Artigo
| Deep eutectic solvents for protein extraction from pumpkin seed meal |
|
Hongke LiuI I. College of Food Science and Technology, Hunan Agricultural University, 410128 Changsha, China Received: 04/08/2025 *e-mail: huaiyun88@126.com Pumpkin seed meal, a by-product of pumpkin seed oil extraction, is recognized for its high protein content. Traditional extraction methods, such as alkaline extraction and acid precipitation, are associated with significant drawbacks, including high chemical consumption, low yield, and equipment corrosion. This study investigates the efficacy of a deep eutectic solvent (DES) system, composed of choline chloride and urea (1:2 molar ratio), for protein extraction from pumpkin seed meal, comparing it with conventional alkaline methods. The optimized conditions included 30% (m/m) water content in the DES, ultrasound treatment for 40 min at 50 °C, and a liquid-to-solid ratio of 20 mL g-1. The protein content in the extract reached 66.09 ± 2.82%, demonstrating the potential of DES as a sustainable and efficient alternative. Furthermore, the DES could be recovered and reused, highlighting its environmental and economic benefits. This study confirms the viability of choline chloride-urea DES for protein extraction, offering significant improvements over traditional methods. INTRODUCTION Pumpkin seed meal, a by-product of oil extraction, is an underutilized resource rich in proteins with promising nutritional and functional properties.1,2 While plant proteins are increasingly sought for sustainable food applications, current research predominantly focuses on defatted pumpkin seed flour or commercial concentrates, leaving the direct extraction and functional characterization of proteins from raw pumpkin seed meal largely unexplored.3-5 Addressing this gap is critical to unlocking the full economic and industrial potential of this agro-industrial residue. Ultrasound-assisted extraction has emerged as a key technology for enhancing plant protein recovery. Studies on sunflower protein6 and selenium-rich rice bran protein7 demonstrate that ultrasonic treatments (e.g., dual-frequency or microwave-coupled modes) improve solubility, structural homogeneity, and bioactivity by disrupting aggregates and modifying surface properties. Similarly, high-intensity ultrasound (HIU) optimizes foaming capacity in pumpkin seed protein isolates (PSPIs) through controlled particle size reduction.8 However, these advancements remain confined to refined substrates, with limited adaptation to complex by-products like pumpkin seed meal.9 Deep eutectic solvents (DES), as green alternatives to conventional solvents, offer further opportunities for sustainable protein extraction.10,11 Their tunable hydrophilicity/hydrophobicity and mild processing conditions preserve protein functionality while achieving high yields, as evidenced in soybean12,13 and algae14,15 studies. Yet, the synergy between DES and ultrasound in extracting proteins from lipid-rich by-products remains uninvestigated. This study innovatively combines ultrasonic-assisted extraction with DES to optimize protein recovery from pumpkin seed meal. We systematically evaluate the effects of extraction parameters (e.g., DES composition, ultrasonic power, and time of reaction) on protein yield and characterize the functional properties of the extracted proteins. The findings of this study provide a theoretical foundation for the advanced processing of pumpkin seed meal and contribute to its comprehensive utilization, thereby enhancing its economic value and sustainability.
