Educação
| An inquiry into structuring concepts in organic chemistry based on the analysis of textbooks and the perspectives of teachers from brazilian universities |
|
Gabrielle O. P. Costa; Pedro C. de Araujo* Instituto de Química de São Carlos, Universidade de São Paulo, 13566-590 São Carlos - SP, Brasil Received: 05/15/2025 *e-mail: pedrocaraujo@usp.br This study investigated, from the perspective of university professors, the fundamental structuring concepts in the teaching of organic chemistry and how these concepts appear in secondary school and university textbooks. The research stems from the observation that conceptual fragmentation compromises learning and contributes to high failure rates in undergraduate courses. A gap in the literature was identified concerning the systematization and articulation of organic chemistry concepts in education. Adopting a qualitative and exploratory approach, the study occurred in two stages: (i) a questionnaire was administered to forty university professors; (ii) chapters from organic chemistry textbooks were analyzed to construct conceptual maps that revealed concept hierarchies and interrelations. The findings show that professors consider chemical bonding, stereochemistry, structure, and acidity-basicity as key structuring concepts. Although these are present in textbooks, their treatment is often fragmented and poorly contextualized, hindering student understanding. The study concludes that effectively integrating organic chemistry concepts into the teaching-learning process requires a more coherent pedagogical approach. It also highlights the need for curriculum revision and the development of teaching materials that support integrated conceptual understanding. Future research should include perspectives of secondary teachers and undergraduate students to better understand the learning challenges in organic chemistry. INTRODUCTION Regarded as an abstract science, most students find Chemistry, especially organic chemistry, very difficult to understand.1-3 In both high school classes and higher education lectures, students find it difficult to visualize three-dimensional molecules, or understand and relate them to everyday phenomena.4 The difficulties encountered in Chemistry teaching end up affecting student learning, and this causes alarmingly undesirable consequences in higher education; in many cases, this situation leads to high failure rates in the study of organic chemistry, for example, contributing to high dropout rates in bachelor's degree courses.5 A huge part of the student learning difficulties essentially stems from the way chemistry is taught.6 In the case of organic chemistry, for example, one needs to understand that laws and representations must act in a co-dependent manner, because if a student has difficulty observing the structures of organic molecules, it becomes difficult for them to understand the types of reactions that can occur. In the discussion on the teaching of scientific concepts, Ausubel7 proposes in his theory of meaningful learning a form of conceptual hierarchy, in which the previous concepts assimilated by students serve as an anchor for the new concepts presented. Novak and Cañas8 argue that levels of hierarchy and systematization are essential for knowledge acquisition, reinforcing the importance of effectively understanding laws and theories. This idea of hierarchy promotes the notion of concepts that are structuring, since they act as support for the understanding of other concepts, as well as in the transformation and evolution of knowledge. In this context, Liebscht and Wahren9 proposed the "Theory of nuclear concepts", where the structuring or nuclear concepts represent "landmarks" or strengths of the cognitive structure, which allow us to "progressively establish a set of connections between them that constitute the "routes" (relationships between concepts) and "least cost pathways" (associations between groups of concepts that are established for a given purpose, and which tend to be as simple as possible). In essence, this way of understanding concepts leads to the understanding of a "comprehensive picture" (when the entire field of knowledge has been mastered, and different associations can be established as the need arises)". The understanding of scientific concepts in a more comprehensive way (of the area) is what would, effectively, translate into the real learning of the concepts. Literature Review The difficulties encountered in the learning of organic chemistry may specifically be linked to the lack of connection with other chemical concepts seen in general chemistry, in addition to the lack of proximity to the reality of the students and their daily lives or even the difficulty in understanding chemical language.5 When we consider the learning process as an active action in the mind of the individual, in which information from the environment is reinterpreted as a form of knowledge,6 it clearly becomes crucially important to ensure that students effectively understand the initial chemical concepts. For understanding to be effective, the concepts need to be related, and this depends on their parity.9 In this sense, knowing that the university environment is devoted toward advancing human development and allows the student to develop as a subject and as a professional,10 it is necessary to understand the reasons why there are lags in the learning of concepts; this quest for understanding should primarily be aimed at seeking to comprehend to what extent the concepts are misunderstood by the student and in which situations the lag occurs in visual and representational aspects. Regarding the concepts in organic chemistry, while many works have demonstrated the application of concepts of chemical bonding and stereochemistry, little or no attention has been devoted to conceptual studies. For the concept of acidity and basicity, to date, there is a scarcity of studies related to the application of these concepts in organic chemistry , in particular, with a focus on inorganic and analytical chemistry in general. Because of this, students do not learn/are not taught the concept of acidity and basicity relative to everyday phenomena, and this makes organic chemistry seem even more abstract. Marques1 and, Gomes and Oliveira10 have shown that students take their mental models to university, so that errors and inconsistencies end up being taken along with them, promoting what Bachelard11 calls epistemological obstacles, which are intrinsic to knowledge acquisition. Studies have shown that one of the main obstacles to students understanding is the polysemy of chemical concepts;1,12 a case in mind here is that of acidity and basicity - seen as a concept, theory or law, which is presented through different approaches, including