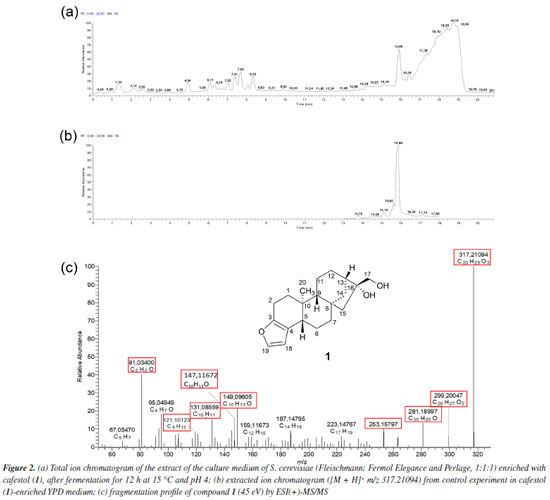
RESULTS AND DISCUSSION Figure 2a shows the total ion chromatogram in positive electrospray ion mode from the biotransformation assay with S. cerevisiae (Fleischmann, Fermol Elegance and Perlage, 1:1:1) in YPD medium enriched with diterpene 1 (25 μg mL-1). This was the condition previously described as responsible for reducing 54% of compound 1 content.18 Andriolo et al.25 and Brand et al.22 showed that positive electrospray ion mode was more efficient in investigating 1 and its metabolites in zebrafish tank and in compound 1 bioaccessibility.
The extracted ion chromatogram from compound 1 ([M + H]+ m/z 317.21094, retention time (tR) 15.86 min) spiked in YPD medium at 25 μg mL-1 without S. cerevisiae (control experiment) is shown in Figure 2b. The fragmentation mass spectrum of compound 1 ion [M + H]+ m/z 317.21094, by ESI(+)-MS/MS (Figure 2c) was similar to those found in the literature.22,25,27 The fragments at m/z 299.20047 [M + H - H2O]+ and m/z 281.18997 [M + H - 2H2O]+ were related to the loss of one and two water molecules, respectively. Fragments at m/z 253.15797, 149.09605, and 147.11672, typical of the furan moiety, were also observed by Andriolo et al.,25 who proposed an opening of the B and C rings of compound 1 via a retro-Diels-Alder reaction followed by elimination of the side chain by remote hydrogen rearrangement for the fragment m/z 253.15797. In addition, fragment m/z 81.03400 has molecular formula C5H5O and an unsaturation index equal to 4, as shown in Figure 3.
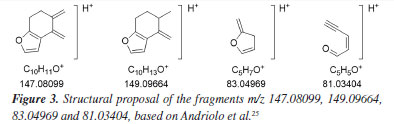
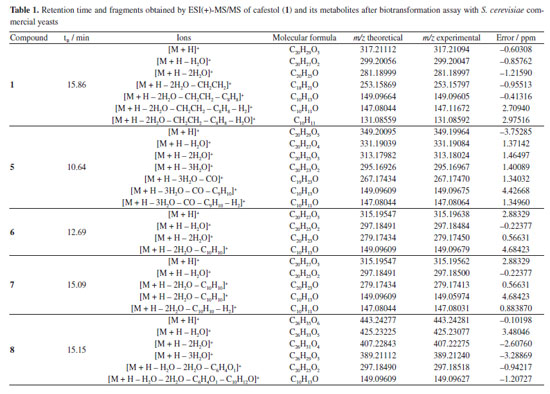
Table 1 presents the retention time and fragments obtained by ESI(+)-MS/MS of compound 1 and its metabolites after biotransformation assay with commercial S. cerevisiae yeast.
