Artigo
| Determination of brodifacoum by gas chromatography coupled to mass spectrometry: a new analytical methodology for the determination of coumarin rodenticides in poisoned foods |
|
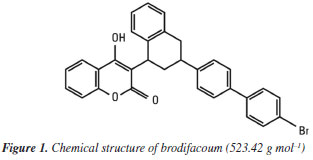
Daniel B. SouzaI,II,* I. Programa de Pós-Graduação em Química, Instituto de Química, Universidade do Estado do Rio de Janeiro, 20550-900 Rio de Janeiro - RJ, Brasil Received: 06/12/2025 *e-mail: busquetiq1985@gmail.com The use of efficient and reliable methods is extremely important for analyzing food samples suspected of containing toxic substances. Due to the difficulty in obtaining highly toxic illicit poisons of the carbamate type (aldicarb/carbofuran) and organophosphates (terbufos), popularly known as chumbinho, which were banned by Agência Nacional de Vigilância Sanitária (ANVISA), there is an increase in cases of suicide and attempted murder using another type of poison: commercial coumarin rodenticides based on brodifacoum which are freely sold in pesticide product stores. The low concentration of the active ingredient (0.005%, m/m) in formulated products containing coumarins makes it difficult to identify these substances in samples with a small amount of poison or when mixed in food, requiring more complex extraction methodologies and instrumentation that many forensic investigations do not have, often generating inconclusive reports. In this work we studied a new analytical methodology for the extraction and identification of brodifacoum, one of the coumarins commonly found in these poisons, using gas chromatography coupled with mass spectrometry (GCMS). In extraction by sonification, we use acetone and methanol/formic acid 50:1 as extracting solvents, followed by concentration and subsequent analysis by chromatography, obtaining excellent results in identifying the main degradation product of brodifacoum in the formulated products, even when mixed with food in cases of poisoning, relating its concentration to that of the commercial standard. This work provides an alternative method of analyzing these materials, helping scientific police prepare more conclusive reports in cases of coumarin poisoning, thus reducing impunity. INTRODUCTION The use of pesticides as agents of deliberate self-poisoning is on the rise, with more than 500,000 cases reported worldwide annually.1 The rate of poisoning cases varies around the world, with carbamates and organophosphates, due to their high toxicity and lack of taste, being the most common cause of death from pesticide poisoning in the world.2 Individuals living in rural areas are more likely to be contaminated by pesticides, while in cities there is a predominance of deaths due to poisoning with illicit drugs.3 In the United States, the coumarin brodifacoum is responsible for the deaths of an average of 10,000 people per year, reaching a peak of 17,000 deaths in 2002.4 Contamination can occur accidentally or intentionally, with intentional poisoning in the USA occurring in a small number of cases involving suicide attempts.5 In India, it is the second highest rate of self-poisoning with rodenticides, accounting for around 12% of deaths. These cases of coumarin poisoning in humans also occur in many other countries and are very difficult to diagnose, as the symptoms of poisoning are confused with other types of diseases and there is a need for specific analytical techniques to identify the poison in the blood.6,7 From 2007 to 2017, Brazil recorded around 40,000 cases of acute pesticide poisoning, causing almost 2,000 deaths.8 These pesticides are responsible for the majority of poisoning deaths in Brazil, accounting for approximately 24% of all poisoning deaths.9 These substances are mixed into food in several cases of homicide and suicide and, although aldicarb and carbofuran have been banned in Brazil since 2012,10 they are still sold at low cost, in fractions, on the black market as rodenticides in small glass bottles. In Rio de Janeiro, on average, 25% of the causes of poisoning are caused by some pesticide, 3/4 of these by chumbinho, representing around 20% of poisoning cases, and also being involved in around 20% of cases of suicide attempts.11 Chumbinho is a poison in the form of plaster spheres with a thin layer of the active ingredient, which can be carbamates such as aldicarb and carbofuran, as well as organophosphates such as terbufos, originally produced to combat crop pests.11 Currently, an increase in the occurrence of poisoning attempts with coumarin derivatives (brodifacoum) has been observed, which despite having lower toxicity when compared to carbamates and organophosphates, can lead to death in high concentrations.12 One of the reasons for this increase is its high commercial availability, unlike carbamates and organophosphates, making it possible to purchase products in a wide range of commercial establishments.13 The LD50 (lethal dose 50%) of brodifacoum is around 14 μg kg-1.14 Coumarin derivatives are anticoagulants based on 4-hydroxycoumarin and are classified into multiple-dose (first-generation) and single-dose (second-generation) compounds. In the first group, the compounds do not produce effects after a single ingestion, even in large amounts, as their action is cumulative. In contrast, second-generation compounds act after a single ingestion and are more toxic, with brodifacoum being the most commonly sold coumarin in this group.15 The chemical structure of brodifacoum (3-[3-[4-(4-bromophenyl)phenyl]-1,2,3,4-tetrahydronaphthalen-1-yl]-4-hydroxychromen-2-one) can be seen in Figure 1.