the definition of Arrhenius, Bronsted-Lowry and Lewis.13 Fiorucci et al.14 have shed light on the fact that "with the exception of hydrochloric acid, the most common acids we live with are organic". Knowing the importance of acids and bases in organic chemistry, new conceptual studies on these topics are necessary in order to prevent previous mistakes in student learning from being perpetuated. Thus, it is understood that without general chemistry concepts, learning organic chemistry may be difficult, since the conceptual hierarchy depends on satisfactory assimilation of the concepts by students. Furthermore, apart from the conceptual aspects, the difficulty in understanding the representational aspects ends up hindering student learning, since these representations are capable of creating connections and guides for understanding Chemistry,15 considering that organic chemistry requires visualization of orbitals, structures, and reaction mechanisms. In general, the main difficulties of the students stem from the lack of abstraction capacity and the difficulty in understanding the conceptual polysemy of some terms, which affects understanding at macroscopic and submicroscopic levels, deemed to be necessary when it comes to learning Chemistry.12 Once students learn these concepts in an incorrectly or inadequately way, it becomes difficult to correct them, as they have already been inadequately assimilated.16 Chemical bonding is considered one of the most important concepts in Chemistry, as it involves understanding the interactions of atoms and the consequences of their transformations.17 Some studies18 have shown that many students confuse the concepts of covalent bonding with ionic bonding, and this can even make it difficult for students to understand molecular geometry; this evidently demonstrates that a lack of understanding of a concept generates a cascade of difficulties in the long run. Other studies19,20 have also shown that spatial visualization is a huge problem for students, and, because of that, they are unable to form a consolidated teaching base, in other words, the inadequate learning of concepts such as stereochemistry tends to affect the quality of learning/teaching in organic chemistry. Theoretical reference Ausubel21 formulated the Theory of Meaningful Learning based on Jean Piaget's Genetic Epistemology, based on the cognitivist line, which is an epistemological conception focused on the learning process through the transformation of information based on the reality in which the individual is inserted.22 The theory formulated by Ausubel guides teaching and recognizes learning as a non-literal and non-arbitrary interaction involving knowledge.23,8 For meaningful learning to occur, Ausubel points out that, on the part of the student, there must be a predisposition to learn, but that it is the responsibility of educators to offer potentially meaningful materials. In this perspective, Ausubel points out that learning occurs from the organization and hierarchization of information in the mind of learner, and that the socioeconomic context of the students should be taken into account.24 This hierarchy occurs through the structure referred to Ausubel as subsuming, where the so-called subsuming concepts are those that serve as facilitators for learning.22 Thus, subsuming knowledge is necessary from learning by reception to learning by discovery, so that, in the long run, these concepts become increasingly stable, further facilitating learning.8 It is important that the concepts, especially the fundamental ones, are well assimilated in order to avoid mistakes; according to Kaanklao and Suwathanpornkul,16 this can be understood as obstruction of the learning of new knowledge from other preliminary knowledge. Once the misunderstanding of these concepts is pre-established, learning difficulties may occur; this reflects the difficulty observed in understanding subjects with more than one explanation, such as acids and bases or oxidation-reduction, for example.6 The learning difficulty experienced by students associated with the confusion of models can also be understood as a difficulty in assimilating concepts that exhibit conceptual polysemy.12 In this context, the use of conceptual maps provides a representation of the relationships of concepts in a hierarchical way; this is a tool that helps to present the notions the individual has about a topic.22 Lima24 defines a conceptual map as "a representation that describes the relationship of ideas of thoughts, a relationship pre-acquired throughout the learning process in the construction of knowledge, which is archived in memory"; essentially, the author shows that conceptual maps can be used as a visual support for information. Thus, from this representation, it becomes possible to promote changes in the structure of learning, contradicting learning techniques considered mechanical.8 Vanides et al.25 understand that the use of activities with unrestricted conceptual maps allows for a greater capacity to observe the connections made between concepts, allowing for comparisons and recording repetitions, such as the work in question. In addition, maps support researchers in their attempts to ensure that qualitative data are incorporated into a specific context. In support of this line of thought, Daley26 demonstrates in his work "Using Concept Maps in Qualitative Research" how the use of maps allows the incorporation of data and the guarantee that they are incorporated into the context of the research. Based on all the aforementioned observations, Rowley and Slack27 point out that conceptual maps allow for the identification of the main concepts in a research area, allowing for mapping and clarifying thinking about the organizational structure of a subject. Research questions and study objectives The main objective of this study was to investigate, from the perspective of university chemistry professors, the structuring concepts in organic chemistry courses in higher education. As part of this objective, the study explored which concepts are addressed in organic chemistry chapters or textbooks used in secondary school classes and in higher education lectures. Throughout the work, the following research questions are explored:
