Artigo
| Enhancement of curcumin bioavailability using a controlled drug delivery system based on chitosan and green iron oxide nanoparticles (Fe3O4) |
|
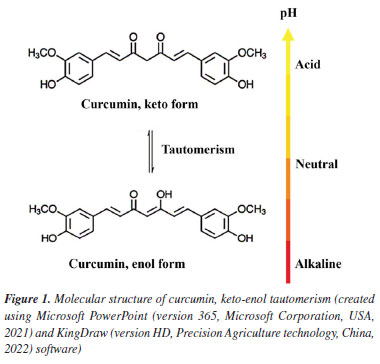
Fernanda K. Alves de SouzaI I. Departamento de Química, Faculdade de Ciências, Universidade Estadual Paulista, 17033-360 Bauru - SP, Brasil Received: 12/17/2024 *e-mail: aroldo.magdalena@unesp.br Curcumin (Cur), extracted from turmeric rhizomes (Curcuma longa), exhibits antiparasitic properties, making it a promising candidate for schistosomiasis mansoni treatment due to its low cost and absence of side effects. However, its chemical instability and water insolubility limit its therapeutic application. Green iron oxide nanoparticles (Fe3O4-green NPs), synthesized following Green Chemistry principles, serve as biocompatible carriers with magnetic properties. When combined with chitosan (Chit), a biopolymer capable of encapsulating curcumin, they create a controlled release system to enhance curcumin's stability and bioavailability. The objective of this study is to develop the Chit-Cur-Fe3O4-green controlled release system. Fe3O4-green NPs were synthesized using Fe2+ ions, potato peel, and sodium carbonate. The system was prepared by combining chitosan, Fe3O4-green NPs, curcumin, N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC), and N-hydroxysuccinimide (NHS). Characterization included X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), ultraviolet-visible spectroscopy (UV-Vis), fluorescence, thermal analysis and magnetic property. Controlled release was tested over 500 h in phosphate-buffered saline (PBS), with curcumin concentration determined by UV-Vis. Pharmacokinetics were analyzed using kinetics models. The system achieved 100% encapsulation efficiency and released 50% of curcumin over 400 h, reaching equilibrium at 370 mg L-1. The release followed a diffusion-controlled mechanism. The Chit-Cur-Fe3O4-green system enhanced curcumin's bioavailability and stability, demonstrating potential for therapeutic applications. INTRODUCTION Curcumin (Cur) is a phytochemical substance produced in the rhizomes of turmeric (Curcuma longa), a perennial plant from the Zingiberaceae family, cultivated in Asian countries such as India, Nepal, China, and Indonesia. Curcumin comprises 3% bisdemethoxycurcumin, 17% demethoxycurcumin, and 77% diferuloylmethane, with the latter being the main contributor to its bioactive properties.1-4 The bioactive properties of curcumin include anti-inflammatory,5 antioxidant,6 antiparasitic,7 antimicrobial,8 and anticancer activities.9 Furthermore, curcumin is widely available, low-cost, and non-toxic. Even at high doses, it exhibits minimal side effects, reinforcing its safety for human use and consolidating its potential in pharmacological applications.4 In aqueous solutions, curcumin undergoes keto-enol tautomerism, with the keto form predominating under acidic conditions and the enol form under alkaline conditions. As shown in Figure 1, the molecular structure of curcumin consists of two aromatic rings, each containing a phenolic and a methoxy group, connected by a seven-carbon chain. In the keto form, the carbon chain contains two ketone groups, whereas, in the enol form, it contains a hydroxyl group and a ketone group.4 Curcumin is hydrophobic in acidic aqueous conditions, being soluble only in organic solvents like acetone. However, in alkaline aqueous conditions, curcumin becomes hydrophilic. The color of curcumin solutions varies with pH due to the protonation state of hydroxyl groups. Between pH 2 and 7, curcumin appears yellow; between pH 7.1 and 8.5, the color shifts to orange; and at higher pH levels, it turns red,4 making curcumin a potential acid-base indicator. Curcumin is chemically unstable due to tautomerism, which occurs in response to pH variations. Since the human body exhibits different pH levels depending on the tissue,10 curcumin becomes unstable in the body, compromising its bioavailability for pharmacological applications. Additionally, curcumin is water-insoluble, exacerbating the issue as blood plasma is a predominantly aqueous medium.11 This combination of factors limits curcumin's therapeutic efficacy. To overcome these challenges, current studies aim to develop formulations that enhance its solubility, stability, and bioavailability, making it more effective in the treatment and prevention of diseases.12 An innovative approach involves incorporating curcumin into controlled drug delivery systems through encapsulation in colloidal particles with a hydrophobic interior and a hydrophilic surface, such as biopolymers.13
Chitosan (Chit), an abundant, low-cost, and biodegradable biopolymer, stands out for its biocompatibility, non-toxicity, and antibacterial, antifungal, mucoadhesive, analgesic, hemostatic, and wound-healing properties. These characteristics make it ideal for biomedical applications, such as drug encapsulation. It is obtained by the partial N-deacetylation of chitin extracted from the exoskeleton of crustaceans like crabs, lobsters, and shrimp.14,15 Chitosan is a polysaccharide composed of N-acetyl-D-glucosamine and D-glucosamine units linked by β-(1→4) glycosidic bonds. This composition defines its copolymer structure and its ability to interact with other substances.14,15 The amino groups of D-glucosamine in chitosan, with a pKa of 6.3, become protonated under low pH conditions, giving it a positive charge and solubility in dilute acids, such as acetic acid. This basic character allows chitosan to form films on negatively charged surfaces, such as fats, cholesterol, and proteins.14,15 Chitosan interacts with the cell membrane, facilitating the introduction of active agents through the epithelium. Its amino and hydroxyl groups enable stable covalent bonding and reactions such as esterification, etherification, and quaternization, allowing its functionalization.14,15 Crosslinking of chitosan represents one of the strategies used to modify its structure, enabling this biopolymer to store active agents such as curcumin, thereby forming a controlled release system composed of chitosan and curcumin, known as Quit-Cur. Crosslinked chitosan provides mechanisms that enhance the drug's properties as well as the rate and extent of bioactive compound release. In this context, chitosan is used to create a positively charged coating on curcumin-loaded delivery systems, aiming to improve mucoadhesion and increase bioavailability. This release occurs through ligand-receptor interactions, as chitosan can be chemically modified to specifically recognize and bind to target receptors, facilitating the entry of the system into cells via endocytic pathways.16 Iron(II,III) oxide nanoparticles (NPs) or Fe3O4 are a mixed oxide composed of iron(II) oxide (FeO) and iron(III) oxide (Fe2O3). This material exhibits magnetic properties due to its superparamagnetic behavior. Each nanoparticle has a constant magnetic moment, behaving like a giant paramagnetic atom. As a result, Fe3O4 NPs exhibit ferrofluid behavior, with the ability to remain stable in colloidal suspensions and interact with external magnetic fields.17-19 When administered into the body, a drug does not act selectively on diseased cells, which compromises the effectiveness of the treatment. To overcome this limitation, the use of Fe3O4 NPs as carriers in controlled drug delivery systems has been proposed, enabling the targeted transport of drugs to specific sites. This approach aims to limit the dispersion of the active compound throughout the body, reducing side effects, and to lower the administered dose without compromising therapeutic efficacy. In the case of curcumin carried by Fe3O4 NPs, the application of an external magnetic field allows precise guidance to the desired site of action, preventing it from spreading throughout the organism. This is crucial, as exposure to varying pH levels within the body can alter its chemical structure, impairing its stability and therapeutic potential. Additionally, encapsulating the drug within a polymer matrix along with the Fe3O4 NPs can extend the release time, enhancing the controlled delivery of the drug.20-23 Traditional routes for the synthesis of Fe3O4 NPs often