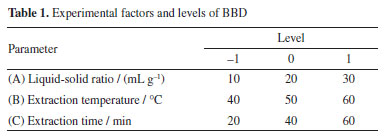
EXPERIMENTAL Materials Sunflower disk powder was purchased from Inner Mongolia Dingshang Jiaren Agricultural Technology Development Co., Ltd. Choline chloride, ethanol, citric acid, glycerol, urea, ethylenediamine, and potassium bromide (KBr) were obtained from Sinopharm Chemical Reagent Co., Ltd. Preparation of DES and single-factor experiments The DES was prepared by combining the hydrogen bond acceptor (HBA) and hydrogen bond donor (HBD) in specific molar ratios and stirring at 80 °C for 30 min to form a homogeneous, transparent liquid.16 The DES was then cooled to room temperature. The types of DES, molar ratios of HBA to HBD (2:1, 1:1, 1:2, 1:3), and water content (10, 20, 30, 40, 50 wt.%) were optimized to identify the most suitable solvent for extraction. Extraction procedures Protein extraction was conducted by treating 1.00 g pumpkin seed meal with 20.0 mL preheated DES (50 °C) via ultrasonication (300 W, 40 kHz, 50 °C, 40 min), followed by cooling, centrifugation (10,000 × g, 15 min, 4 °C) and supernatant collection. Total protein in raw material was determined by Kjeldahl method17 (N-to-protein factor = 6.25), while soluble protein in extracts was quantified using Coomassie Brilliant Blue method. Extraction yield was calculated using Equation 1:  Protein separation and recovery The crude extract was mixed with anhydrous ethanol to precipitate the protein.18 The mixture was centrifuged at 10,000 rpm for 10 min, and the resulting precipitate was sequentially washed three times with 70% (v/v) ethanol and deionized water alternately (each wash was followed by centrifugation at 8,000 rpm for 5 min) to remove soluble impurities. Finally, the purified precipitate was freeze-dried to obtain the protein. Response surface optimization experiments A Box-Behnken design (BBD) was employed to optimize the liquid-to-solid ratio, extraction temperature, and extraction time.19 The experimental factors and levels are presented in Table 1.
DES recycling and reuse The DES was recovered by evaporating ethanol under reduced pressure and reused for subsequent extraction cycles. The recovery rate and protein extraction efficiency were evaluated over four cycles. Fourier transform infrared spectroscopy (FTIR) FTIR analysis was performed using a WQF-600N spectrometer (Beijing Rui Li Analytical Instrument Co., Ltd.). Samples were mixed with KBr and pressed into pellets for analysis. Scanning electron microscopy (SEM) The microstructure of the pumpkin seed meal and extracted proteins was examined using a SEM (Hitachi SU8010, Japan). Samples were mounted on aluminum stubs using carbon tape and sputter-coated with gold to enhance conductivity. The SEM images were acquired at an accelerating voltage of 5 kV and a working distance of 10 mm. Hydrophilicity and hydrophobicity testing The water-holding capacity (WHC) and oil-holding capacity (OHC) of the extracted proteins were determined using standard methods.20
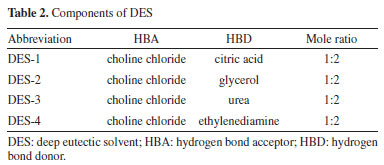
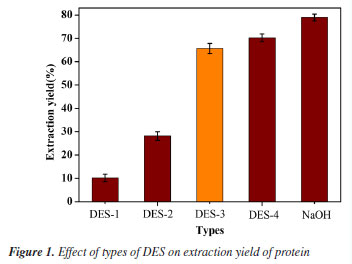
RESULTS AND DISCUSSION Screening of DESs Influence of DES types The extraction efficiency of various DESs was evaluated under optimized conditions (30 wt.% water, 20 mL g-1 liquid-solid ratio, 50 °C, 40 min), with pH 9 NaOH as a control (Table 2).21 As shown in Figure 1, DES-3 achieved a protein extraction rate of 66.09 ± 2.82%, lower than DES-4 (70.22 ± 2.13%) and alkaline extraction (78.95 ± 1.68%). Despite its slightly reduced yield, DES-3 was selected for its near-neutral pH (7.2), which minimizes protein hydrolysis, and the generally recognized as safe (GRAS, U.S. FDA)22 status of urea, ensuring food safety compliance. In contrast, DES-4 posed cytotoxicity risks due to ethylenediamine residues.