METHODOLOGY This study adopted a qualitative and exploratory research design,28 structured in two stages, with the objective of understanding subjective perceptions and organizing conceptual structures based on responses from participants and analysis of textbook content. Qualitative research is suitable for investigating complex educational meanings, experiences and contexts, which, in this study, are represented by the perspectives of teachers and the conceptual structures present in teaching materials. In turn, exploratory research seeks to answer specific questions or address phenomena that have not yet been investigated, offering initial support for more in-depth studies.28 In the first stage, we developed an evaluation questionnaire, which was responded by research professors in the field of organic chemistry. In the second stage, the chapters on organic chemistry in Brazilian high school textbooks and Higher Education Organic Chemistry textbooks were analyzed thoroughly; this was done in order to construct conceptual maps with a view to hierarchically organizing the scientific concepts mentioned in the chapters and books on organic chemistry. Questionnaire for organic chemistry research teachers For the first stage, a questionnaire containing 17 questions was prepared. The first questions were aimed at characterizing the research subjects: their doctoral field and their experience in teaching organic chemistry at the higher education level. The remaining questions asked about: the concepts required to be taught in organic chemistry, such as identifying concepts that the teachers believe to be basic and fundamental in organic chemistry, concepts that can later influence student learning in other Chemistry disciplines and concepts that they believe to be a priority for teaching in an organic chemistry discipline. In addition, there was also a space for the teachers to complement their answers, where they were free to comment on whatever they deemed necessary to add to their previous answers. The questionnaire was placed on the Google Forms online platform and distributed among university professors responsible for teaching organic chemistry disciplines in undergraduate and postgraduate programs in Brazilian public universities. The participants were selected based on the results of a search conducted to find organic chemistry professors from different Brazilian public universities, followed by reading their academic resumes. All professors who identified themselves as being in the field of organic chemistry and were responsible for teaching courses in the area and had published works in the area were contacted and invited to answer the questionnaire. The responses were collected anonymously in order to preserve the integrity of the participants and not to interfere with the results. The comments added by the professors were optional and were studied and treated together with the other responses. Approximately 200 professors from 15 higher education institutions located in different regions of Brazil received the study form, and 40 of them answered the questionnaire (20% of the invited professors). The Southeast and North regions had the highest number of participating professors (57.1 and 28.6% respectively), followed by the Northeast and South regions (each with 7.1%), while no responses were obtained from the Central-West region. The professors who answered the form were between 30 and 50 years old, work specifically in the field of organic chemistry, teach undergraduate and postgraduate courses, and their research focuses on organic synthesis or the formation of natural products. The experience of the respondents in teaching/doing research in the field of organic chemistry varied between 10 and 30 years. Although this study focused exclusively on university professors, this choice was deliberate, as the aim was to identify core concepts from the perspective of those responsible for advanced instruction in organic chemistry. Nevertheless, we acknowledge that including high school teachers could provide complementary insights and will be considered in future stages of this research. Data analysis Each response was read individually by one of the authors of this study and tabulated through simple data categorization. Responses in which the same concepts were written in different ways were placed under the same category; for instance, "chemical bonds" and "covalent and ionic bonds" were evaluated as being the same concept. The responses were tabulated and numbered in increasing order of occurrence, with those with less than 20% frequency being discarded, except for comparison purposes. Constructing the conceptual maps The conceptual maps were constructed using the chapters on organic chemistry from the seven basic education textbooks approved under the Brazilian National Textbook Distribution Program (PNLD) in 2021. The PNLD is a Brazilian public education program aimed at distributing textbooks to public schools in the country. Publishers register their teaching materials and these materials are rigorously evaluated by the Technological Research Institute of the State of São Paulo and the Basic Education Secretariat of the Federal Ministry of Education. The higher education textbooks selected were those most widely used by the teachers who took part in the study, as these were the textbooks they use in their classes/lectures. Each selected book was read in full and the scientific concepts mentioned in the higher education textbooks and in the organic chemistry chapters of the basic education textbooks were listed one by one. These scientific concepts were organized into conceptual maps, which were used to form an organizational chart that indicated the conceptual hierarchy proposed in Ausubel's Theory of Meaningful Learning,29 in line with the training structure proposed by Novak and Cañas:8
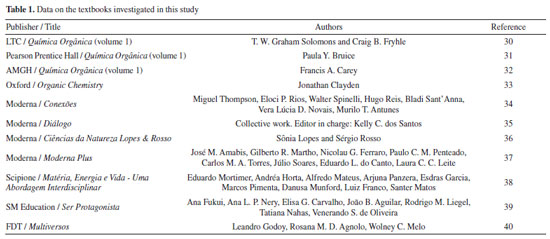
Concept maps were created for each book, based on the arrangement of the concepts presented; in addition, we listed additional resources that could assist in student learning, such as diagrams and representations. Table 1 presents the 4 higher education editions and the 7 secondary education editions used in our study.
In addition to high school textbooks, we also analyzed 4 organic chemistry textbooks used by teachers in higher education. Based on responses from the teachers, the textbooks were selected and the first chapter corresponding to the subjects of organic chemistry was analyzed (without considering initial contextualization chapters).
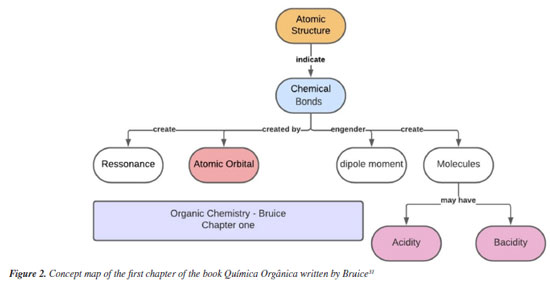
RESULTS AND DISCUSSION Perceptions about structuring concepts in the teaching of organic chemistry The teachers were asked questions on the selection of scientific concepts in organic chemistry in different scenarios: the fundamental concepts in organic chemistry; the concepts that are reviewed at the beginning of a course; the concepts reviewed during the course and the most essential concepts of the area. As can be seen in Table 2, acidity-basicity was the concept that was most cited by the teachers (20 or 50%), followed by stereochemistry (16 or 40%) and chemical bonding (13 or 32.5%). As can be noted, two of the concepts classified as the most important in organic chemistry came from the basic cycle of a Higher Education Chemistry course.