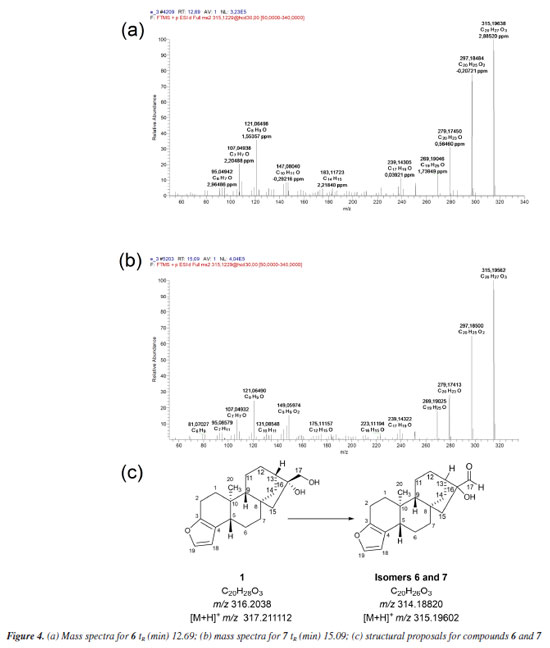
The in silico analyses (Way2Drug platform and Compound Discoverer software) were used to predict coffee metabolites and compare them to the ions found after LC-HRMS analysis, supporting the process of identifying analytes with a high level of accuracy. Starting the discussion for metabolites 6 and 7 (Figures 4a, 4b and 4c), isomers with [M + H]+ m/z 315.19547, a deficiency of two Hs is observed when compared to 1 [M + H]+ m/z 317.21094. In the fragmentation pattern, it can be seen a loss of two water molecules (m/z 297.18491, [M + H - H2O]+ and m/z 279.17434, [M + H - 2H2O]+). These ions, together with the presence of the diagnostic ion of the furan ring m/z 149.09609, suggest the presence of isomeric aldehydes 6 and 7, as proposed in Figure 4c, formed from the oxidation of the primary alcohol at C-17 in cafestol. The fact that two metabolites of the same mass occur at different retention times and with similar fragmentation may be related, in this case, to an epimerization at C-13 or C-16, arising from tertiary alcohol dehydration, which can be followed by hydration. This dehydration is known to occur during coffee roasting, giving rise to dehydrocafestol and dehydrokahweol isomers.28,29 However, to better support this hypothesis, isotopic labeling would be necessary.
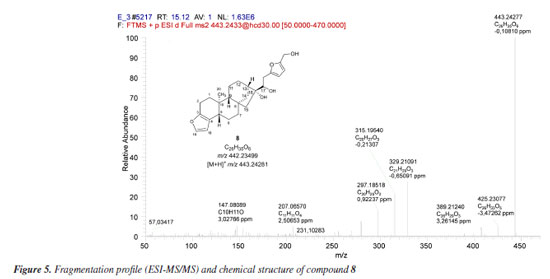
Biotransformation reactions mediated by S. cerevisiae are described as hydrolysis, reduction, and oxidation involving invertases, esterases, lipases, among others.30 Alcohol dehydrogenases (ADH) are oxidoreductases that catalyze the reversible oxidation of alcohols to aldehydes or ketones, and S. cerevisiae has at least seven isoforms of ADH, which vary in their specificities and reactivity. In addition to these, P450 monooxygenases are also responsible for catalyzing oxidation reactions. Cytochrome P450 is a set of proteins containing a heme group, whose most common reaction is the hydroxylation of organic structures by cleavage at the C-H bond, forming compounds that are more polar than their precursors. Cytochrome P450 has several isoforms that catalyze the same type of oxidation reaction on different substrates. Furthermore, knowing that: (i) P450 is the main enzyme complex responsible for the biotransformation of drugs in the human organism, being present mainly in the liver, lungs and kidneys;31 (ii) the strains of S. cerevisiae have three isoforms of P450s, and (iii) that CYP51 is also found in humans,32 it seemed rational to join these approaches together with the software proposals, in an attempt to elucidate the metabolites present in diterpene 1 reduction promoted by S. cerevisiae yeasts. The MS/MS spectrum of compound 8 (Figure 5) shows the ion [M + H]+ m/z 443.24277. The ions m/z 425.23077 [M + H - H2O]+, m/z 407.22275 [M + H - 2H2O]+, and m/z 389.21240 [M + H - 3H2O]+ suggest the presence of three OH groups in the structure. The fragment [M + H]+ m/z 315.19540 refers to the loss of a C6H8O3 moiety.