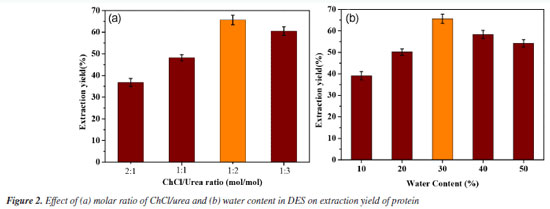
Effect of the molar ratio of HBA to HBD The choline chloride-urea molar ratio optimization under aqueous DES conditions revealed distinct mechanistic limitations at non-optimal ratios (Figure 2a). At 2:1 HBA:HBD, although 30% water prevented solidification above 42 °C, excessive chloride ions competed with urea for hydrogen-bonding sites, disrupting the solvent network and reducing protein extraction to 36.77 ± 1.84%.23 The 1:1 ratio suffered from insufficient charge delocalization and elevated microviscosity due to substoichiometric HBD availability, limiting mass transfer (48.19 ± 1.41% efficiency).24 In contrast, the 1:2 ratio achieved peak efficiency (66.09 ± 2.82%) through balanced polarity-viscosity compromise, while urea-rich systems (1:3) declined to 60.48 ± 2.02% due to self-association of excess HBD that weakened protein-solvent interactions.25
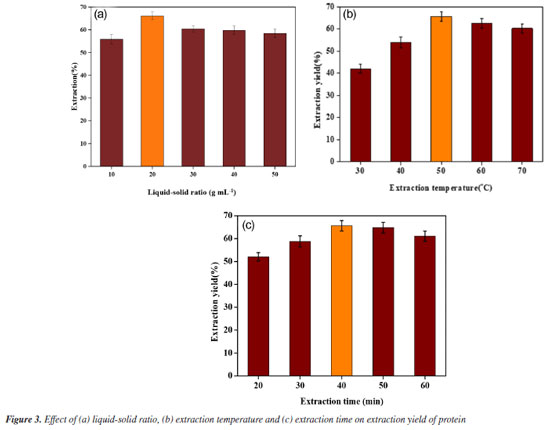
Effect of DES water content The effect of water content in ChCl-urea DES on protein extraction efficiency was evaluated under optimized conditions. As shown in Figure 2b, the maximum extraction efficiency (66.09 ± 2.82%) occurred at 30 wt.% water, where viscosity reduction and lowered surface tension synergistically enhanced solvent penetration.26 Single-factor experiments Effect of liquid-to-solid ratio The effect of the liquid-to-solid ratio on protein extraction was investigated using a ChCl/urea solvent. The experiments were conducted at 50 °C with ultrasound for 40 min, varying the liquid-to-solid ratio from 10 to 50 mL g-1. As shown in Figure 3a, a lower liquid-to-solid ratio may limit solvent-sample contact, thus hindering the extraction efficiency. Conversely, an excessively high ratio can decrease extraction efficiency, likely due to the over-dispersion of ultrasound energy, which weakens cavitation.