Regarding the reviews carried out, the concept of chemical bonding came in first place (20 or 50%), followed by acidity-basicity (19 or 47.5%), atomic structure (11 or 27.5%), and theories of atomic and molecular orbitals (10 or 25%). Regarding the reviews required to be conducted during the semester, acidity-basicity came in first place - in terms of the number of responses (13 or 32.5%), followed by stereochemistry (11 or 27.5%), reaction mechanisms (8 or 20%), and resonance (6 or 15%). Finally, for the responses relative to the contents considered essential to be applied in the classroom, stereochemistry (17 or 42.5%) and reaction mechanism (16 or 40%) recorded the highest frequencies of responses, followed by acidity-basicity (13 or 32.5%) and atomic structure (12 or 30%). From the results presented in Table 2, one can observe the hierarchy proposed by Ausubel7 and, Novak and Cañas8 in the Theory of Meaningful Learning. Considering that this theory proposes that the student must be endowed with pre-existing knowledge, which will serve as an anchor in the assimilation process in university education,8,18 one will observe that the teachers indicate fundamental concepts, such as chemical bonding and acidity-basicity, which are applied in the basic cycle of a chemistry course at the higher education level; these concepts are considered to be essential and are reviewed at the beginning of the course, though they tend to receive less attention during the school year. Mullins41 considers some concepts to be fundamental in organic chemistry; these concepts include electronegativity, polar covalent bond, steric and inductive effect, resonance and aromaticity. It is worth noting that, in their responses, the teachers also pointed out the concept of acidity-basicity as a structuring concept in organic chemistry. The concept of acidity-basicity stands out as one of the key structuring concepts in chemistry in general.42 According to Novak and Cañas8 and Lima et al.,43 the cognitive structure of the student exhibits two main forms of connection when it comes to knowledge acquisition; one of these forms is the subordinate connection, which presents a horizontal connection between concepts to obtain a branch of one knowledge under the other. The difficulty that students encounter when learning Chemistry can be mainly explained by the high level of abstraction and polysemy presented by some concepts,12 such as the concept of acidity-basicity, in which there is already prior knowledge and the student learns about this same concept in a complementary way, with a focus on organic chemistry, which can generate confusion between the definitions used. According to Karakoyun and Asiltürk42 and Tumay,44 the main problem regarding the concept of acidity-basicity is that most students have a fundamental misunderstanding regarding what an acid and a base actually are; this occurs mainly because this concept occupies a nonspecific position, since they are not exactly theories, but rather a form of classification, as presented by Jensen.45 Studies show that teachers prefer textbooks that present definitions of the acid-base concepts, following the Arrhenius, Bronsted-Lowry and Lewis order, so that the presentation of these concepts is carried out in a progressive and cumulative manner;13 this may cause difficulties when students try to assimilate the concept from a new perspective. The concept of atomic structure, indicated in Table 2, was mentioned by 30% of teachers; this concept is regarded as a fundamental concept in organic chemistry. Rodrigues et al.46 showed that the excessive use of the octet rule causes students to have difficulty in correctly schematizing Lewis structures. Although the concept was not highlighted as needing review, it is noted that concepts that depend on spatial visualization suffer from a lag of understanding, which indicates that students have difficulty in understanding and reproducing visual diagrams. Regarding learning difficulties, Kleinman et al.46 point out that students may have struggle to learn Chemistry due to the difficulty in establishing connections between visual and conceptual components; in other words, due to the difficulty in assimilating contents that are more visual than theoretical. This lack of spatial visualization skills has a direct impact on the ability to manipulate molecular representations.48 The same observations can be made to the concept of stereochemistry, since this concept also requires new explanations from 15% of teachers who teach it. Stereochemistry can be considered a structuring element of the Mullins' model,41 since without an effective understanding of this concept, students tend to have difficulty working on activities involving reaction mechanisms that are extremely important in organic chemistry.19 Bringing this into the context of responses from teachers, the difficulty in understanding stereochemistry can be explained by the difficulty students have in representing structures effectively, which causes a cascade of conceptual lags, leading to difficulties in representing mechanisms. This causes teachers to prioritize the teaching of stereochemistry, due to its structuring capacity over other concepts in organic chemistry. Given the structuring characteristic of stereochemistry, in the perception of teachers, it can be argued that its relative importance in chemistry teaching can contribute to the reduction of what Taber6 defines as pedagogical impediments, which affect student learning. This prioritization may also be related to the perception of the difficulties faced by students in understanding stereochemistry, considering its implications for learning reaction mechanisms, considered fundamental to the teaching of organic chemistry;49 thus, this emerges as one of the priorities recognized by teachers when it comes to teaching organic chemistry (16%). As indicated by other studies, the understanding and use of reaction mechanisms is fundamental for the transfer and acquisition of knowledge, as this concept acts as a "unifying content" in organic chemistry.50 Concept maps It is important to note that the parallel treatment of secondary and higher education textbooks may suggest an implicit equivalence in their conceptual presentation. However, these textbooks are developed with distinct educational purposes. While secondary education materials aim to foster general scientific literacy, higher education textbooks are designed for in-depth disciplinary training. This distinction must be recognized to avoid assuming a linear progression in concept comprehension between the two levels. Analysis of organic chemistry textbooks in higher education In the second stage, we analyzed the structure of the four textbooks indicated as the most widely used by teachers (Table 3). Concurrently, the textbooks approved under the PNLD 2021 for teaching natural sciences and aimed at students in the second year of high school were also analyzed.