involve the use of toxic chemical reagents, whose improper handling and disposal generate hazardous byproducts that are harmful to the environment. In this context, the adoption of green synthesis represents a significant and promising innovation. Based on the Principles of Green Chemistry, this approach seeks to minimize or eliminate the use and generation of substances that are harmful to human health and the ecosystem.24,25 Unlike conventional methodologies, green synthesis replaces harsh chemical reagents with plant extracts derived from agro-industrial waste such as leaves, peels, and bagasse. These materials, in addition to being renewable and low-cost, are non-toxic and widely available, making the technique not only environmentally sustainable but also economically viable.24-27 Plant extracts are rich in bioactive compounds - such as carboxylic acids, aldehydes, ketones, sugars, terpenoids, and flavonoids - that act as reducing, stabilizing, and mediating agents in the Fe3O4 NPs formation reaction.24,26,27 This natural synthesis mechanism imparts physicochemical properties to the Fe3O4 NPs that are suitable for biomedical applications, while significantly reducing the environmental impact of the process. Furthermore, this innovative strategy is aligned with the United Nations Sustainable Development Goals (SDGs), particularly SDG 12, which aims to ensure sustainable consumption and production patterns. The target is to reduce waste generation by 2030 through sustainable practices such as prevention, recycling, and reuse.28 In recent decades, schistosomiasis mansoni has been frequently diagnosed in northeastern Brazil, particularly in the states of Alagoas, Pernambuco, and Sergipe, where it accounts for 10% of hospital admissions in the public health system. This region, characterized by a low human development index (HDI), faces a lack of basic sanitation and education, which facilitates the prevalence of neglected diseases as defined by the World Health Organization (WHO), such as Chagas disease, leishmaniasis, tuberculosis, dengue, malaria, leprosy, and schistosomiasis.29 Schistosomiasis mansoni is a disease caused by the parasite Schistosoma mansoni, common in tropical regions. It is transmitted through water contaminated with human feces and involves snails of the genus Biomphalaria as intermediate hosts. Schistosomiasis is generally asymptomatic but can present in its acute phase (itching, fever, pain, and liver swelling) or chronic phase, with intestinal (diarrhea), hepato-intestinal (enlarged liver), and hepatosplenic forms (enlarged liver and spleen, ascites, and risk of death).29 Praziquantel is the main drug used in the treatment of schistosomiasis, however, it has significant limitations. This medication does not prevent new infections, is ineffective against the juvenile forms of the parasite, and has little impact on liver and spleen lesions. Moreover, there have been reports of emerging resistant strains, which further compromise its long-term efficacy. The continued implementation of praziquantel-based treatment programs faces major obstacles, such as high costs and logistical challenges, making large-scale application difficult. Other drugs, such as metrifonate and oxamniquine, also present limitations and are rarely used due to their low availability, unsatisfactory efficacy under field conditions, and the resistance observed in laboratory experiments. Given this scenario, the need to develop new, more effective, safer, and accessible schistosomicidal compounds becomes evident. In this context, curcumin emerges as a promising drug. It is a natural compound known for its antiparasitic properties. Curcumin stands out due to its low cost, wide availability, and low toxicity, making it a viable alternative for low-income populations and for use in public health programs. These characteristics, combined with its action against various parasites, justify the growing scientific interest in its potential for schistosomiasis control.30 This study, therefore, aims to synthesize a controlled release system composed of curcumin encapsulated in chitosan and carried by Fe3O4-green NPs, referred to as Chit-Cur-Fe3O4-green. Thus, the proposed study emerges as a promising solution to enhance the stability, solubility, and targeting of curcumin, thereby boosting its effectiveness. This approach has the potential to revolutionize the treatment of schistosomiasis mansoni, which still relies on conventional therapies with high costs and risks.
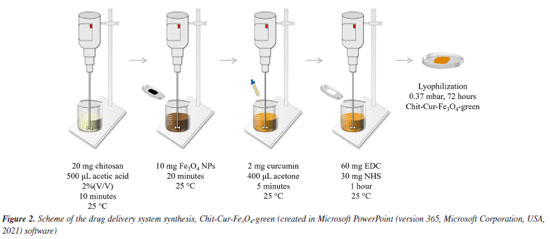
EXPERIMENTAL All experiments were conducted in triplicate to ensure the reproducibility and reliability of the obtained results. Chemical reagents Iron(II) chloride tetrahydrate (FeCl2.4H2O), iron(III) chloride hexahydrate (FeCl3.6H2O), chitosan (low molecular weight), N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), and phosphate-buffered saline (PBS, pH 7.4) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nitrogen gas (N2) was obtained from White Martins (Bauru, SP, Brazil). Ammonium hydroxide (NH4OH) and sodium carbonate (Na2CO3) were obtained from Synth (Diadema, SP, Brazil). Potatoes (Solanum tuberosum L.) were purchased from a local supermarket. Acetic acid (CH3COOH) was obtained from Hexis Científica (Indaiatuba, SP, Brazil). Curcumin was extracted in a research laboratory. Acetone was purchased from Dinâmica (Indaiatuba, SP, Brazil). All aqueous solutions were prepared with distilled water. All chemicals used were of analytical grade and did not undergo any additional purification. Synthesis Synthesis of Fe3O4 NPs via the coprecipitation method The Fe3O4 NPs synthesized via the coprecipitation method were prepared by mixing aqueous solutions of FeCl2·4H2O (0.05 mol L-1, 50 mL) and FeCl3·6H2O (0.1 mol L-1, 50 mL) in a molar ratio of 1Fe2+:2Fe3+. The mixture, which displayed an orange color, was stirred magnetically for 15 min at 25 ± 2 ºC under a nitrogen (N2) stream to remove O2 from the reaction medium, preventing the oxidation of iron salts. Subsequently, 5 mL of 28% NH4OH, the precipitating agent, was added. The iron salts instantly precipitated into Fe3O4 NPs, as described by Equation 1, causing the reaction medium to turn black and reach a pH of 10. The reaction was maintained for 1 h to ensure maximum yield. Finally, the Fe3O4 NPs were separated magnetically, washed with distilled water until reaching pH 7, dried at 75 ± 2 ºC for 24 h, and ground into a fine powder.31,32 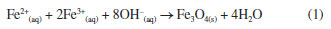 Synthesis of Fe3O4 NPs via the green chemistry method The Fe3O4 NPs synthesized via the green chemistry method were prepared starting with the preparation of potato peel extract. Six English potatoes (Solanum tuberosum L.) were washed and peeled. The peels were added to 500 mL of distilled water under mechanical stirring for 30 min at 100 ± 2 ºC. The resulting extract was separated using a sieve, and 100 mL of this extract was mixed with 2 g of FeCl2·4H2O under magnetic stirring for 30 min at 75 ± 2 ºC. Subsequently, 5 g of Na2CO3, the precipitating agent, was added. The iron salt instantly precipitated into Fe3O4-green NPs, resulting in a navy-blue coloration of the medium and a pH of 9. The reaction was maintained for 30 min to ensure maximum yield. Finally, the Fe3O4-green NPs were separated by centrifugation, washed with distilled water until reaching pH 7, dried at 75 ± 2 ºC for 24 h, and ground into a fine powder. The difference observed in the final coloration between the coprecipitation and green synthesis methodologies is related to the nature of the precipitating agent used. In the coprecipitation method, the use of NH4OH raises the pH of the reaction medium to approximately 10, promoting a more intense precipitation compared to Na2CO3, which is employed in the green synthesis and reaches a maximum pH of around 9. As a result, the material obtained via the green methodology exhibits a navy-blue color, whereas the product from coprecipitation is completely black, suggesting possible differences in the composition or structure of the formed material. Nevertheless, it is important to highlight that, after thermal treatment, the Fe3O4-green NPs synthesized through the green approach also