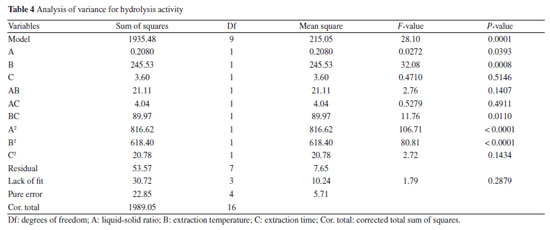
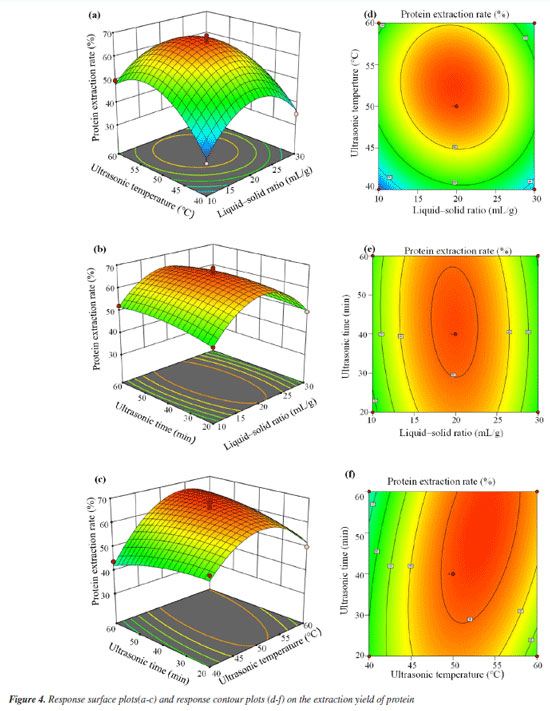
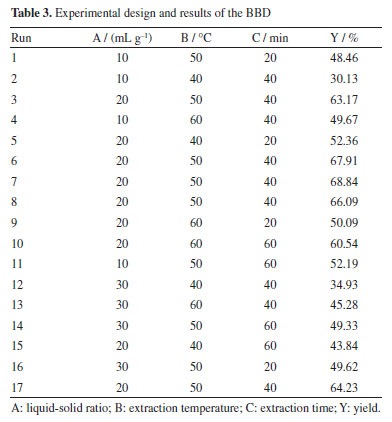
Effect of temperature The effect of temperature on protein extraction was investigated using a DES solvent. The experiments were conducted at temperatures ranging from 30 to 70 °C for 40 min under ultrasound. As shown in Figure 3b, the protein extraction rate initially increased with temperature, likely due to the reduction in viscosity and surface tension of the DES, which facilitated mass transfer and improved extraction efficiency. However, further increases in temperature resulted in a decline in extraction efficiency, possibly due to protein denaturation or hydrolysis. Effect of extraction time This study aimed to investigate the effect of extraction time on protein extraction efficiency using a DES solvent with ultrasonication at 50 °C. Extraction times ranged from 20 to 60 min. As shown in the Figure 3c, protein extraction efficiency increased with extraction time up to a certain point. However, excessive extraction time led to a decrease in efficiency, likely due to protein hydrolysis induced by prolonged ultrasonic exposure. Additionally, extended treatment may facilitate the extraction of other bioactive compounds, which could interfere with protein dissolution. Response surface optimization results To overcome the limitations of single-factor experiments in identifying interactive effects, the parameter ranges established in “Single-factor experiments” sub-section (derived from single-factor experiments) were used to design a BBD. The results of BBD study are shown in Table 3, and the model variance analysis is presented in Table 4. The overall P-value of the model is < 0.0001, indicating significance, while the P-value for lack-of-fit is > 0.05, confirming model validity. The coefficient of determination (R2) value is 0.9731, suggesting a good fit, and both the adjusted R2 (0.9384) and predicted R2 (0.7350) show high agreement, with a difference of less than 0.2, indicating the model's reliability (Figure 4).