These rodenticides especially inhibit vitamin K 2,3-epoxide reductase, leading to an increase in the inactive form of vitamin K and an interference with the carboxylation of vitamin K-dependent vitamins from clotting factors (II, VII, IX and X) in the liver.16 Symptoms of exposure to high concentrations of these substances are: hematuria, epistaxis, gingival bleeding, ecchymosis, bleeding in the central nervous system and other bleeding.17 The effects of second-generation anticoagulants may persist for more than 2 years due to their long biological half-life.4 Rodenticide formulations with coumarins have been developed for rodent control with the aim of improving their palatability so that they are suitable for the environments that rodents frequent and are highly attractive. They have a high concentration of paraffin to avoid problems with humidity and can be found in the form of pellets, grain and cereals, pressed formulation, waterproof block, contact powder and, more recently, impregnated sunflower.18 Samples suspected of containing coumarin contamination presented food-permeated, colored powdery material (bran), insoluble in water, originating from the fragmentation of "pellets" from commercial products. In the analysis of these pesticides, chromatographic techniques are mostly used (51%), followed by electroanalytical techniques (31%) and spectroscopic techniques (18%).19 The low concentration of the active ingredient in coumarin derivatives and the vehicle used in the bait (cereal bran such as wheat, soybeans, corn and rice) make this extraction and analysis a challenge.20 Attempts at analysis by ultraviolet spectrophotometry and fluorimetry were not effective in determining the active ingredient in rodenticides because the excipients present in the material interfere with the analysis results.21 Brodifacoum has a known methodology for analysis by high-performance liquid chromatography (HPLC) with diode array detector (DAD) or liquid chromatography coupled to mass spectrometry (LC-MS), achieving good results in coumarin research in blood samples in the ppb (ng mL-1) range.22 In the case of gas chromatography-mass spectrometry (GC-MS) analyses, dog poisoning analyses were performed by extracting a liver sample using a 9:1 chloroform:methanol mixture and subsequently derivatizing it with trimethylanilinium hydroxide. The detection limit was approximately 60 mg kg-1.23 Due to its labile nature, brodifacoum is not commonly applied by gas chromatography (GC) without derivatization as it is not thermally stable, being partially decomposed when heated in the injector.24 Nevertheless, the degradation products of brodifacoum, formed by thermal breakdown, may be more volatile and thermally stable. These degradation products can be amenable to GC-MS analysis, allowing for indirect monitoring of brodifacoum concentration.25,26 The objective of this article is to demonstrate the application of advanced sample preparation techniques and gas chromatography coupled with mass spectrometry in the detection of brodifacoum in foods without derivatization, through the identification of its main degradation product, in an investigative study conducted by the Scientific Police of the State of Rio de Janeiro. Although in some cases contamination is clear, identification of the active ingredient is necessary to provide technical support to the Public Prosecutor Office and the Civil Police of the state, contributing to the conclusion of a criminal investigation. The method described in this work can be adapted and applied to other related forensic studies.