exhibited magnetic properties similar to those obtained by the conventional method. This indicates that the magnetic material was indeed formed, albeit through a different reaction mechanism, with the added advantage of using more environmentally friendly reagents.25 Synthesis of the controlled drug delivery system As described in Figure 2, the controlled release system was synthesized starting with the dissolution of 20 mg of chitosan in 500 μL of a 2% (v/v) acetic acid aqueous solution, under mechanical stirring for 10 min at 25 ± 2 ºC. The experimental setup was adapted to be suitable for the synthesis of the system: a 5 mL beaker was used, and the mechanical stirrer was fitted with a thinner blade to allow effective stirring of the small reaction volume involved in the synthesis. Then, 10 mg of Fe3O4 NPs (synthesized by coprecipitation and green chemistry for comparison) were added, and the mixture was stirred for 20 min. Next, 2 mg of curcumin, dissolved in 400 μL of acetone, was added, and the mixture was stirred for 5 min. Finally, 60 mg of EDC was added, and the mixture was stirred for 5 min, followed by the addition of 30 mg of NHS. The reaction was maintained for 1 h to ensure maximum yield. At the end, the system was lyophilized (0.37 mbar, 72 h) and ground into a fine powder.20-22,33
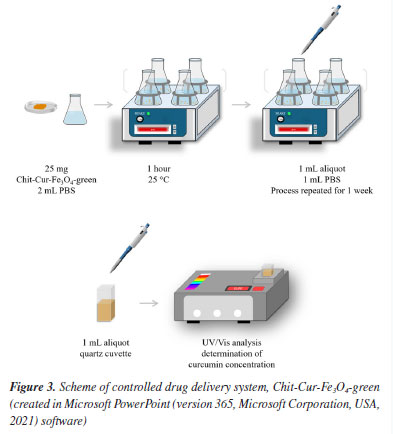
Characterization X-ray diffraction (XRD) The diffractograms were obtained using a Rigaku MiniFlex 600 diffractometer, by the powder method, with Cu-Kα radiation (λ = 1.54056 Å), at 40 kV, with 20 mA, and in the range of 10º ≤ 2θ ≤ 80º at 10º min-1. Scanning electron microscopy (SEM) The micrographs were obtained using a FEI Helios NanoLab 600i scanning electron microscope, with an integrated EDS, and the powder samples were metallized by carbon evaporation, with 3000 kV, 0.17 nA, and resolutions ranging from 100 to 5000 times. Fourier transform infrared spectroscopy (FTIR) The infrared spectra were obtained using a PerkinElmer Spectrum IR spectrophotometer, with ATR (attenuated total reflection), in the range of 4000-400 cm-1, with 32 scans and a resolution of 4 cm-1. Thermal analysis The thermograms were obtained using a TA Instruments thermogravimetric analyzer, model SDT 2960, under a dry air atmosphere with a flow rate of 50 mL min-1, using a Pt crucible, within the temperature range of 30-800 ºC, at a heating rate of 10 ºC min-1. Zeta potential (ZP) The zeta potential graphs were obtained using a Malvern Panalytical equipment, Zetasizer Nano, ZEN3500 Nano ZS, with a 175º angle, red laser (633 nm), light source (He-Ne), and 4 mW. Ultraviolet-visible spectroscopy (UV-Vis) The ultraviolet-visible spectra were obtained using an HP spectrophotometer, Kayak XA, in the range of 700-200 nm. Fluorescence spectroscopy (FLU) The fluorescence spectra were obtained using a spectrofluorometer, Molecular Devices, Spectramax M2, in the range of 700-420 nm. Qualitative magnetic property The qualitative magnetic property was evaluated based on the behavior of the materials in relation to the presence of a magnet. Quantitative magnetic property The magnetic measurements were done in a Physical Properties Measurement System - PPMS from Quantum Design, equipped with a vibrating sample magnetometer. The magnetization as a function of temperature was done in the warming mode and under a magnetic field of 100 Oe. The zero field cooling magnetization (Mzfc) was done cooling the sample in absence of a magnetic field, and the field cooling magnetization was done after cooling the sample in presence of a magnetic field of 100 Oe. Controlled release As described in Figure 3, the controlled release was performed by mixing 25 mg of the system with 2 mL of PBS, under agitation in a water bath at 25 ± 2 ºC. Aliquots of 1 mL were collected at 1-h intervals for 4 weeks, and 1 mL of PBS was added to maintain a constant volume. The collected aliquot was centrifuged; the solid phase - the system - was returned to the controlled release medium, and the liquid phase was analyzed by UV-Vis spectroscopy to determine the concentration of released curcumin.20,33
To determine the concentration of curcumin released by UV-Vis spectroscopy, a calibration curve of curcumin was constructed when mixed with the synthesis and release reagents, which includes 2% (v/v) acetic acid, acetone, EDC, NHS, and PBS, in the same proportions and concentrations established in the synthesis methodology. Five dilutions of curcumin mixed with the synthesis and release reagents were made using PBS as a solvent (0.03125, 0.0625, 0.125, 0.25, 0.5 g L-1). This is because the characteristic maximum wavelength of curcumin in acetone is modified when curcumin is dissolved in PBS due to the pH increase, being equal to 407 nm. From the calibration curve, Figure 4, the line equation was obtained, Equation 2, which was associated with the Lambert-Beer law, Equation 3. 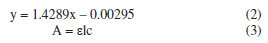
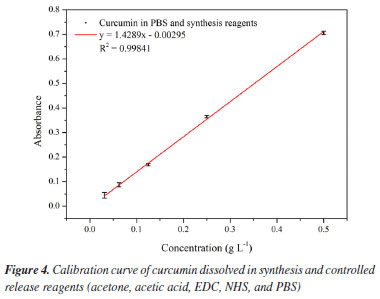
where: y is the absorbance, x is the curcumin concentration (g L-1), εl is the molar absorptivity coefficient of curcumin (g L-1 cm-1) multiplied by l, which is the path length, corresponding to the slope coefficient equal to 1.4289, and 0.00295 is the intercept coefficient. The line equation was manipulated to determine the curcumin concentration in the aliquots collected during the controlled release, Equation 4. 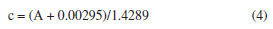 Based on the released curcumin concentration, the following were calculated: dilution concentration, concentration difference, accumulated concentration, and accumulated mass. Then, the drug loading (DL, in percentage) was calculated, as described by Equation 5, which is the percentage of the proportion of curcumin in the system, and the encapsulation efficiency (EE, in percentage) was also calculated, as described by Equation 6, which is the percentage of the amount of drug encapsulated that was added during the synthesis.20  where: mCur is the mass of curcumin in the system (mg), msystem is the mass of the system (mg), and mCur initial is the initial mass of curcumin added to the system (mg). The kinetic models of controlled drug release represent a methodology for analyzing the drug release mechanism. In this study, controlled release will be analyzed through the zero-order, first-order, Higuchi, Korsmeyer-Peppas, and Hixson-Crowell models, as described by Equations 7-11.20,33 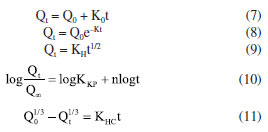 where: Qt is the amount of drug released at time t (mg), Q0 is the initial amount of drug in the solution (mg), Q∞ is the amount of drug released at infinite time t (mg), t is time (h), n is the diffusion exponent (drug release mechanism), and K0, K, KH, KKP and KHC are rate constants for the zero-order, first-order, Higuchi, Korsmeyer-Peppas, and Hixson-Crowell models, respectively.
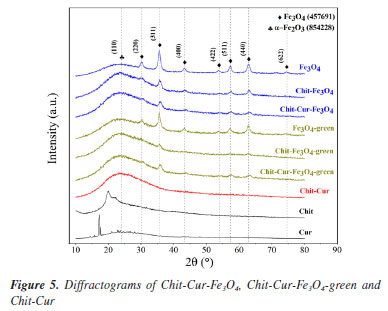
RESULTS AND DISCUSSION Characterization The diffractograms in Figure 5 show the characteristic peaks of Fe3O4 NPs in all the samples containing this compound, with reference to the Crystmet database, crystal structure file number 457691,34 as reported by Silveira et al.32 and Neves et al.20 As shown, the samples of the Chit-Cur-Fe3O4 system are identified in blue, while the corresponding Chit-Cur-Fe3O4-green samples are highlighted in green, and those related to Chit-Cur are marked in red. Furthermore, the individual samples that form the systems are represented in black. Therefore, it can be considered that the methodologies for synthesizing the Fe3O4 and Fe3O4-green NPs were effective. Additionally, characteristic peaks of α-Fe2O3 were observed in the samples containing Fe3O4-green NPs (Crystmet 854228). This compound is one of the intermediates in the reaction for forming Fe3O4 NPs, meaning the synthesis reaction of Fe3O4-green NPs may not have reached the molar ratio of 1Fe2+:2Fe3+ required for complete Fe3O4 NP formation. As mentioned by Magdalena et al.,35 in a conventional coprecipitation synthesis, this ion ratio is typically controlled by an inert N2 atmosphere. It was observed that the characteristic peaks of Fe3O4 NPs decreased in intensity when added to the Chit-Cur-Fe3O4, Chit-Cur-Fe3O4-green, and Chit-Cur systems.