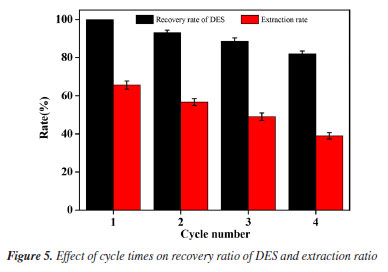
The P-values of the three independent variables demonstrate all exert a significant influence on the protein extraction rate. By means of data fitting, the regression equation for the protein extraction rate in relation to the three factors can be expressed as follows: 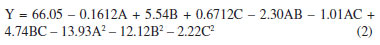 where Y: the protein extraction rate, expressed as a percentage; A: the liquid-to-solid ratio, in mL g-1; B: the extraction temperature, in °C; C: the extraction time, in min. The Design-Expert 12 software (StatEase, USA) predicted optimal conditions for protein extraction as 26.38 mL g-1 (liquid-to-solid ratio), 52.24 °C, and 45.39 min (ultrasonication time), with a yield of 60.41%. For practical operability, parameters were adjusted to 25 mL g-1, 52 °C, and 45 min. Experimental validation under these adjusted conditions yielded an average extraction rate of 67.19 ± 1.24% (triplicate tests). The slight deviation (60.70% vs. 67.19%) likely stemmed from minor operational variations (e.g., temperature control and sample handling), confirming the model's applicability in practical extraction processes. Crucially, the close proximity between predicted and observed results, coupled with the high model validity metrics, confirms the BBD model's effectiveness in optimizing the extraction process within the parameter space identified by the foundational single-factor studies. Reusability of DES After the protein extraction process using DES, centrifugation was employed to separate the protein-rich phase from the crude DES extract. The supernatant was then subjected to reduced pressure to evaporate water and ethanol, allowing for the recovery of DES. This recovered DES was subsequently reused for additional protein extraction cycles from pumpkin seed meal (Figure 5). Following four cycles of this procedure, the recovery rate of DES was determined to be 82.05 ± 1.69%, while the protein extraction efficiency reached 38.96 ± 1.53%. These findings confirm the structural stability of DES, underscoring its suitability for multiple reuses in protein extraction processes. The observed reduction in extraction efficiency during cycles 3-4 primarily stems from progressive accumulation of non-protein impurities (e.g., lipids/polysaccharides) in recycled DES, which compete for hydrogen-bonding sites, coupled with slight compositional drift due to differential volatility of DES components during recovery.27
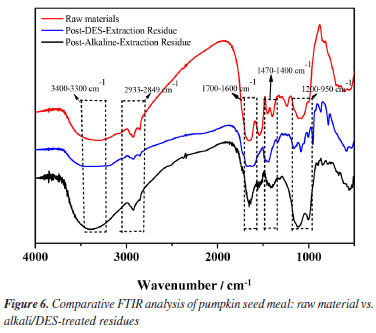
Structural characterization FTIR spectroscopy The chemical structural changes in pumpkin seed meal induced by alkali and DES treatments were analyzed via FTIR. As shown in Figure 6, characteristic absorption peaks were identified:28 (i) a broad peak at 3400-3300 cm-1 corresponding to O-H stretching vibrations, attributed to hydroxyl groups in polysaccharides or bound water in proteins; (ii) peaks at 2933-2849 cm-1 assigned to C-H stretching in aliphatic chains of lipids or protein side chains; (iii) a distinct peak at 1700-1600 cm-1 (amide I band) arising from C=O stretching vibrations in protein amide bonds, indicative of preserved α-helix/β-sheet secondary structures; (iv) peaks at 1470-1400 cm-1 (C-H bending) and 1200-950 cm-1 (C-O stretching), suggesting interactions with carbohydrates or lipids.29 Notably, the DES-treated residues retained a pronounced amide I band intensity, implying minimal protein denaturation, whereas alkali treatment reduced this signal, likely due to partial protein degradation.30 These results demonstrate that DES extraction maintains protein structural integrity more effectively than alkaline methods, aligning with its higher extraction efficiency observed experimentally.
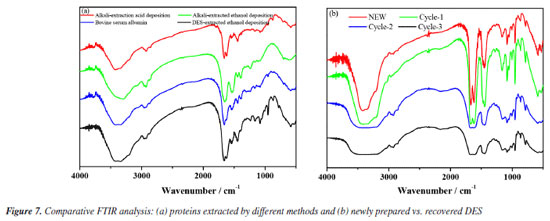
FTIR was utilized to investigate the functional group variations and conformational attributes of proteins extracted via the direct extraction method (DES), alkaline extraction (alkali-acid and alkali-ethanol), and bovine serum protein as references. As depicted in Figure 7a, the FTIR spectra across all samples revealed consistent patterns,31 with significant absorption peaks at 3430, 2927, 1661, 1544, and 1239 cm-1. These peaks represent the bending vibrations of free and bound OH and NH groups (amide A), the asymmetric and symmetric stretching vibrations of CH2 and CH3 (amide B), the stretching vibration of the C=O bond (amide I), the bending vibration of N-H alongside the stretching vibration of C-N (amide II), and the stretching vibration of C-N coupled with N-H vibration (amide III). The analysis suggests that both the alkaline and DES extraction methods exert similar effects on the peptide bonds of pumpkin seed meal protein. However, distinct structural variations were noted between proteins precipitated using acid and ethanol, with the latter showing a closer resemblance to the structure of bovine serum protein.