The C6H8O3 moiety could be associated with a 2,5-dihydroxymethylfuran (10) unit, probably generated by the hydrogenation of 5-hydroxymethylfurfural (HMF) (9), a product of dehydration of monosaccharides such as glucose and fructose. Liu et al.33 investigated the ability of S. cerevisiae and Pichia stipitis strains to biotransform HMF, and showed 2,5-dihydroxymethylfuran as the end product of the hydrogenation of HMF by these yeasts.33 Later, Liu and Moon34 characterized the enzyme responsible for this biotransformation, ARIP1 (activin receptor-interacting protein 1, an aldehyde-dependent reductase NADPH - nicotinamide adenine dinucleotide phosphate hydrogen) as shown in Figure 6.
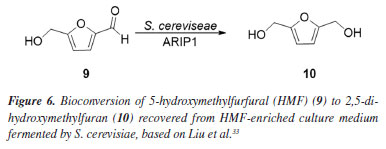
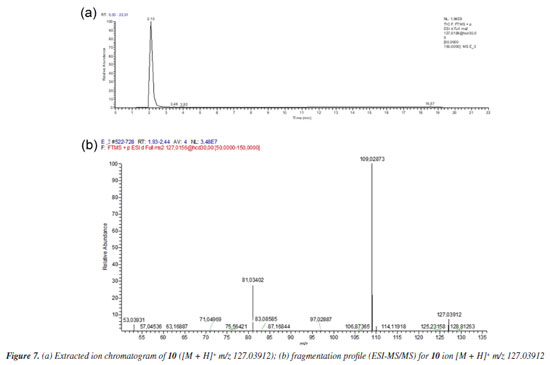
Figure 7a shows the chromatogram of the extracted ion m/z 127.03912 [M + H]+ referring to the 2,5-dihydroxymethylfuran (10), and its fragmentation spectrum (Figure 7b).
The MS profile of ion [M + H]+ m/z 127.03912, in Figure 7b, is similar to that observed by Lucioli et al.,35 who analyzed the chemical composition of some culture media by HPLC-UV and UPLC-ESI-QTOF-MS/MS, looking for bioactive compounds likely to exert cytotoxic activity. For 5-hydroxymethylfurfural (9), ions at m/z 109.0209 (C6H5O2), m/z 81.0346 (C5H5O) and m/z 53.0410 (C4H5) were described, as also observed in MS/MS fragmentation of ion m/z 127.0317, as shown in Figure 7b. Thus, analyzing the MS/MS spectrum of substance 8, besides the considerations already discussed, it is reasonable to suppose the condensation of 2,5-hydroxymethylfuran to the aldehydes 6 and 7 to give 8, as proposed in Figure 5. The formation of hemi-acetals is known in yeasts,36 as also specifically demonstrated by Kusano et al.,37 in S. cerevisiae. Besides the proposal of metabolites 6, 7 and 8, compound 5 (C20H28O5) also showed fragment m/z 149.09627 and could also be associated with a furanoid compound. However, the fragmentation did not allow us to propose a reasonable structure for this metabolite, unless the incorporation of two OH units to complete the observed ion [M + H]+ m/z 349.20095.
CONCLUSIONS In 1983, Thelle et al.38 reported the relationship between the consumption of boiled coffee and an increased cholesterol level in Norwegians, which was later associated with cafestol.20 Despite this negative aspect, cafestol shows a multi-target anticancer activity and other positive biological activities such as anti-inflammatory.19 It is also known that cafestol undergoes structural changes when subjected to more intense roasting conditions, favoring the dehydration of the molecule through its tertiary hydroxyl at C-16,26 whose biological activity still needs to be understood. Although many foods undergo fermentation processes for commercialization, studies employing highly sensitive and high-resolution analytical techniques to elucidate the metabolites produced during fermentation remain scarce, particularly in the case of coffee. The application of mass spectrometry techniques can clarify the chemical transformations that food constituents experience. The results showed open perspectives for further studies, as little is known about the biotransformation reactions of endogenous compounds in green coffee beans during wet processing.
DATA AVAILABILITY STATEMENT All data are available in the text.
ACKNOWLEDGMENTS The authors thank EMBRAPA CAFÉ. This study was financially supported by FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro), process SEI -E26/211.375/2021, E-200.862/2021 and E-26/202.941/2017. The authors also acknowledge CNPq (process 401749/2019-3 and 309212/2021-9.