EXPERIMENTAL Reagents Acetone P.A. and methanol P.A. were obtained from Modern Chemistry (São Paulo, Brazil) and formic acid P.A. was obtained from Êxodo Scientific (São Paulo, Brazil). Brodifacoum standard 95% was obtained from Krodec (São Paulo, Brazil), batch 20240220, manufactured in 02/2024. The commercial rodenticide, in the form of pellets, was obtained from the regular pesticide trade in 25 g bags with a brodifacoum concentration of 0.005%. Preparation of standards The standards were made from brodifacoum stock solution prepared with 0.0242 g of the standard 95% in 5 mL of P.A. acetone, resulting in a concentration of 4.60 g L-1. Aliquots of the stock solution were removed to obtain solutions with brodifacoum concentrations ranging from 0.4 to 2 g L-1. Instrumental conditions of analysis The degradation products of brodifacoum were identified through a chromatogram obtained using a Thermo Scientific Trace 1310 gas chromatograph coupled to an ISQ 7000 single quadrupole mass spectrometer (Thermo Fisher Scientific Inc., Waltham, USA). Chromatographic separation was performed using a Thermo Scientific NA-5MS capillary column (30 m × 0.25 mm internal diameter (i.d.), 0.25 µm film thickness) with helium (99.999%) as the carrier gas at a flow rate of 1.0 mL min-1. The sample solution (1.0 µL) was injected in splitless mode at 250 ºC using a Thermo Scientific TriPlus RSH autosampler. The analysis was performed with the following temperature gradient: isotherm at 50 ºC for 1 min; increase to 325 ºC at a rate of 20 ºC min-1; isotherm at 325 ºC for 3 min. The GC-MS interface temperature was 300 ºC, and the solvent delay was set to 4 min. Electron ionization mass spectrometry was performed at 70 eV, and the mass spectra were acquired in full-scan mode. The full-scan acquisition range was m/z 40-550. Method validation The method was validated for the following parameters: linearity, limit of quantitation (LOQ), limit of detection (LOD), precision and selectivity.27 Linearity Linearity test solutions for the assay method were prepared from a stock solution at five levels of analyte concentration (0.4, 0.5, 1.0, 1.5 and 2.0 g L-1). The peak area vs. concentration data was analyzed with least squares linear regression. The slope and y-intercept of the calibration curve was reported. Limit of quantification and limit of detection The limit of quantification (LOQ) and limit of detection (LOD) were based on the standard deviation of the blank response and the slope of the constructed calibration curve.27 Precision The precision of the assay method was evaluated by carrying out three independent assays of a reference standard of brodifacoum at a concentration of 1.0 g L-1. The percentage of the relative standard deviation (%RSD) of three obtained assay values was calculated.27 Selectivity The proposed selectivity method was evaluated to verify whether any excipients commonly found in rodenticide products interfere with the analysis when eluted during the retention time of the substance of interest, which must be well separated from the other compounds present in the sample.28 Extraction procedure in rodenticides and poisoned foods In the analysis of rodenticides in pellet form, these were previously macerated and mixed with the extraction solvent, which in this case was acetone P.A. or a 50:1 solution of methanol and formic acid. In the case of foods permeated with the rodenticide, aliquots of the material were separated and the extraction process carried out. Aliquots of 10 g of the formulated product or 30 g of the contaminated food were used, and 50 mL of the extracting solvent was added. Analyses were performed in triplicates. The mixtures were sonified for 10 min, filtered, and the liquids were evaporated to 1 mL at room temperature (25 ºC).21
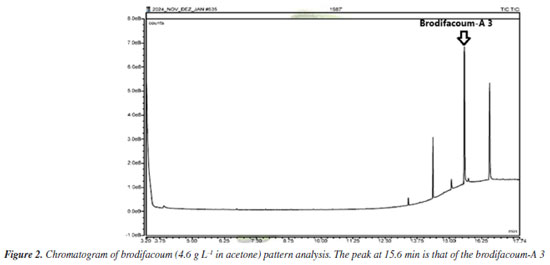
RESULTS AND DISCUSSION Brodifacoum-A 3 identification Under the chromatographic conditions described, the chromatogram with the degradation products of brodifacoum is shown in Figure 2.