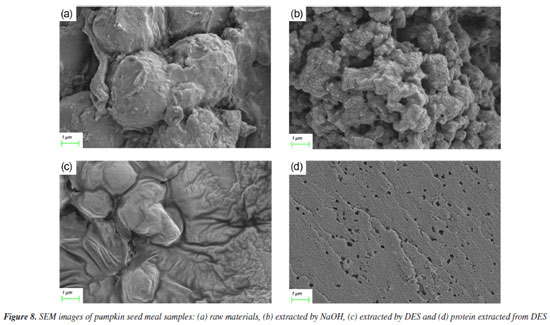
Figure 7b illustrates the infrared spectral analysis of DES following multiple extraction cycles. With an increase in the number of cycles, the absorption bands observed at 3410, 3348, and 3210 cm-1 exhibit broadening, which is attributed to the enhanced formation of hydrogen bonds between urea and ChCl. Additionally, the absorption band at 1453 cm-1, indicative of the interaction between methyl groups (-CH3) and chlorine atoms (Cl), remains consistent across cycles. Notably, the stable absorption band at 962 cm-1, corresponding to the C=O stretching vibration, confirms that the structural integrity of the Ch+ ion in the urea-choline chloride system is maintained throughout the cycling process. SEM To evaluate the influence of different extraction techniques on the microstructure of pumpkin seed meal, SEM was used to examine both the raw material and the extracted samples. As shown in Figure 8, the surface structure of the raw pumpkin seed meal is predominantly smooth. In contrast, the sample extracted using ultrasound-assisted alkaline solution exhibits a distinct porous structure, which enhances protein dissolution. The sample extracted with ultrasound-assisted DES displays a damaged surface with noticeable grooves and pores, accompanied by visible cracks. These observations suggest that ultrasound-assisted extraction disrupts the cell wall, thereby reducing mass transfer resistance and promoting the dissolution of proteins.
Hydrophilicity and hydrophobicity testing As shown in Figure 9, the protein extracted via choline chloride-urea deep eutectic solvent (DES) exhibited a significantly higher water-holding capacity (WHC, 1.94 g g-1) compared to alkaline-extracted protein (1.34 g g-1) (P < 0.05). The enhancement is attributed to DES' unique hydrogen-bonding network: urea acts as a hydrogen-bond disruptor, mitigating excessive protein aggregation while preserving hydrophilic domains. This aligns with the observation that DES maintains protein hydration shells better than ionic liquids.32 In contrast, alkaline extraction induced irreversible denaturation, exposing hydrophobic residues and reducing water-binding sites - a common limitation noted in legume protein extractions.33 The superior WHC suggests an optimized hydrophilic-lipophilic balance, positioning DES-extracted proteins as viable green alternatives for moisture-sensitive foods, with potential to reduce synthetic hydrocolloid usage.
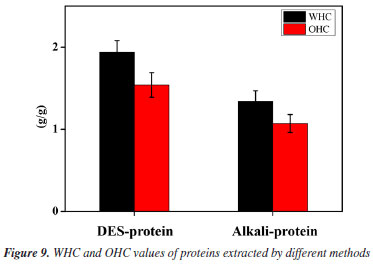
The DES method yielded pumpkin seed meal protein with a significantly higher oil-holding capacity (OHC, 1.54 g g-1) compared to alkaline extraction (1.07 g g-1) (P < 0.05, Figure 9). Choline chloride stabilizes amphiphilic domains through charge shielding - a mechanism superior to ionic liquids in minimizing structural collapse.34 In contrast, alkaline treatment induced partial denaturation, exposing buried hydrophobic regions that promoted irreversible aggregation and reduced effective lipid-binding sites.35 The superior OHC reflects an optimized balance between surface hydrophobicity and structural flexibility, positioning DES-extracted proteins as promising emulsifiers to replace synthetic surfactants in plant-based meat analogs.