AUTHOR CONTRIBUTIONS Natália A. B. Tinoco was responsible for investigation, formal analysis, data curation, methodology, and writing the original draft; Natália A. B. Tinoco, Ana Laura M. Brand, Ana Carolina R. Silva and Cyrus V. Andriolo for formal analysis and data curation; Natália A. B. Tinoco and Ana Laura M. Brand for data curation, and writing the original draft; Rafael Garrett and Claudia M. Rezende for conceptualization, data curation, funding acquisition, project administration, resources, and writing the original draft.
REFERENCES 1. Avallone, S.; Guyot, B.; Brillouet, J.-M.; Olguin, E.; Guiraud, J.-P.; Curr. Microbiol. 2001, 42, 252. [Crossref] 2. Pereira, G. V. M.; Soccol, V. T.; Soccol, C. R.; Curr. Opin. Food Sci. 2016, 7, 50. [Crossref] 3. Elhalis, H.; Cox, J.; Zhao, J.; Appl. Food Res. 2023, 3, 100253. [Crossref] 4. Tarzia, A.; Scholz, M. B. S.; Petkowicz, C. L. O.; Int. J. Food Sci. Technol. 2010, 45, 2167. [Crossref] 5. Pereira, G. V. M.; Sampaio, V. M.; Wiele, N.; Vale, A. S.; de Carvalho Neto, D. P.; de Souza, A. F. D.; dos Santos, D. V. N.; Ruiz, I. R.; Rogez, H.; Soccol, C. R.; Trends Food Sci. Technol. 2024, 151, 104641. [Crossref] 6. Joët, T.; Laffargue, A.; Descroix, F.; Doulbeau, S.; Bertrand, B.; de kochko, A.; Dussert, S.; Food Chem. 2010, 118, 693. [Crossref] 7. Vaughan, M. J.; Mitchell, T.; Gardener, B. B. M.; Appl. Environ. Microbiol. 2015, 81, 6518. [Crossref] 8. Febrianto, N. A.; Zhu, F.; Food Chem. 2023, 412, 135489. [Crossref] 9. Selmar, D.; Bytof, G.; Knopp, S.-E.; Breitenstein, B.; Plant Biol. 2006, 8, 260. [Crossref] 10. Evangelista, S. R.; Miguel, M. G. C. P.; Cordeiro, C. S.; Silva, C. F.; Pinheiro, A. C. M.; Schwan, R. F.; Food Microbiol. 2014, 44, 87. [Crossref] 11. Waters, D. M.; Arendt, E. K.; Moroni, A. V.; Crit. Rev. Food Sci. Nutr. 2017, 57, 259. [Crossref] 12. Torres-Valenzuela, L. S.; Serna-Jiménez, J. A.; Martínez, K. In Coffee - Production and Research; Castanheira, D. T., ed.; IntechOpen: Rijeka, Croatia, 2019, ch. 7. [Crossref] 13. Madrid-Restrepo, M. A.; Leon-Inga, A. M.; Peñuela-Martínez, A. E.; Cala, M. P.; Reyes, A.; bioRxiv, 2025. [Crossref] 14. Pereira, G. V. M.; Neto, E.; Soccol, V. T.; Medeiros, A. B. P.; Woiciechowski, A. L.; Soccol, C. R.; Food Res. Int. 2015, 75, 348. [Crossref] 15. Feng, X.; Dong, H.; Yang, P.; Yang, R.; Lu, J.; Lv, J.; Sheng, J.; Curr. Microbiol. 2016, 73, 190. [Crossref] 16. da Silva, M. C. S.; da Luz, J. M. R.; Veloso, T. G. R.; Gomes, W. S.; Oliveira, E. C. S.; Anastácio, L. M.; Cunha Neto, A.; Moreli, A. P.; Guarçoni, R. C.; Kasuya, M. C. M.; Pereira, L. L.; Eur. Food Res. Technol. 