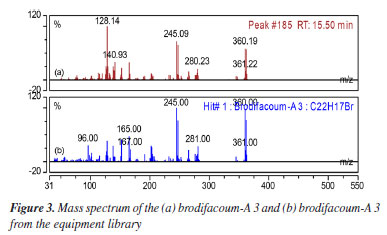
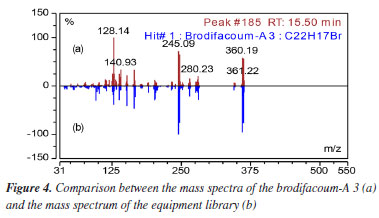
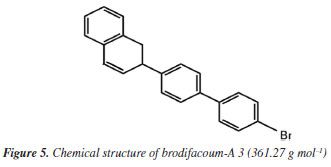
The highest intensity peak at 15.6 min was identified by the spectral library of the equipment (Designer Drugs Library, DD2020) as brodifacoum-A 3 (2-[4-(4-bromophenyl)phenyl]-1,2-dihydronaphthalene) as shown in Figures 3 and 4. This molecule is a degradation product of the brodifacoum standard at high temperatures by rearrangement and loss of the coumarin structure,29 with a molecular formula of C22H17Br and a chemical structure shown in Figure 5. The other peaks observed in the chromatogram are impurities present in the standard and other degradation products of brodifacoum.
The use of selected ion monitoring (SIM) would provide a higher level of selectivity than full-scan; however, for the analysis of brodifacoum in food, full-scan mass spectrometry is more suitable than SIM because it allows simultaneous detection of multiple compounds. This is especially important in complex food matrices, where unexpected interferences may occur.30 It has been demonstrated that GC-MS in full-scan mode provided high precision and broad linearity for pesticide quantification in food samples.31 Linearity The calibration curve for brodifacoum-A 3 was linear over the concentration range of 0.4-2.0 g L-1. The data for brodifacoum-A 3 peak area versus brodifacoum standard concentration were treated by linear regression analysis and the correlation coefficient (r) of 0.992 was obtained as can be seen in Figure 6. The regression equation for the calibration curve was found to be y = 6738318.4x + 1138106.5. The results revealed an excellent correlation between the peak area and analyte concentration.28
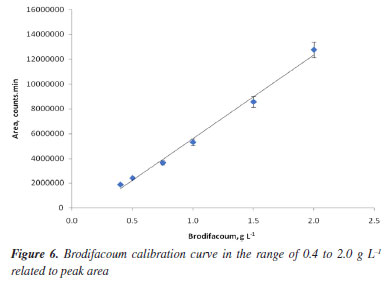
LOQ and LOD The LOQ and LOD were determined in triplicate, based on 10 and 3.3 times the standard deviation of the response, respectively, divided by the slope of the calibration curve. The LOD was found to be 0.09 g L-1 and the LOQ was found to be 0.27 g L-1.28 Precision The precision of the method was determined by the repeatability (intra-day precision) of the brodifacoum standard solutions. Repeatability was calculated by analyzing three samples of the 100% standard concentration (1.0 g L-1) on the same day. The %RSD of intraday assays values obtained was 5.14, which is an accepted value in trace or impurity analysis methods.28 Selectivity The selectivity test demonstrated that the excipients present in the rodenticide formulations did not interfere with the identification of brodifacoum, as can be seen in Figure 7. Rodenticide analysis Samples of commercial rodenticides in the form of pellets were extracted with acetone or a 50:1 methanol:formic acid mixture and analyzed by chromatography, comparing the results to verify the efficiency of the extraction process. Acetone was chosen because it is a solvent that solubilizes brodifacoum well and the 50:1 methanol:formic acid mixture was compared based on the findings of Yuen,21 who achieved good recovery of the analyte and had less carryover of oily substances and dyes that could interfere with the analysis. The recovery results were calculated from the concentration reported on the rodenticide packaging (0.005%, m/m) and can be seen in Table 1.

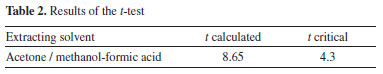
The results show that the extraction of brodifacoum-A 3 from rodenticide samples using acetone was more efficient than with a mixture of methanol and formic acid. The confidence interval shown in the table was calculated with a 95% confidence level. A paired t-test was then performed between the results, and a statistical difference was found in the means, suggesting a difference between the mean values,32 as can be seen in Table 2.