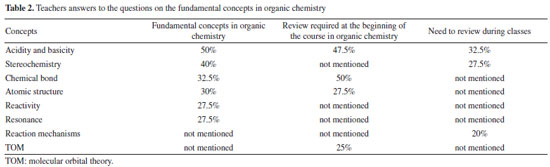
For a thorough analysis of the contents of the textbooks presented, a map was created containing the main topics of the initial chapters of each work; this allowed us to perform a comparative analysis of their structures and approaches. The Solomons edition is divided into two volumes; the first of which is a more introductory general content while the second volume covers more specific contents, where many of the reaction mechanisms are discussed in greater depth. The first volume, which was the focus of this study, begins with a chapter on chemical bonding and atomic/molecular structure entitled "The basics". Acidity and basicity and stereochemistry are found in chapters 3 and 5 of the book, respectively. A unique feature of this book is that at the end of the chapters, there are mind maps that help the student understand the subject studied. In the map of the first chapter of the book Química Orgânica by Solomons et al.,30 presented in Figure 1, one will observe that the book begins with atomic structure as part of its presentation of more abstract concepts, such as atomic and molecular orbitals, and even chemical bonding, which in turn appears as an introductory concept for the other concepts presented in the book, as Mullins41 suggested. The volume of the book by Paula Bruice is divided into 3 main parts; the first chapter of the book - "Electronic structure and bonding - Acids and Bases", talks about chemical bonds, hybridization, Lewis structure, and acidity and basicity within organic matter; stereochemistry is found in the fifth chapter of the book, in the second part of the division.
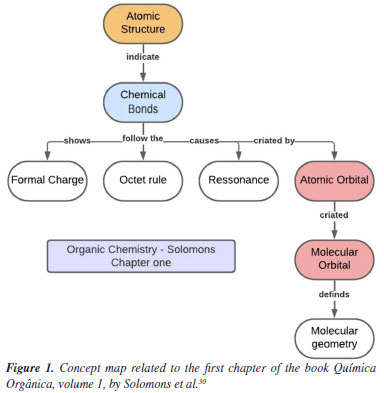
Figure 2 shows the concepts related to the first chapter of the book by Paula Bruice, where structure (atomic and molecular) and chemical bonding appear in the same sequence, followed by explanations on acids and bases and electron distribution.
The organic chemistry book written by Clayden et al.33 is the only book that is not composed of more than one volume. The first chapter explains why it is important to study organic chemistry; interestingly, it does not present the concepts that are intended to be analyzed, so we had to organize the conceptual map of this book in a different way. The concepts begin to appear effectively in the second chapter, entitled "Organic structures", where the focus is solely on explaining how organic molecular structures are formed. For this reason, the map related to this book, presented in Figure 3, was constructed from the reading of the second chapter, since the focus of this study was to analyze and understand the conceptual organization and how the structuring concepts in organic chemistry are approached. Acidity-basicity can be found in chapter 8 and stereochemistry in chapter 16. This book also presents shorter chapters, with more direct information; this explains the differences observed in the chapters of this book compared with the others.
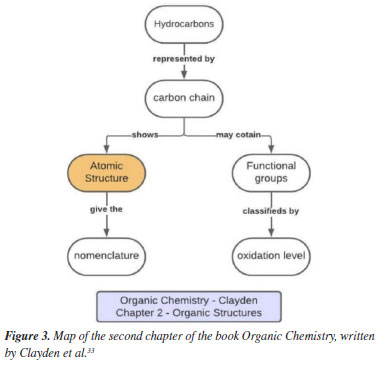
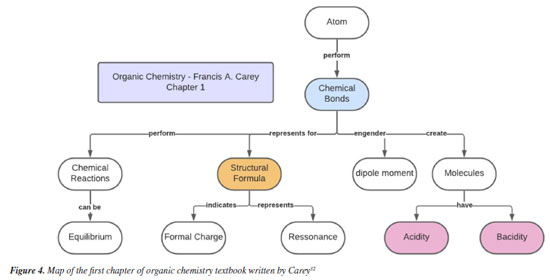
The organic chemistry book written by Clayden et al.33 begins the chapter by defining the meaning of hydrocarbons, then moves on to chains, and ends with (molecular) structure. The section on "chains" leads to the explanation of functional groups, which had not been presented until then. The map shows an organization of the book in a manner that is different from the others, with the presentation of fewer concepts and less similarity to the maps presented previously. This difference stems primarily from the different size of the chapters and the form of organization chosen by the authors.33 Like in the other books, Carey32 presents a summary at the end of the chapter and uses tables and information boxes to highlight important points. The first chapter of the book, entitled "Structure determines properties," presents concepts of atomic structure, acidity-basicity, and chemical bonds. Figure 4 shows the map of the first chapter of the book by Carey. Atomic structure appears again in other chapters, in a specific way, while stereochemistry is covered in chapter 7.
In the book written by Carey,32 the presentation begins with atoms as the initial concept, followed by bonds, molecules and the concept of acidity-basicity. Even though the chapter is entitled "Atomic structure", this concept is presented together with bonds; in essence, this shows that the two concepts are dependent. These small conceptual and organizational differences occur because each book presents a specific organization and each presentation is made in a different order. However, there is a certain similarity in relation to the chemical concepts that are considered basic by the teachers - these include chemical bonding, acidity-basicity, atomic/molecular structure and stereochemistry. To facilitate understanding, Table 4 highlights the concepts that were indicated by the teachers.