The micrographs with EDS and the size histogram of Fe3O4 and Fe3O4-green NPs in Figure 6 show the surface characteristics of the Chit-Cur-Fe3O4, Chit-Cur-Fe3O4-green, and Chit-Cur systems. The histogram was constructed using ImageJ Ops software (version 1.51k, National Institutes of Health, USA, 2017), based on the counting of one hundred Fe3O4 and Fe3O4-green NPs shown in the micrographs in Figures 6a-6b, indicating that the Fe3O4 and Fe3O4-green NPs measured 20 and 67 nm, respectively. In addition, the Kruskal-Wallis statistical test was performed using Stata software (version 14.0, StataCorp, USA, 2015). The mean size values of the Fe3O4 NPs showed a statistically significant difference (P ≤ 0.001). This indicates that size control, as well as size homogeneity, was affected by the synthetic methodology (coprecipitation and green chemistry).
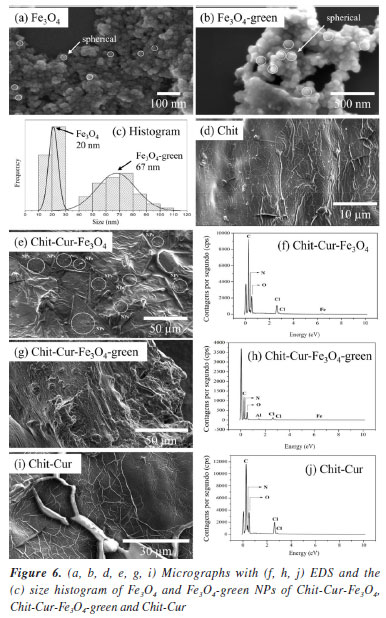
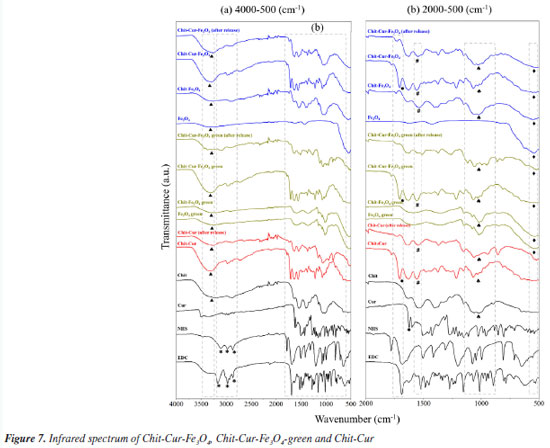
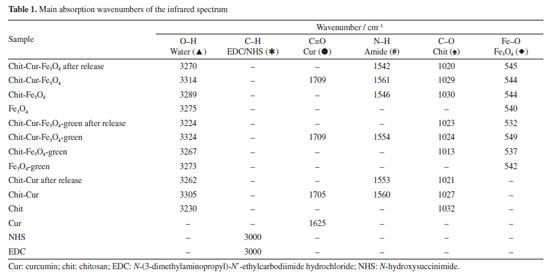
Thus, it was observed that although the Fe3O4 NPs and Fe3O4-green NPs exhibit a spherical shape and nanometric size, the Fe3O4 NPs are smaller than the Fe3O4-green NPs, indicating that the size of the NPs can be influenced by the synthetic methodology, such as coprecipitation and green chemistry. That is, the green synthesis can alter the nucleation and formation mechanism of the NPs. As stated by Magdalena et al.,35 the formation of Fe3O4 NPs depends on the molar ratio of Fe2+ and Fe3+ ions in the reaction medium, as well as the reaction rate. Therefore, the addition of sodium carbonate to increase the pH of the reaction medium may have influenced the nucleation and growth mechanism of the NPs. Regarding chitosan, it has a smooth morphology with interconnections and regions in the shape of air pockets, a characteristic that remains present in the Chit-Cur system. The Chit-Cur-Fe3O4 system has the characteristics of chitosan combined with dispersed NPs. In contrast, in the Chit-Cur-Fe3O4-green system, dispersed NPs were not observed. The systems exhibited the expected chemical elements in their composition, such as carbon (C), nitrogen (N), and oxygen (O), along with iron (Fe) and chlorine (Cl) for the systems containing Fe3O4 NPs derived from chlorinated iron salts. The presence of aluminum (Al) was also noted in the green system, which, according to Salman and Rashid,36 originates from the potato peel extract. The infrared spectra presented in Figures 7a and 7b, along with the assignments of the bonds described in Table 1, highlight relevant aspects of the molecular composition of the Chit-Cur-Fe3O4, Chit-Cur-Fe3O4-green, and Chit-Cur systems. As illustrated, the samples associated with the Chit-Cur-Fe3O4 system are represented in blue, while those of the Chit-Cur-Fe3O4-green system appear in green, and the Chit-Cur system is displayed in red. Additionally, the individual samples that compose the systems are indicated in black. This color differentiation facilitates comparative analysis between the systems and their individual components. In this context, it was noted in the region around 3300 cm-1, a broad band of variable size in most materials, which, according to Leandro-Silva et al.,37 is related to the stretching vibrations of the O-H bond, characteristic of hydroxyl (OH) groups present in water molecules (H2O). This suggests that the materials exhibited a certain degree of hydration from the synthesis process, which remained present even after thermal treatment (for Fe3O4 and Fe3O4-green NPs) and lyophilization (for the systems). For the samples of EDC, NHS, and Cur, the O-H band was not identified, as expected, since these materials were characterized in their purified form, not subjected to synthesis processes or subsequent drying. For the EDC and NHS samples, three bands were identified in the 3000 cm-1 region, associated with the stretching vibrations of C-H bonds, typical of aromatic hydrocarbons. In the other materials containing EDC and NHS in their composition, i.e., the systems, these three bands appeared with reduced intensity, suggesting that EDC and NHS may have been consumed in the synthesis reaction of the systems, forming new bonds. For the systems after controlled release, these bands were not identified, suggesting the release of the compound formed by these new bonds. At 1625 cm-1, a band characteristic only of curcumin was observed, and Nosrati et al.21 also identified this band, associating it with the stretching vibration of the C=O (carbonyl) bond. In systems containing curcumin, this band was shifted to the 1700 cm-1 region, suggesting the formation of a bond between curcumin and the reagents from the synthesis of the systems. In the systems characterized after controlled release, the same band was identified with low intensity, indicating the release of curcumin from the system. Around 1550 cm-1, a band appeared only in the systems. According to Nosrati et al.,21 this region is characteristic of bands related to N-H bonds that form amide groups. The presence of an amide group was expected in the systems due to the addition of EDC and NHS in the synthesis. Keleştemur et al.38 explains that EDC is a coupling agent used to activate carboxyl groups present in curcumin so they can react with free amine groups from chitosan. This process generates an amide bond, which is stable and resistant to hydrolysis. NHS is added to stabilize the reactive intermediate formed by EDC, enhancing the efficiency of the reaction. Chitosan has a characteristic band at 1032 cm-1, which was also identified by Silveira et al.32 and Bhatia et al.39 and is associated with the stretching vibration of the C-O bond in the chitosan polymer structure. In materials containing chitosan, this band is noticeable, especially in systems after controlled release, suggesting that chitosan remains in the systems and is not dissolved, demonstrating that only the drug, curcumin, is released. Moreover, a characteristic band of Fe-O bonds was identified at 540 cm-1. According to Malika et al.,25 this band is specifically related to the Fe-O bond in the tetrahedral site of the crystalline structure, confirming the formation of Fe3O4 NPs. This behavior was also observed in other materials containing these NPs, indicating that, even after the controlled release of the drug, the systems continued to exhibit this band. This suggests that the Fe3O4 NPs and chitosan remain retained in the system, releasing only the drug. Regarding the Fe3O4-green NPs, when the infrared spectrum was compared with that of Fe3O4 NPs, a significant difference was observed. In addition to the Fe-O bond band, other bands were identified at wave numbers characteristic of organic bonds. Based on the studies of Karami et al.,40 this suggests that the Fe3O4-green NPs may have been functionalized by organic groups present in the potato peel extract used in the green synthesis. Girigoswami et al.41 and Karami et al.40 attributed the bands at 1151, 1077, and 1014 cm-1 to stretching vibrations of C-O bonds, and the band at 841 cm-1 to C-H bond vibrations. These bonds compose the organic groups present in the Fe3O4-green NPs.