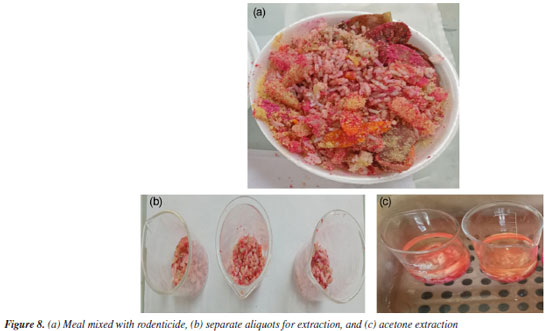
Analysis of food mixed with rodenticide In order to test the identification of brodifacoum in foods contaminated by commercial rodenticides, 25 g of commercial rodenticide were mixed on the surface of 450 g of a meal, as shown in Figure 8, simulating a real case of poisoning. It is common to grind rodenticides and mix them into food to disguise their presence and flavor. Three aliquots of 30 g of the sample were separated and 50 mL of acetone was added to extract the brodifacoum. Since the solubility of brodifacoum in acetone is 6-20 g L-1,33 the use of 50 mL of the extracting solvent is sufficient to extract all the active ingredient from the formulated product permeated in the food.
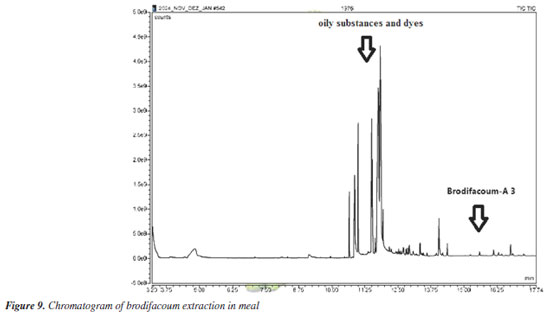
The mixtures were sonicated for 10 min, concentrated to 1 mL at room temperature (25 ºC), filtered and analyzed directly without dilution. The analyses were performed and the presence of brodifacoum-A 3 was identified in all aliquots, demonstrating that its mixture in foods does not interfere with its detection, as can be seen in Figure 9.
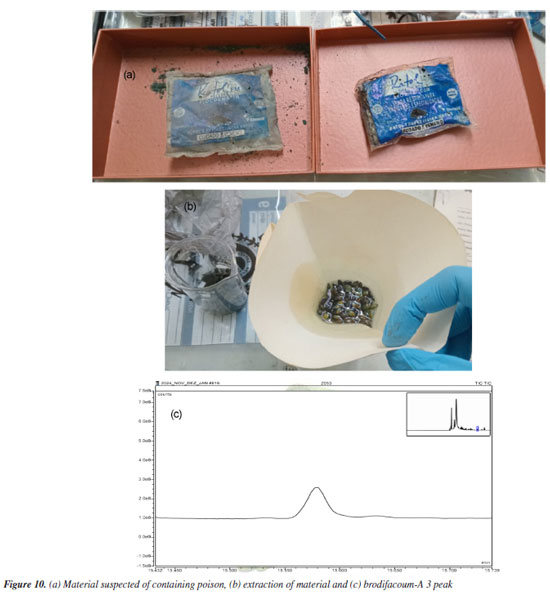
The quantification of brodifacoum in the three samples was performed using the calibration curve, obtaining an average value of 0.45 ± 0.07 g L-1. The relative standard deviation of approximately 14% is due to the fact that, even when homogenizing the powdered rodenticide on the surface of the material, it is not possible to guaranteed that each aliquot analyzed contains the same quantity of the product, which generates differences in each aliquot of the triplicate analyzed. In this case, the main objective was to verify the effectiveness of the method in identifying brodifacoum-A 3 in the food matrix, which is very common in cases of poisoning. Analysis of a real case of poisoning One food sample suspected of having been poisoned was analyzed using the method developed here to identify whether brodifacoum had been used. The police investigation suspected the death of a dog, indicating that some poisoned food could have been used. Preliminary tests did not identify some of the most commonly used poisons in the state of Rio, such as chumbinho. After reports from investigators and extensive research in the databases, the hypothesis arose that the poison in question was brodifacoum, in the form of sunflower seed. A 10 g aliquot of the suspect material was separated and 50 mL of acetone was added to extract brodifacoum. The mixture was sonified for 10 min, concentrated to 1 mL at room temperature (25 ºC), filtered and analyzed directly without dilution. The analysis performed identified the presence of brodifacoum-A 3, providing a conclusive expert report for the investigation (Figure 10).