Associating the concepts highlighted by the teachers with those addressed in the initial chapters of Higher Education Organic Chemistry textbooks, two main aspects are noted: (i) general chemistry concepts, such as chemical bonds and atomic structures, are equally essential for understanding organic chemistry - this is clearly in line with the theories of conceptual hierarchy;7 and (ii) the concepts of acids and bases should be considered structuring concepts of organic chemistry, since they were widely mentioned by the teachers, and they appear in the initial chapters of two of the four books analyzed (Bruice and Carey),31,32 in addition to being covered in separate chapters throughout the book by Solomons et al.30 Analysis of the textbooks used in basic education A careful analysis of the different conceptual maps shows how the main concepts highlighted by teachers are treated in the textbooks used in high school. Table 5 shows the concepts considered fundamental in organic chemistry, identified both by teachers and in higher education textbooks, appear in the works approved under the PNLD 2021. Another relevant finding is the contrast between the macroscopic emphasis in high school textbooks and the submicroscopic and representational focus in higher education materials. This misalignment can hinder conceptual progression of the students, especially when they transition to more abstract content in university-level organic chemistry, such as orbital theory or stereochemical analysis.
As indicated in Table 5, chemical bonding plays a relevant role not only in organic chemistry, but also in all other disciplines; this concept serves as a basis for the presentation of many other concepts.12,2 Freitas et al.2 argue that to understand the concept of chemical bonding, students must understand previous basic concepts such as atoms and molecules, and they should exhibit a high level of abstraction skills; this evidently points to the importance of logical sequencing of the concepts in the textbooks. Toma17 argues that learning chemical bonding contributes to the understanding of transformations and changes that occur not only at the microscopic level, but also at the macroscopic level. Based on the analysis of the responses from the teachers and the textbooks, chemical bonding can be considered a subsuming or structuring concept of organic chemistry. Regarding the difficulty in learning chemical bonding, Fernandez and Marcondes51 point out that students have conceptual difficulties related to the topic; these difficulties range from understanding molecular geometry to confusion in defining and distinguishing between ionic and covalent bonds. Lima et al.43 claim that the difficulty in understanding chemical bonding lies in the fact that the presentation of bonds follows the sequence "ionic bond, covalent bond, polarity and geometry". The level of abstraction makes it difficult for students to apply the concepts of chemical bonding to other models and situations, in different areas of chemistry, such as organic chemistry.43 Some studies have argued that there are very few works in the literature that are focused on the methodologies for teaching chemical bonding,12 especially when it comes to teaching ionic bonds - to date, there are few publications on this subject matter.43 Regarding the polysemy presented in the concepts of acidity-basicity, some studies52,53 claim that the use of different definitions for these concepts has been one of the main factors that make it difficult for students to assimilate these concepts in higher education. In this regard, one of the professors participating in the research pointed out that: "The failures observed in the learning of General Chemistry become evident in organic chemistry courses. This creates a domino effect, with catastrophic consequences. Our education system fails to instill critical thinking in students. The dissociation that arises is very difficult to reverse. I take the concept of acidity and basicity applied to organic chemistry as a clear example of the problem. I see that, with very rare exceptions, students simply do not know what it is about. Because they know (or almost know) how to define acidity and basicity, they deduce that they know what it is. The difference between the index of a book and its content. They learned to "memorize" the concept, but they did not understand it." In his studies on teaching the concept of acidity-basicity, Oversby54 suggests that the concept in question can be expanded in different ways so that learning can occur. Of the books investigated in this study, only one presented a chapter solely focused on acidity-basicity in organic chemistry, where it sought to present the concept in a specific way. In the map shown in Figure 5, the concept of acidity and basicity is explored focusing only on organic chemistry; the idea here is to compare the differences in approach.
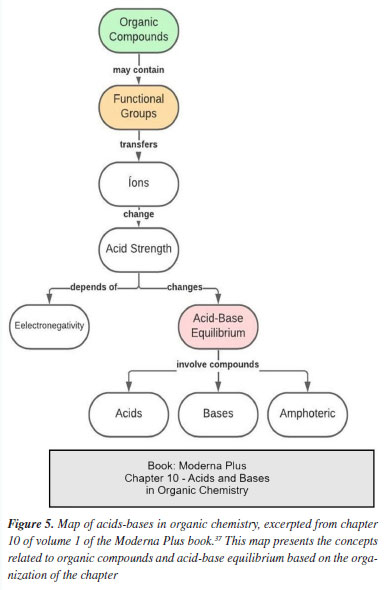
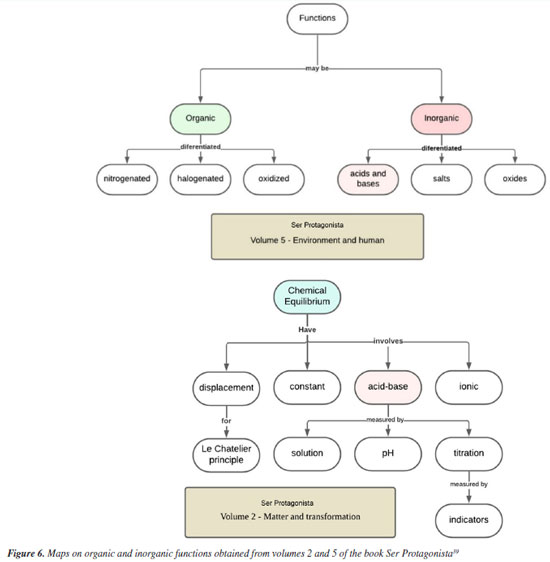
Figure 5 presents a map on organic compounds, taken from the book approved under the PNLD 2021, Moderna Plus, which contains a specific chapter on acids and bases in organic chemistry. Figure 6 presents the acid-base concept exclusively from an inorganic perspective, thereby highlighting how the same concept can be approached from different perspectives.