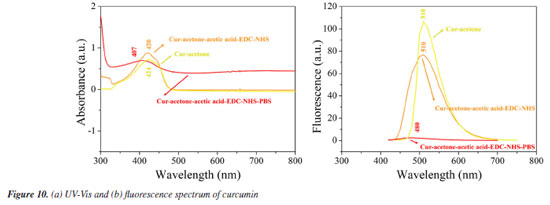
The thermograms in Figure 8 show that Fe3O4 NPs lose mass at a constant rate, with a noticeable weight loss occurring around 100 ºC, which, according to Fumis et al.,42 indicates the loss of water from the sample. Subsequently, a mass gain is observed at approximately 700 ºC. Fumis et al.42 discusses that this may be due to the formation of the compound γ-Fe2O3, an intermediate product of the oxidation reaction of Fe3O4 NPs, resulting from the incorporation of oxygen gas. In the case of the Fe3O4-green NPs, a similar mass loss around 100 ºC is also attributed to water. Additionally, a mass loss around 400 ºC may be related to the degradation of organic matter present in the material, as reported by Fumis et al.42 and Nosrati et al.21 In this case, the organic matter likely originates from the potato peel extract used during the synthesis. Thus, the thermogram suggests a possible functionalization of the Fe3O4-green NPs by functional groups present in the potato peel extract, as established by Popescu et al.43 Moreover, the loss of organic mass accounts for approximately 40% of the total mass loss of the material. This result indicates that the majority of the mass of the Fe3O4-green NPs is composed of organic matter. The zeta potential shown in Figure 9 reveals that the isoelectric points of curcumin, Fe3O4 NPs, and the systems Chit-Cur-Fe3O4, Chit-Cur-Fe3O4-green, and Chit-Cur are 4.4, 5.7, 7.0, 6.5, and 7.4, respectively. The isoelectric point of the Fe3O4-green NPs could not be determined. The zeta potential data for the Fe3O4 NPs indicate a modification in the surface functionalization properties depending on the synthesis method used. The results showed that, with the green chemistry approach, the zero charge point shifted to lower pH values. Above pH 6.0, the system showed better colloidal properties, with values below -30 mV, in comparison to the Fe3O4 NPs. This change is likely related to the functional groups from the potato peel extract. França et al.29 note that in its adult stage, Schistosoma mansoni resides in the veins of the intestine and liver. According to Neves et al.,20 the pH of the blood vessels corresponds to the physiological pH, ranging from 7.4 to 8.0. Within this pH range, the systems Chit-Cur-Fe3O4, Chit-Cur-Fe3O4-green, and Chit-Cur exhibit a reduced negative charge. Szlasa et al.44 highlight that the membrane of the endothelial cells lining the blood vessels carries a negative charge due to the presence of glycocalyx, a layer composed of carbohydrates and proteins. As a result, the electrostatic interaction between the systems and the membrane will be limited, reducing the chance of non-specific adhesion. Although the Chit-Cur-Fe3O4-green system exhibits a negative or neutral charge at physiological pH, its hydrophobic surface, observed through micrographs, infrared spectra, and thermograms, facilitates significant interactions. Based on the studies of Kang and Loverde,45 systems with a hydrophobic surface are capable of interacting with the cell membranes of both blood vessels and Schistosoma mansoni cells. This is because the membrane of endothelial cells, composed of lipids and glycolipids, can promote interaction with hydrophobic molecules, allowing the system to be adsorbed or penetrated. However, Abath and Werkhauser,46 in their deeper study of the parasite, show that this interaction is more limited compared to the parasite's membrane, which has a tegument rich in highly hydrophobic lipids and glycolipids. Mafud et al.47 indicate that this hydrophobic environment in the parasite's membrane facilitates the adsorption and potential penetration of the drug, compromising the integrity of the tegument and interfering with the parasite's vital processes. Therefore, while drug interaction with both membranes is feasible, its effectiveness is more pronounced in the case of the parasite, where the lipid structure is more favorable to the drug's action. The UV-Vis and fluorescence spectra in Figures 10a and 10b show the behavior of curcumin in three different reaction media: (i) acetone, (ii) acetone-acetic acid-EDC-NHS, and (iii) acetone-acetic acid-EDC-PBS. These spectra allow for the analysis of how curcumin reacts to the reagents used in the synthesis of the systems Chit-Cur-Fe3O4, Chit-Cur-Fe3O4-green, and Chit-Cur. The maximum absorption for curcumin in acetone was 424 nm, in acetone-acetic acid-EDC-NHS it was 420 nm, and in acetone-acetic acid-EDC-NHS-PBS it was 407 nm. The maximum fluorescence emission for curcumin in acetone was 510 nm, in acetone-acetic acid-EDC-NHS it was 510 nm, and in acetone-acetic acid-EDC-NHS-PBS it was 480 nm. As can be observed, the absorbance and fluorescence of curcumin in PBS decreased when compared to curcumin in acetone. There was also a shift in the absorbance curve in PBS. For curcumin in acetone and curcumin in acetone with the synthesis reagents, the difference was minimal, suggesting that the reagents did not significantly interfere with the absorbance and fluorescence of curcumin. It was possible to infer that the decrease in absorbance and fluorescence of curcumin in PBS was due to the presence of PBS. This reduction in absorbance and fluorescence may be related to the degradation of curcumin caused by PBS, as Wang et al.48 reported that 90% of curcumin was degraded in PBS buffer (pH 7.4), with degradation continuing in higher pH, meaning that curcumin undergoes degradation in neutral-alkaline pH. In this context, the molecular structure of curcumin in neutral-alkaline media is altered, as a proton is removed from the phenolic group. One way to prevent curcumin degradation is to protect it with another compound before exposure to neutral-alkaline media. Since 1997, the literature has reported that curcumin is unstable in neutral-basic media and can degrade. However, more recent studies on controlled release of curcumin in PBS buffer do not always account for degradation, leading to some inconsistency in the more recent data, like Aguiar et al.,49 Allam,30 Amani et al.,50 Chakraborti et al.,51 Kazemi et al.,52 Nosrati et al.,21 Salehiabar et al.,53 Yang et al.54 According to Nardo et al.,55 the maximum absorption for curcumin in acetone is 420 nm, a value that closely matches the experimental values. According to Bansal and Rosenholm,56 the maximum fluorescence emission for curcumin in acetone is 524 nm, which is also close to the experimental values.
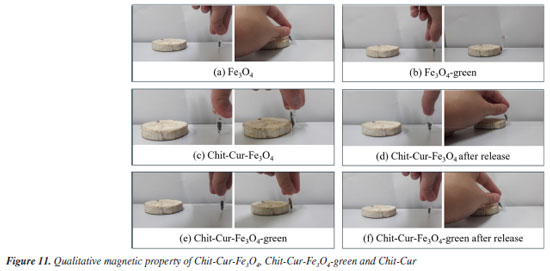
The images of the qualitative magnetic property in Figures 11a-11f show that both Fe3O4 and Fe3O4-green NPs exhibited attraction to an external magnetic field provided by a magnet, both individually and in the systems before and after controlled release, which can be considered a satisfactory result. However, the intensity of the magnetic attraction varied between the individual NPs and the aggregated samples in the Chit-Cur-Fe3O4, Chit-Cur-Fe3O4-green, and Chit-Cur systems. Specifically, the individual samples exhibited a higher intensity of magnetic attraction compared to the samples from the systems, which, in turn, showed lower intensity than the samples after release. This observed behavior can be interpreted as follows: the higher the purity of the NPs, the greater the intensity of magnetic attraction. Therefore, the individual samples exhibited a higher intensity of attraction, as did the samples after release. In the latter case, the release process removed reagents from the system, making the NPs purer than before the release.