2022, 248, 1499. [Crossref] 17. Ferreira, L. J. C.; Gomes, M. S.; de Oliveira, L. M.; Santos, L. D.; Food Res. Int. 2023, 169, 112793. [Crossref] 18. Tinoco, N. A. B.; Pacheco, S.; Godoy, R. L. O.; Bizzo, H. R.; de Aguiar, P. F.; Leite, S. G. F.; Rezende, C. M.; Food Res. Int. 2019, 115, 487. [Crossref] 19. Silva, M. A. E.; Brand, A. L. M.; Novaes, F. J. M.; Rezende, C. M.; Food Rev. Int. 2023, 39, 7048. [Crossref] 20. Urgert, R.; van der Weg, G.; Kosmeijer-Schuil, T. G.; van de Bovenkamp, P.; Hovenier, R.; Katan, M. B.; J. Agric. Food Chem. 1995, 43, 2167. [Crossref] 21. Muguruma, Y.; Nunome, M.; Inoue, K.; Chem. Pharm. Bull. 2022, 70, 12. [Crossref] 22. Brand, A.; Silva, A.; Andriolo, C.; Mellinger, C.; Uekane, T.; Garrett, R.; Rezende, C.; J. Agric. Food Chem. 2024, 72, 27876. [Crossref] 23. Novaes, F. J. M.; Lima, F. A.; Calado, V.; Marriott, P. J.; de Aquino Neto, F. R.; Rezende, C. M.; Ind. Crops Prod. 2020, 152, 112494. [Crossref] 24. Lima, F. A.; Bezerra, M. A. M.; Souza, R.; Itabaiana Jr., I.; Haynes, T.; Hermans, S.; Wojcieszak, R.; Novaes, F. J. M.; Rezende, C. M.; ACS Omega 2020, 5, 25712. [Crossref] 25. Andriolo, C. V.; Novaes, F. J. M.; Pereira, H. M. G.; Sardela, V. F.; Rezende, C. M.; J. Chromatogr. B 2021, 1186, 123028. [Crossref] 26. Druzhilovskiy, D. S.; Rudik, A. V.; Filimonov, D. A.; Gloriozova, T. A.; Lagunin, A. A.; Dmitriev, A. V.; Pogodin, P. V.; Dubovskaya, V. I.; Ivanov, S. M.; Tarasova, O. A.; Bezhentsev, V. M.; Murtazalieva, K. A.; Semin, M. I.; Maiorov, I. S.; Gaur, A. S.; Sastry, G. N.; Poroikov, V. V.; Russ. Chem. Bull. 2017, 66, 1832. [Crossref] 27. Dias, R. C. E.; de Faria, A. F.; Mercadante, A. Z.; Bragagnolo, N.; Benassi, M. T.; J. Braz. Chem. Soc. 2013, 24, 492. [Crossref] 28. Novaes, F. J. M.; da Silva, M. A. E.; Silva, D. C.; de Aquino Neto, F. R.; Rezende, C. M.; Plants 2023, 12, 1580. [Crossref] 29. Speer, K.; Kölling-Speer, I.; Braz. J. Plant Physiol. 2006, 18, 201. [Crossref] 30. Csuk, R.; Glaenzer, B. I.; Chem. Rev. 1991, 91, 49. [Crossref] 31. Honkakoski, P.; Negishi, M.; Drug Metab. Rev. 1997, 29, 977. [Crossref] 32. van Leeuwen, J. S.; Vermeulen, N. P. E.; Vos, J. C.; Curr. Drug Metab. 2012, 13, 1464. [Crossref] 33. Liu, Z. L.; Slininger, P. J.; Dien, B. S.; Berhow, M. A.; Kurtzman, C. P.; Gorsich, S. W.; J. Ind. Microbiol. Biotechnol. 2004, 31, 345. [Crossref] 34. Liu, Z. L.; Moon, J.; Gene 2009, 446, 1. [Crossref] 35. Lucioli, S.; Pastorino, F.; Nota, P.; Ballan, G.; Frattarelli, A.; Fabbri, A.; Forni, C.; Caboni, E.; Molecules 2019, 24, 1738. [Crossref] 36. Park, Y. C.; Shaffer, C. E. H.; Bennett, G. N.; Appl. Microbiol. Biotechnol. 2009, 85, 13. [Crossref] 37. Kusano, M.; Sakai, Y.; Kato, N.; Yoshimoto, H.; Sone, H.; Tamai, Y.; Biosci. Biotechnol. Biochem. 1998, 62, 1956. [Crossref] 38. Thelle, D. S.; Arnesen, E.; Førde, O. H.; N. Engl. J. Med. 1983, 308, 1454. [Crossref]
Guest Editor handled this article: Antonio E. M. Crotti |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access