In the chapter on "Chemical equilibrium", acidity and basicity are also addressed from an analytical rather than organic perspective, while in the chapters on "Functions", the same concept is addressed from an inorganic perspective. Thus, the chapters presented in this map show chemical concepts, but they do not focus necessarily on organic chemistry. Thus, the same concept is presented in two different ways in the maps of the two books analyzed. Based on the observations above, one will notice that the perspective of the teacher follows the same line of thought as Oversby,54 who emphasizes that, in organic chemistry there are no references to the acid-base model or theory used, so that "it is not surprising that some students adopt strategies for learning each model and use them by heart, placing great effort on the individual's mental capacity for memorization". Regarding the concept of stereochemistry, it is important to highlight that one needs to understand atomic and molecular structures in order to make sense of the representation of isomers; this simply shows that the concepts are and must be related to each other. It is believed that some students do not have a consolidated understanding of atomic structure in order to understand stereochemistry; this is indicative that previous learning occurred in a mechanical and non-meaningful way.20 The main difficulty encountered by students when it comes to learning and understanding the concepts lies in the inability to visualize things through a three-dimensional level and to relate the interaction between molecules in space.20 Over time, stereochemistry has become one of the most difficult topics in organic chemistry courses in higher education, as it requires the visualization of molecular structures, which is not always easy for students.19 Vergnaud55 believes that the main cause of problems for students when it comes to solving problems lies in thought operations, based on hypotheses and comparisons, which are necessary to establish relationships between the problem and the resolution; this does not come easily for most students. As can be observed in the maps presented in the Supplementary Material, many chapters that deal with the concept of stereochemistry do not revisit concepts of structure and fail to provide a facilitating ground for understanding problems that involve the concept of isomers without even being able to visualize the three-dimensional structures. Finally, Gabel56 points out that in addition to understanding visualization in space, the difficulty in understanding stereochemistry may be related to the difficulty in understanding phenomena outside the daily life of the students, since stereochemistry is based mainly on scientific concepts. In this sense, it becomes extremely important for students to engage effectively in meaningful learning, as proposed by Ausubel;18 in doing so, students can satisfactorily understand concepts of bonding and atomic/molecular structures and later apply them in stereochemistry, and in fact assimilate this new knowledge in an appropriate manner. It is necessary to clarify that the comparison between the content of high school and university textbooks was not intended to establish a direct equivalence but rather to identify how early concept exposure, or its absence, may influence later learning. Recognizing this dynamic helps to explain conceptual lags and informs the need for curriculum alignment across educational stages.
CONCLUSIONS This study analyzed the scientific concepts considered structuring in organic chemistry from the perspective of university professors, as well as their presentation in textbooks for secondary school and higher education students. The results showed that chemical bonding, stereochemistry, structure and acidity-basicity are considered essential foundations for the construction of knowledge in organic chemistry, in line with the specialized literature. This teaching validation reinforces the need for a continuous curriculum review, guided by more integrative pedagogical approaches. Regarding the analysis of the teaching materials, although the scientific concepts were covered, the presentation was often fragmented and poorly articulated, and this tended to undermine students' understanding. This finding is supported by studies that point to the importance of explicit connections between scientific concepts to avoid cognitive errors. The use of concept maps emerges as a promising strategy, since it allows us to make conceptual relationships visible, thus expanding the learning potential. It is worth noting, however, that this study was limited to the perspective of higher education professors, without including the perceptions of students or teachers in basic education. Future research that incorporates these perspectives may help deepen our understanding of the difficulties encountered by students and teachers alike when it comes to learning general chemistry and organic chemistry, in particular, throughout the academic trajectory; this will clearly help enrich our teaching practices in the area, in addition to enhancing the learning experience of students. Furthermore, studies that evaluate the direct impact of the use of concept maps in the teaching-learning process may help consolidate their effectiveness. Based on the findings of this study, we suggest that integrative pedagogical practices, such as the use of concept maps, guided problem-solving activities, and visualization tools (e.g., molecular models or software-based representations), can support meaningful learning and facilitate conceptual integration in organic chemistry. These strategies may serve as a foundation for instructional interventions aiming to reduce fragmentation and enhance comprehension of abstract content. As a future research direction, we recommend exploring the impact of visual and three-dimensional representations in organic chemistry education. Given the challenges students face in understanding abstract concepts, such as stereochemistry and molecular structures, this line of investigation could contribute to the development of more effective and meaningful teaching resources. In summary, organic chemistry is a key discipline in scientific training; its complexity makes it extremely challenging to learn/teach, though it enriches the formative scientific education of students. The findings of this study highlight the urgent need for devising and implementing pedagogical practices that favor conceptual interconnection and meaningful learning. The study reinforces the role of concept maps not only as an auxiliary tool, but also as a catalyst for a more critical, articulated and transformative education.
SUPPLEMENTARY MATERIAL Additional maps developed during this study, based on high school and college textbooks, in addition to those already presented in this work, which aid in learning and corroborate the information discussed, are available at http://quimicanova.sbq.org.br/, as a PDF file, with free access.
DATA AVAILABILITY STATEMENT All data are available in the text.
ACKNOWLEDGMENTS We are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant No. 2022/12895-1; No. 2022/05934-0; No. 2023/04176-8; No. 2024/06062-2); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant No. 304087/2021-1; No. 407164/2022-7); and Instituto Nacional de Ciência e Tecnologia (INCT) Syntheses of Amazon Biodiversity (CNPq/MCTIC/INCT-2022 58/2022 grant 406767/2022-0) for the financial support.