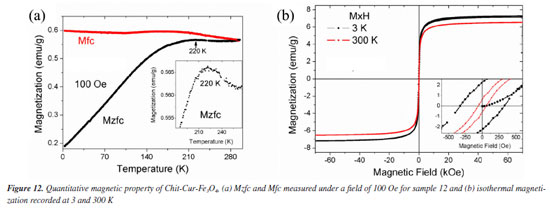
Figure 12a shows the Mzfc and Mfc measurements recorded on the warming mode under a magnet field of 100 Oe. The Mzfc for system Chit-Cur-Fe3O4 has an increasing trend up to 220 K, then it decreases slightly, the peak at 220 K that may be assigned to the thermal blocking temperature (Tb) of superparamagnetic (SPM) nanoparticles. For temperatures T > Tb the system behaves like paramagnetic particles, i.e., they will have a random orientation of their super moments in absence of a magnetic field and they will order and be attracted when applied an external magnetic field. The isothermal magnetization at 3 and 300 K are shown in Figure 12b. At 3 K, the saturation magnetization (Ms) of this sample is 7.21 emu g-1, the coercivity field (Hc) is 325 Oe and the remanence magnetization is 2.22 emu g-1. At room temperature the Ms, Hc and Mr are 6.52 emu g-1, 58 Oe and 0.61 emu g-1, respectively. The ratio of Mr/Ms for the measurements recorded at 3 and 300 K are 0.31 and 0.09, these values indicates that at 300 K the sample is mostly in the SPM regime. The small value of coercivity obtained at 300 K is because the largest particles are blocked, while the smaller particles are SPM. It is known that Ms for bulk magnetite samples is about 90 emu g-1 according to Cornell and Schwertmann.57 For system Chit-Cur-Fe3O4, the Ms value is smaller than the bulk magnetite. It is because the mass considered to normalize the magnetic moment was the total mass of the sample due to magnetite, chitosan and curcumin.
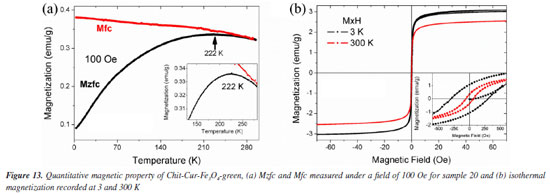
Figure 13a shows the Mzfc and Mfc for system Chit-Cur-Fe3O4-green. The Mzfc shows a peak at 222 K, this peak is due to the blocking temperature and is similar to the found in system Chit-Cur-Fe3O4. It is known that, for no interacting single domain magnetic nanoparticles, the blocking temperature is given by: Tb = KeffV/(25KB), where Keff is the effective magnetocrystalline anisotropy, V is the volume of nanoparticles and KB is Boltzmann's constant. Following this equation the volume of nanoparticles in system Chit-Cur-Fe3O4 and system Chit-Cur-Fe3O4-green should be similar, i.e., the nanoparticles in both samples have similar diameter. The Ms value for system Chit-Cur-Fe3O4-green is 3.05 emu g-1, it is smaller than the found in system Chit-Cur-Fe3O4 and it may be due to a larger mass contribution of chitosan and curcumin for system Chit-Cur-Fe3O4-green. The Hc at 3 and 300 K are 305 and 61 Oe, respectively. The ratio Mr/Ms for the measurements recorded at 3 and 300 K are 0.33 and 0.12. The Hc and Mr/Ms are similar in both systems. These results suggest that the magnetic nanoparticles in system Chit-Cur-Fe3O4 and system Chit-Cur-Fe3O4-green have similar volumes and are mostly superparamagnetic at 300 K as shown in Figure 13b.
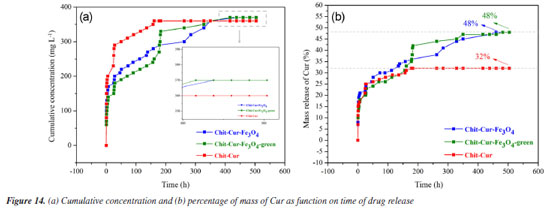
The quantitative magnetic properties, Figures 12 and 13, have significant implications for controlled drug delivery systems using magnetic nanoparticles, such as Chit-Cur-Fe3O4 and Chit-Cur-Fe3O4-green. The observed superparamagnetic behavior should be especially emphasized. Firstly, superparamagnetic nanoparticles do not retain magnetization in the absence of a magnetic field, which prevents permanent aggregation between nanoparticles and contributes to the colloidal stability of the suspension - an essential factor for intravenous application and safe circulation of the system within the body. Furthermore, the fact that they only respond in the presence of an external magnetic field allows these nanoparticles to be precisely guided to the target site, without the risk of unwanted residual magnetization. In this way, the system can be designed to release the active ingredient in a targeted manner, enhancing therapeutic efficacy and reducing side effects. Thus, the superparamagnetic behavior not only confirms the suitability of these nanoparticles for therapeutic use but also represents one of their main functional advantages.17,20 Controlled release Figures 14a and 14b shows that the Chit-Cur-Fe3O4, Chit-Cur-Fe3O4-green, and Chit-Cur systems reached equilibrium at approximately 400, 400, and 100 h, respectively. These systems exhibited cumulative concentrations of 370, 370, and 360 mg L-1, and cumulative masses of 0.75, 0.75, and 0.73 mg, respectively. As observed, the performance of the green system was equivalent to that of the conventional system. According to Malika et al.,25 the reduction of toxic reagents in synthesis processes is highly relevant today, making this result significant. The system without NPs reached equilibrium more quickly than the systems with NPs, indicating that the presence of NPs slows down the release of curcumin. This behavior is consistent with the observations of Neves et al.,20 who report a controlled and gradual release in systems containing NPs.
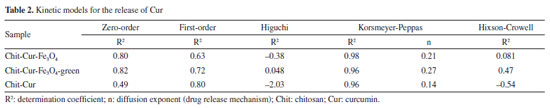
The three systems demonstrated an incorporation efficiency of 100%, a value higher than that obtained by Kazemi et al.,52 which was 96%. This result indicates that all the curcumin mass added during the synthesis was completely incorporated by the reagents. At the end of the process, no curcumin residues were observed, as the mixture of reagents formed a consistent, almost solid gel, without the presence of supernatant-unlike what usually occurs, as described by Neves et al.,20 where drug residues in the supernatant result in incorporation efficiencies lower than 100%. The systems with Fe3O4 and Fe3O4-green NPs showed a drug loading value of 6.25%, close to the value reported by Nosrati et al.,21 which was 6.89%. However, the value obtained by Kazemi et al.,52 of 63%, indicates that the methodology used in this study has potential for optimizations that could increase this parameter. In comparison, the release system without NPs showed a drug loading of 9.09%, higher than the systems with nanoparticles. This behavior is expected, as the absence of NPs reduces the total mass of the system, proportionally increasing the curcumin concentration and, consequently, the drug loading value. The systems with Fe3O4 and Fe3O4-green NPs showed a curcumin release mass percentage of 48%, higher than the values reported by Nosrati et al.,21 which was 35%, and by Kazemi et al.,52 which was 40%. In contrast, the release system without NPs showed a lower curcumin release mass percentage of 32%. It is important to discuss the fact that the drug loading of the systems with NPs was lower than that of the system without NPs, even though the mass percentage released was higher in the systems with NPs. This behavior can be interpreted as follows: in the system without NPs, the higher proportion of curcumin relative to the total mass (as indicated by the drug loading) did not translate into higher release efficiency. The absence of NPs reduced the release performance, suggesting that despite a lower proportion of curcumin, the NPs contributed to increasing the efficiency and the controlled release time of the drug, a desirable feature for release systems, as evidenced in the studies by Neves et al.20 Thus, a possible explanation for the lower drug loading associated with a higher mass percentage released in the systems with NPs is that the NPs delay the release process, allowing for gradual and efficient release over time. In contrast, the system without NPs, by reaching equilibrium more quickly, showed a reduced curcumin release. Studies by Kazemi et al.52 and Nosrati et al.21 also pointed out that NPs influence both the release time and the amount of drug released. Among the kinetic models evaluated, the Korsmeyer-Peppas model best fitted the experimental data, with coefficient of determination (R2) values of 0.98, 0.96, and 0.96 for the Chit-Cur-Fe3O4, Chit-Cur-Fe3O4-green, and Chit-Cur systems, respectively, as shown in Figure 15 and Table 2. This model fit well to the controlled release of the three systems because its Equation 10 generates an exponential curve, indicating that the release mechanism is characterized by a gradual increase in the mass of drug released over time. As described by Bhowmik et al.,58 Dash et al.,59 and Yadav et al.,60 the Korsmeyer-Peppas model is empirical and is widely used to describe drug release from polymeric systems, especially when the release mechanism does not follow simple diffusion and involves multiple processes. This model is particularly useful for polymeric dosage forms with complex or anomalous release behaviors.