CONCLUSIONS This study demonstrates that choline chloride-urea DES is an effective and sustainable method for protein extraction from pumpkin seed meal. The DES exhibited high extraction efficiency, reusability, and minimal environmental impact, making it a viable alternative to conventional methods. The extracted proteins showed superior functional properties, highlighting their potential for food and industrial applications.
DATA AVAILABILITY STATEMENT We state that all data are available in the text.
REFERENCES 1. Stahn, T.; Storandt, R.; Grebenteuch, S.; Rohn, S.; May, D.; Dolsdorf, C.; Pleissner, D.; Sustainability 2023, 15, 13774. [Crossref] 2. Rakita, S.; Kokić, B.; Manoni, M.; Mazzoleni, S.; Lin, P.; Luciano, A.; Ottoboni, M.; Cheli, F.; Pinotti, L.; Foods 2023, 12, 432. [Crossref] 3. Wal, A.; Singh, M. R.; Gupta, A.; Rathore, S.; Rout, R. R.; Wal, P.; The Natural Products Journal 2024, 14, e160523216963. [Crossref] 4. Pandey, V. K.; Singh, K.; Suthar, T.; Srivastava, S.; Rustagi, S.; Ungai, D.; Kovács, B.; Shaikh, A. M.; Horticulturae 2024, 10, 1194. [Crossref] 5. Habib, M.; Singh, S.; Sagar, N. A.; Ahmad, S.; Qureshi, I.; Jan, S.; Jan, K.; Bashir, K.; Sustainable Food Technol. 2025, 3, 445. [Crossref] 6. Li, Y.; He, Y.; Zhou, H.; Kinyoro, I. S.; Ma, S.; Wang, J.; Zhang, S.; Wu, C.; Liu, X.; LWT--Food Sci. Technol. 2024, 212, 116940. [Crossref] 7. Dabbour, M.; Jiang, H.; Mintah, B. K.; Wahia, H.; He, R.; Innovative Food Sci. Emerging Technol. 2021, 74, 102824. [Crossref] 8. Du, H.; Zhang, J.; Wang, S.; Manyande, A.; Wang, J.; LWT--Food Sci. Technol. 2022, 155, 112952. [Crossref] 9. Gavril, R. N.; Stoica, F.; Lipșa, F. D.; Constantin, O. E.; Stănciuc, N.; Aprodu, I.; Râpeanu, G.; Foods 2024, 13, 2694. [Crossref] 10. Frommhagen, M.; Hutnik, N.; Schols, H. A.; Food Hydrocolloids 2025, 160, 110714. [Crossref] 11. Belviso, B. D.; Perna, F. M.; Carrozzini, B.; Trotta, M.; Capriati, V.; Caliandro, R.; ACS Sustainable Chem. Eng. 2021, 9, 8435. [Crossref] 12. Uribarrena, M.; Cabezudo, S.; Núñez, R. N.; Copello, G. J.; de la Caba, K.; Guerrero, P.; Int. J. Biol. Macromol. 