The police authority did not request a quantitative analysis of the active ingredient, as its identification alone was sufficient to clarify the facts; however, its quantification was carried out using the calibration curve, obtaining a value of 0.41 g L-1. As the packaging was damaged, damp and the material was in poor condition, the concentration obtained was approximately 20% lower than that shown on the product label (0.005%, m/m).
CONCLUSIONS In this work, brodifacoum determinations were studied by gas chromatography coupled to mass spectrometry. The chromatogram obtained identified brodifacoum-A3 as the main degradation product, and a calibration curve was created relating the degradation product to the concentration of the brodifacoum standard, which showed good linearity, allowing identification of the active ingredient with smaller amounts of sample. The qualitative identification tests for brodifacoum-A 3 in a simulated contaminated food sample were satisfactory, allowing us to conclude that the active ingredient was present even when mixed with organic matter. The method was applied to a real case of poisoning and it was possible to identify brodifacoum-A 3 in the sample, assisting in the preparation of the expert report. The method has limitations due to the degradation of brodifacoum at high temperatures and its quantification in complex matrices, with the liquid chromatography technique being the most suitable for thermolabile compounds. However, the results show that the method is effective for qualitative analysis of materials suspected of containing poison, which are commonly contaminated with large amounts of rodenticide, identifying the active ingredient and becoming an option in forensic laboratories. Forensic laboratories that do not have liquid chromatography can use the methodology described in this article to identify brodifacoum in samples suspected of having been contaminated with coumarins, helping criminal investigations with more trustworthy reports.
DATA AVAILABILITY STATEMENT Any additional data may be obtained from the corresponding author upon reasonable request.
ACKNOWLEDGMENTS Daniel B. de Souza thanks the Postgraduate Program in Chemistry at the State University of Rio de Janeiro for supporting his postdoctoral studies.
REFERENCES 1. Abhilash, K. P. P.; Chandran, J.; Murugan, S.; Rabbi, N. A. S.; Selvan, J.; Jindal, A.; Gunasekaran, K.; Medical Journal Armed Forces India 2022, 78, S139. [Crossref] 2. Ferreira, A.; Maroco, E.; Yonamine, M.; Oliveira, M. L. F.; Braz. J. Pharm. Sci. 2008, 44, 3. [Crossref] 3. Soltaninejad, K.; International Journal of Forensic Sciences 2023, 8, 1. [Crossref] 4. Van Sittert, N. J.; Tuinman, C. P.; Toxicology 1994, 91, 71. [Crossref] 5. King, N.; Tran, M.; Transfusion Medicine Reviews 2015, 29, 250. [Crossref] 6. Papin, F.; Clarot, F.; Vicomte, C.; Gaulier, J. M.; Daubin, C.; Chapon, F.; Vaz, E.; Proust, B.; Forensic Sci. Int. 2007, 166, 85. [Crossref] 7. Watson, W. A.; Litovitz, T. L.; Klein-Schwartz, W.; Rodgers Junior, G. C.; Youniss, J.; Reid, N.; Rouse, W. G.; Rembert, R. S.; Borys, D.; The American Journal of Emergency Medicine 2003, 22, 335. [Crossref] 8. Lopes, C. V. A.; Albuquerque, G. S. C.; Health Debate 2018, 42, 117. [Crossref] 9. Bochner, R.; Freire, M. M.; Ciência & Saúde Coletiva 2020, 25, 761. [Crossref] 