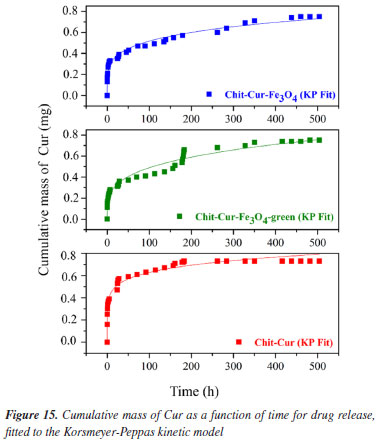
In the Korsmeyer-Peppas model, the value of n characterizes the drug release mechanism. According to Dash et al.59 and Yadav et al.,60 n ≤ 0.45 indicates Fickian diffusion, 0.45 < n < 0.89 corresponds to non-Fickian transport, n = 0.89 characterizes relaxational transport (case II), and n > 0.89 suggests super case II transport. The observed values of n for the three systems, 0.21, 0.27, and 0.14, respectively, are all less than 0.45, classifying them within the Fickian diffusion mechanism. This indicates that the drug release is dominated by diffusion through the polymeric system, according to Fick's law for diffusion in solids. Neves et al.20 discusses that the diffusion process is influenced by crucial factors such as the thickness of the polymeric matrix, its hydrophilic nature, and the drug's solubilization capacity within the matrix. The drug molecules can either be incorporated into the polymer through covalent bonds or dispersed within the polymeric matrix. Only the dissolved drugs contribute to the diffusion process, indicating that curcumin in the controlled release system may have its solubility enhanced in aqueous environments. Thus, the Korsmeyer-Peppas kinetic model is suitable for representing the controlled release systems in this study, as the chitosan matrix with Fe3O4 NPs provides a gradual and exponential release of curcumin. This controlled release behavior is complex and may follow a Fickian diffusion mechanism, although influenced by multiple interactive factors.
CONCLUSIONS The results indicated that the controlled release system Chit-Cur-Fe3O4-green was successfully synthesized, achieving an incorporation efficiency of 100%. The functionalized Fe3O4-green nanoparticles provided a controlled, slow, and gradual release of curcumin over 400 hours, reaching equilibrium at 370 mg L-1 and 0.75 mg of curcumin. This system resulted in an approximately 50% increase in the bioavailability of curcumin, compared to free curcumin, which is insoluble and chemically unstable. Furthermore, the developed system proved to be characteristic of diffusive processes.
DATA AVAILABILITY STATEMENT All data generated or analyzed during this study are included in this published article.
ACKNOWLEDGMENTS The authors thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for the financial support.
REFERENCES 1. Jurenka, J. S.; Alternative Medicine Review 2009, 14, 141. [Link] accessed in August 2025 2. Karunagaran, D.; Rashmi, R.; Kumar, T. R. S.; Curr. Cancer Drug Targets 2005, 5, 117. [Crossref] 3. Nasery, M. M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G.; Molecules 2020, 25, 689. [Crossref] 4. Zheng, B.; McClements, D. J.; Molecules 2020, 25, 2791. [Crossref] 5. Abdul‐Rahman, T.; Awuah, W. A.; Mikhailova, T.; Kalmanovich, J.; Mehta, A.; Ng, J. C.; Coghlan, M. A.; Zivcevska, M.; Tedeschi, A. J.; Costa, E.; Kumar, A.; Cantu‐Herrera, E.; Lyndin, M.; Sikora, K.; Alexiou, A.; Bilgrami, A. L.; Al‐Ghamdi, K. M.; Perveen, A.; Papadakis, M.; Ashraf, G. M.; BioFactors 2024, 50, 693. [Crossref] 6. Silva, F. K. S.; Orlandi, C. B. C.; Fernandes, M. A.; Brasil, G. S. P.; Mussagy, C. U.; Scontri, M.; Sasaki, J. C. S.; Abreu, A. P. S.; Guerra, N. B.; Floriano, J. F.; de Mendonça, R. J.; Caetano, G. F.; Farhadi, N.; Gómez, A.; Huang, S.; Farias, A. M.; Primo, F. L.; Li, B.; Fusco-Almeida, A. M.; Dokmeci, M. R.; Jucaud, V.; Mendes-Giannini, M. J. S.; Cardoso, M. R.; Herculano, R. D.; Int. J. Biol. Macromol. 2023, 242, 124778. [Crossref] 7. Sahebi, K.; Shahsavani, F.; Mehravar, F.; Hatam, G.; Alimi, R.; Radfar, A.; Bahreini, M. S.; Pouryousef, A.; Teimouri, A.; BMC Complementary Med. Ther. 2024, 24, 238. [Crossref] 8. Zamanidehyaghoubi, G.; Shahidi, F.; Dovom, M. R. E.; Mohebbi, M.; Roshanak, S.; LWT--Food Sci. Technol. 2024, 205, 116553. [Crossref] 9. Rahim, U. A.; Mustapa, M.; Shakrin, N. N. S. M.; Nurdin, A.; Taridi, N. M.; Yusof, Y. A. M.; Nordin, M. F. M.; Roos, N. A. C.; PLoS One 2024, 19, e0314280. [Crossref] 10. Proksch, E.; J. Dermatol. 2018, 45, 1044. [Crossref] 11. Brust, M.; Schaefer, C.; Doerr, R.; Pan, L.; Garcia, M.; Arratia, P. E.; Wagner, C.; Phys. Rev. Lett. 2013, 110, 078305. [Crossref] 12. Azad, A. K.; Lai, J.; Sulaiman, W. M. A. W.; Almoustafa, H.; Al-Shehade, S. A.; Kumarasamy, V.; Subramaniyan, V.; Pharmaceutics 2024, 16, 160. [Crossref] 13. Jacob, S.; Kather, F. S.; Morsy, M. A.; Boddu, S. H. S.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A. B.; Nanomaterials 2024, 14, 672. [Crossref] 14. Islam, M. M.; Shahruzzaman, M.; Biswas, S.; Sakib, M. N.; Rashid, T. U.; Bioact. Mater. 2020, 5, 164. [Crossref] 15. Shukla, S. K.; Mishra, A. K.; Arotiba, O. A.; Mamba, B. B.; Int. J. Biol. Macromol. 2013, 59, 46. [Crossref] 16. Saheb, M.; Fereydouni, N.; Nemati, S.; Barreto, G. E.; Johnston, T. P.; Sahebkar, A.; J. Cell. Physiol. 2019, 234, 12325. [Crossref] 17. Bini, R. A.; Marques, R. F. C.; Santos, F. J.; Chaker, J. A.; Jafelicci Jr., M.; J. Magn. Magn. Mater. 2012, 324, 534. [Crossref] 18. Lu, A.