2025, 291, 139045. [Crossref] 13. Song, X.; Xu, Z.; Sun, H.; Cao, C.; Chang, S.; Su, G.; Zhang, J.; Food Chem. 2025, 468, 142438. [Crossref] 14. Moldes, D.; Requejo, P. F.; Vega, M.; Bolado, S.; Wijffels, R. H.; Kazbar, A.; Microchem. J. 2024, 205, 111275. [Crossref] 15. Xu, Y.; Wang, Q.; Hou, Y.; Mar. Drugs 2020, 18, 618. [Crossref] 16. Dou, W.; Yu, J.; Wang, X.; J. Mol. Liq. 2023, 382, 121923. [Crossref] 17. Marcó, A.; Rubio, R.; Compañó, R.; Casals, I.; Talanta 2002, 57, 1019. [Crossref] 18. Meng, Y.; Sui, X.; Pan, X.; Zhang, X.; Sui, H.; Xu, T.; Zhang, H.; Liu, T.; Liu, J.; Ge, P.; Ultrason. Sonochem. 2023, 98, 106522. [Crossref] 19. Wu, J.; Su, M.; Hu, A.; Wang, H.; J. Food Process. Preserv. 2022, 46, e16856. [Crossref] 20. Olalere, O. A.; Gan, C.-Y.; Sustainable Chem. Pharm. 2023, 32, 101002. [Crossref] 21. Gao, Z.; Shen, P.; Lan, Y.; Cui, L.; Ohm, J.-B.; Chen, B.; Rao, J.; Food Res. Int. 2020, 131, 109045. [Crossref] 22. Huang, H.; Ding, L.; Lu, J.; Wang, N.; Cai, M.; J. Food Sci. 2020, 85, 3061. [Crossref] 23. Kumar, A.; Bhakuni, K.; Venkatesu, P.; Phys. Chem. Chem. Phys. 2019, 21, 23269. [Crossref] 24. Rozas, S.; Benito, C.; Alcalde, R.; Atilhan, M.; Aparicio, S.; J. Mol. Liq. 2021, 344, 117717. [Crossref] 25. Zhang, N.; Liu, F.-F.; Dong, X.-Y.; Sun, Y.; J. Phys. Chem. B 2012, 116, 7040. [Crossref] 26. Zhong, G.; Li, S.; Xu, S.; Liao, W.; Fu, X.; Peng, F.; ACS Sustainable Chem. Eng. 2018, 6, 15108. [Crossref] 27. Picciolini, E.; Pastore, G.; Del Giacco, T.; Ciancaleoni, G.; Tiecco, M.; Germani, R.; J. Mol. Liq. 2023, 383, 122057. [Crossref] 28. Marquez-Rodriguez, A. S.; Guimarães, M.; Mateus, N.; de Freitas, V.; Ballinas-Casarrubias, M. L.; Fuentes-Montero, M. E.; Salas, E.; Cruz, L.; Food Chem. 2021, 344, 128603. [Crossref] 29. Talari, A. C. S.; Garcia Martinez, M. A.; Movasaghi, Z.; Rehman, S.; Rehman, I. U.; Appl. Spectrosc. Rev. 2017, 52, 456. [Crossref] 30. Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y.; ACS Sustainable Chem. Eng. 2015, 3, 2746. [Crossref] 31. Lan, Y.; Ohm, J.-B.; Chen, B.; Rao, J.; Food Hydrocolloids 2021, 113, 106423. [Crossref] 32. Hanafi, M. A.; Anwar, F.; Saari, N.; Food Front. 2024, 5, 1265. [Crossref] 33. Jarpa-Parra, M.; Bamdad, F.; Wang, Y.; Tian, Z.; Temelli, F.; Han, J.; Chen, L.; LWT--Food Sci. Technol. 2014, 57, 461. [Crossref] 34. Edler, K. J.; Warr, G. G.; Djerdjev, A. M.; Lam, M. T.; Hawley, A. M.; Mudie, S.; Faraday Discuss. 2025, 260, 94. [Crossref] 35. Adams, S. L.; Barnett, D.; Walsh, B. J.; Pearce, R. J.; Hill, D. J.; Howden, M. E. H.; Immunology and Cell Biology 1991, 69, 191. [Crossref]
Editor handled this article: Jorge M. David |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access