10. Passos, L. S.; Farias, M. T.; Teixeira, M. T.; Revista Brasileira de Criminalística 2023, 12, 81. [Crossref] 11. Santos, S. A.; Legay, L. F.; Lovisi, G. M.; Santos, J. F. C.; Lima, L. A.; Revista Brasileira de Epidemiologia 2013, 16, 376. [Crossref] 12. Feinstein, D. L.; Gierzal, K.; Iqbal, A.; Kalinina, S.; Ripper, R.; Lindeblad, M.; Zahkarov, A.; Lyubimov, A.; Breemend, R. V.; Weinberg, G.; Rubinstein, I.; Toxicol. Lett. 2019, 306, 61. [Crossref] 13. Feinstein, D. L.; Brodsky, S.; Weinberg, G.; Breeman, R. V.; Rubinstein, I.; Toxicol. Lett. 2017, 268, 71. [Crossref]. 14. Kalinin, S.; Marangoni, N.; Kowal, K.; Dey, A.; Lis, K.; Brodsky, S.; Breemen, R. V.; Hauck, Z.; Ripper, R.; Rubinstein, I.; Weinberg, G.; Feinstein, D. L.; Toxicol. Sci. 2017, 159, 224. [Crossref] 15. Gallocchio, F.; Basilicata, L.; Benetti, C.; Angeletti, R.; Binato, G.; Forensic Sci. Int. 2014, 244, 63. [Crossref] 16. Chua, J. D.; Friedenberg, W. R.; Arch. Intern. Med. 1998, 158, 1929. [Crossref] 17. Sarin, S.; Mukhtar, H.; Mirza, M. A.; Ann. Intern. Med. 2005, 142, 156. [Crossref] 18. Papini, S.; Prisco, R. C. B.; Savoy, V. L. T.; Vieira, E.; Luchini, L. C.; Am. J. Anal. Chem. 2012, 3, 266. [Crossref] 19. Almeida, E. M. F.: Determinação Eletroanalítica de Pesticidas Carbamatos e Ditiocarbamatos; Tese de Mestrado, Universidade Federal de Uberlândia, Patos de Minas, Brasil, 2021. [Link] accessed in August 2025 20. Hegdal, P. L.; Blaskiewicz, R. W.; Environ. Toxicol. Chem. 1984, 3, 167. [Crossref] 21. Yuen, S. H.; Analyst 1978, 103, 1229. [Crossref] 22. Breemen, R. B. V.; Hafner, J. W.; Nosal, D. G.; Feinstein, D. L.; Unmet, I. R.; Toxicol. Commun. 2021, 5, 93. [Crossref] 23. DuVall, M. D.; Murphy, M. J.; Ray, A. C.; Reagor, J. C.; J. Vet. Diagn. Invest. 1989, 1, 66. [Crossref] 24. Logan, B.; Fiorentin, T. R.; Identification of Anticoagulant Adulterants in Seized Material and Biological Samples; National Criminal Justice Reference Service (NCJRS): Washington, 2022. [Link] accessed in August 2025 25. Bodur, S.; Tutar, B. K.; Tutar, Ö. F.; Bakirdere, S.; Anal. Methods 2024, 16, 1363. [Crossref] 26. Herrero-Hernández, E.; Pose-Juan, E.; Álvarez-Martin, A.; Andrades, M. S.; Rodrígues-Cruz, M. S.; Sánchez-Martin, M. J.; J. Sep. Sci. 2012, 35, 3492. [Crossref] 27. US Food and Drug Administration (US FDA); Center for Drug Evaluation and Research (CDER); Bioanalytical Method Validation: Guidance for Industry; Office of Communications, Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration: Silver Spring, 2018. [Link] accessed in August 2025 28. Ribani, M.; Bottoli, C. B. G.; Collins, C. H.; Jardim, I. C. S. F.; Melo, L. F. C.; Quim. Nova 2004, 27, 771. [Crossref] 29. Tosti, T.; Rakić, G.; Natić, M.; Milojković-Opsenica, D.; Husinec, S.; Savić, V.; Tešić, Ž.; J. Planar Chromatogr.--Mod. TLC 2009, 22, 333. [Crossref] 30. Kaufmann, A.; Anal. Bioanal. Chem. 2012, 403, 1233. [Crossref] 31. Vargas-Pérez, M.; Domínguez, I.; González, F. J. E.; Frenich, A. G.; J. Chromatogr. A 2020, 1622, 461118. [Crossref] 32. Martelli, J. A.; Maltagliati, L. A.; Trevisan, F.; Gil, C. T. L. A.; Revista Dental Press de Ortodontia e Ortopedia Facial 2005, 10, 122. [Crossref] 33. Murphy, M. J.; Vet. Toxicology 2018, 46, 583. [Crossref]
Associate Editor handled this article: Eduardo M. Richter |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access