-H.; Salabas, E. L.; Schüth, F.; Angew. Chem., Int. Ed. 2007, 46, 1222. [Crossref] 19. Santana, G. P.; Ramos, A. M.; Fabris, J. D.; Quim. Nova 2008, 31, 430. [Crossref] 20. Neves, R.; Bronze-Uhle, E.; Santos, P.; Lisboa-Filho, P.; Magdalena, A.; Quim. Nova 2021, 44, 824. [Crossref] 21. Nosrati, H.; Sefidi, N.; Sharafi, A.; Danafar, H.; Manjili, H. K.; Bioorg. Chem. 2018, 76, 501. [Crossref] 22. Pham, X. N.; Nguyen, T. P.; Pham, T. N.; Tran, T. T. N.; Tran, T. V. T.; Adv. Nat. Sci.: Nanosci. Nanotechnol. 2016, 7, 045010. [Crossref] 23. Pourasgar, S.; Ranji, N.; Asadpour, L.; Shahriarinour, M.; Nikpassand, M.; Arabian J. Sci. Eng. 2025, 50, 6231. [Crossref] 24. León-Flores, J.; Pérez-Mazariego, J. L.; Marquina, M.; Gómez, R.; Escamilla, R.; Tehuacanero-Cuapa, S.; Reyes-Damián, C.; Arenas-Alatorre, J.; J. Cluster Sci. 2023, 34, 2381. [Crossref] 25. Malika, M.; Jhadav, P. G.; Parate, V. R.; Sonawane, S. S.; Chem. Pap. 2023, 77, 1081. [Crossref] 26. Aswathi, V. P.; Meera, S.; Maria, C. G. A.; Nidhin, M.; Nanotechnol. Environ. Eng. 2023, 8, 377. [Crossref] 27. Soltys, L.; Olkhovyy, O.; Tatarchuk, T.; Naushad, M.; Magnetochemistry 2021, 7, 145. [Crossref] 28. Martínez, J.; Cortés, J. F.; Miranda, R.; Processes 2022, 10, 1274. [Crossref] 29. de França, F. S.; da Silva, A. S.; Magalhães, C. M. M.; Benevides, K. S.; Rev. Bras. Anal. Clin. 2020, 52, 224. [Crossref] 30. Allam, G.; Immunobiology 2009, 214, 712. [Crossref] 31. Niculescu, A.-G.; Chircov, C.; Grumezescu, A. M.; Methods 2022, 199, 16. [Crossref] 32. Silveira, M. L. D. C.; Silva, I. M. B.; Magdalena, A. G.; Cerâmica 2021, 67, 295. [Crossref] 33. Bronze-Uhle, E. S.; Costa, B. P.; Ximenes, V. F.; Lisboa-Filho, P. N.; Nanotechnol., Sci. Appl. 2017, 10, 11. [Crossref] 34. BdEC, Bases de Estruturas Cristalinas, https://bdec.dotlib.com.br/bases, accessed in August 2025. 35. Magdalena, A. G.; Silva, I. M. B.; Marques, R. F. C.; Pipi, A. R. F.; Lisboa-Filho, P. N.; Jafelicci Jr., M.; J. Phys. Chem. Solids 2018, 113, 5. [Crossref] 36. Salman, S. D.; Rashid, I. M.; Environ. Prog. Sustainable Energy 2024, 43, e14260. [Crossref] 37. Leandro-Silva, E.; Silveira, M. L. D. C.; Pipi, A.; Piacenti-Silva, M.; Magdalena, A. G.; Rev. Virtual Quim. 2024, 16, 98. [Crossref] 38. Keleştemur, S.; Altunbek, M.; Culha, M.; Appl. Surf. Sci. 2017, 403, 455. [Crossref] 39. Bhatia, S.; Shah, Y. A.; Al-Harrasi, A.; Jawad, M.; Koca, E.; Aydemir, L. Y.; Int. J. Biol. Macromol. 2024, 254, 128045. [Crossref] 40. Karami, N.; Mohammadpour, A.; Samaei, M. R.; Amani, A. M.; Dehghani, M.; Varma, R. S.; Sahu, J. N.; Int. J. Biol. Macromol. 2024, 254, 127663. [Crossref] 41. Girigoswami, A.; Deepika, B.; Pandurangan, A. K.; Girigoswami, K.; Artif. Cells, Nanomed., Biotechnol. 2024, 52, 59. [Crossref] 42. Fumis, D. B.; Silveira, M. L. D. C.; Gaglieri, C.; Ferreira, L. T.; Marques, R. F. C.; Magdalena, A. G.; Mater. Res. 2022, 25, e20220312. [Crossref] 43. Popescu, R. C.; Andronescu, E.; Vasile, B. S.; Nanomaterials 2019, 9, 1791. [Crossref] 44. Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J.; J. Bioenerg. Biomembr. 2020, 52, 321. [Crossref] 45. Kang, M.; Loverde, S. M.; J. Phys. Chem. B 2014, 118, 11965. [Crossref] 46. Abath, F. G. C.; Werkhauser, R. C.; Parasite Immunol. 1996, 18, 15. [Crossref] 47. Mafud, A. C.; Silva, M. P. N.; Monteiro, D. C.; Oliveira, M. F.; Resende, J. G.; Coelho, M. L.; de Sousa, D. P.; Mendonça, R. Z.; Pinto, P. L. S.; Freitas, R. M.; Mascarenhas, Y. P.; de Moraes, J.; Chem.-Biol. Interact. 2016, 244, 129. [Crossref] 48. Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K.; J. Pharm. Biomed. Anal. 1997, 15, 1867. [Crossref] 49. Aguiar, D. P.; Moscardini, M. B. M.; Morais, E. R.; de Paula, R. G.; Ferreira, P. M.; Afonso, A.; Belo, S.; Ouchida, A. T.; Curti, C.; Cunha, W. R.; Rodrigues, V.; Magalhães, L. G.; PLoS One 2016, 11, e0167135. [Crossref] 50. Amani, S.; Mohamadnia, Z.; Mahdavi, A.; Int. J. Biol. Macromol. 2019, 141, 1258. [Crossref] 51. Chakraborti, S.; Dhar, G.; Dwivedi, V.; Das, A.; Poddar, A.; Chakraborti, G.; Basu, G.; Chakrabarti, P.; Surolia, A.; Bhattacharyya, B.; Biochemistry 2013, 52, 7449. [Crossref] 52. Kazemi, S.; Pourmadadi, M.; Yazdian, F.; Ghadami, A.; Int. J. Biol. Macromol. 2021, 186, 554. [Crossref] 53. Salehiabar, M.; Nosrati, H.; Javani, E.; Aliakbarzadeh, F.; Manjili, H. K.; Davaran, S.; Danafar, H.; Int. J. Biol. Macromol. 2018, 115, 83. [Crossref] 54. Yang, R.; Zheng, Y.; Wang, Q.; Zhao, L.; Nanoscale Res. Lett. 2018, 13, 330. [Crossref] 55. Nardo, L.; Paderno, R.; Andreoni, A.; Másson, M.; Haukvik, T.; TØnnesen, H. H.; J. Spectrosc. 2008, 22, 187. [Crossref] 56. Bansal, K. K.; Rosenholm, J. M.; Ther. Delivery 2020, 11, 297. [Crossref] 57. Cornell, R. M.; Schwertmann, U.; The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; Wiley: Weinheim, 2006. 58. Bhowmik, D.; Gopinath, H.; Kumar, B. P.; Duraivel, S.; Kumar, K. S. S.; The Pharma Innovation 2012, 1, 24. [Link] accessed in August 2025 59. Dash, S.; Murthy, P. N.; Nath, L.; Chowdhury, P.; Acta Pol. Pharm. 2010, 67, 217. [Link] accessed in August 2025 60. Yadav, G.; Bansal, M.; Thakur, N.; Khare, S.; Khare, P.; Middle-East Journal of Scientific Research 2013, 16, 782. [Link] accessed in August 2025
Associate Editor handled this article: Marcela M. Oliveira |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access