AUTHOR CONTRIBUTIONS Gabrielle de O. P. Costa was responsible for data curation, visualization; Eliana C. F. Spaziani for data curation, visualization; Renata T. M. P. de Souza for investigation; Matheus S. B. da Silva for investigation; Pedro C. de Araujo for formal analysis, writing original draft; Ana C. Kasseboehmer for conceptualization, funding acquisition, project administration.
REFERENCES 1. Marques, D. D. A.: Estudo do Desenvolvimento de Modelos Mentais sobre o Conceito de Ligações Químicas e sua Relação com Obstáculos Epistemológicos; Dissertação de Mestrado, Universidade Federal do Amazonas, Manaus, Brasil, 2015. [Link] accessed in August 2025 2. Freitas, H. W. S.; Sampaio, C. G.; Barroso, M. C. S.; Research, Society and Development 2022, 11, e16411830638. [Crossref] 3. Fernandes, L. S.; Campos, A. F.; Revista Brasileira de Pesquisa em Educação em Ciências 2013, 12, 153. [Link] accessed in August 2025 4. Raupp, D. T.; Pino, J. C. D.; Acta Sci. 2015, 17, 1. [Link] accessed in August 2025 5. Alves, N. B.; Sangiogo, F. A.; Pastoriza, B. D. S.; Quim. Nova 2021, 44, 773. [Crossref] 6. Taber, K. S.; Chem. Educ. Res. Pract. 2001, 2, 123. [Crossref] 7. Ausubel, D. P. In Analyses of Concept Learning; Klausmeier, H. J.; Harris, C. W., eds.; Academic Press: New York, 1966, p. 157. [Crossref] 8. Novak, J. D.; Cañas, A. J.; Reflecting Education 2007, 3, 29. [Link] accessed in August 2025 9. Liebscht, M.; Wahren, S. In Handbook of Research on Didatic Strategies and Technologies for Education: Incorporating Advancements; Pumilia-Gnarini, P. M., ed.; IGI Global: Hershey, 2013, p. 388; García, L. M. C.; Lopes, V. J. G.; González, R. L.; Catarreira, S. M. V.; Carvalho, J. L. T. In Handbook of Research on Didactic Strategies and Technologies for Education: Incorporating Advancements; Pumilia-Gnarini, P. M., ed.; IGI Global: Hershey, 2013, p. 591. [Link] accessed in August 2025 10. Gomes, H. J. P.; Oliveira, O. B.; Revista Ciência e Cognição 2007, 12, 96. [Link] accessed in August 2025 11. Bachelard, G.; La Formación del Espíritu Científico; Siglo XXI: Paris, 1948. 12. Johnstone, A. H.; Chem. Educ. Res. Pract. 2000, 1, 9. [Crossref] 13. Stoyanovich, C.; Gandhi, A.; Flynn, A. B.; J. Chem. Educ. 2015, 92, 220. [Crossref] 14. Fiorucci, A. R.; Soares, M. H. F. B.; Cavalheiro, E. T. G.; Quim. Nova Esc. 2002, 15, 6. [Link] accessed in August 2025 15. Talanquer, V.; JACSAu 2022, 2, 2658. [Crossref] 16. Kaanklao, N.; Suwathanpornkul, I.; Kasetsart Journal of Social Sciences 2020, 41, 282. [Crossref] 17. Toma, H. E.; Quim. Nova Esc. 1997, 6, 8. [Link] accessed in August 2025 18. Vargas-Hernández, J. G.; Vargas-González, O. C.; Journal of Research in Instructional 2022, 2, 47. [Crossref] 19. Vishnumolakala, V. R.; Southam, D. C.; Treagust, D. F.; Mocerino, M.; Chem. Educ. Res. Pract. 2016, 17, 309. [Crossref] 20. Bongers, A.; Northoff, G.; Flynn, A. B.; Chem. Educ. Res. Pract. 2019, 20, 554. [Crossref] 21. Ausubel, D. P.; Educational Psychology: A Cognitive View; Holt, Rinehart and Winston: New York, 1968. 22. Farias, G. B. D.; Perspectivas em Ciência da Informação 2022, 27, 58. [Crossref] 23. Agra, G.; Formiga, N. S.; Oliveira, P. S.; Costa, M. M. L.; Fernandes, M. G. M.; Nóbrega, M. M. L.; Revista Brasileira de Enfermagem 2019, 72, 248. [Crossref] 24. Lima, G. A. B. O.; Perspectivas em Ciência da Informação 2004, 9, 134. [Link] accessed in August 2025 25. Vanides, J.; Yin, Y.; Tomita, M.; Ruiz-Primo, M. A.; Science Scope 2005, 28, 27. [Link] accessed in August 2025 26. Daley, B. J.; Proceedings of the First International Conference on Concept Mapping; Pamplona, Spain, 2004. [Link] accessed in August 2025 27. Rowley, J.; Slack, F.; Management Research Review 2004, 27, 31. [Crossref] 28. Singh, A.; SSRN 2021, 3789360. [Crossref] 29. Ausubel, D. P.; The Psychology of Meaningful Verbal Learning; Grune & Stratton: New York, 1980. 30. Solomons, T. W. G.; Fryhle, C. B.; Snyder, S. A.; Química Orgânica, 9ª ed.; LTC: Rio de Janeiro, 2009. 31. Bruice, P. Y.; Química Orgânica, 4ª ed.; Pearson: São Paulo, 2006. 32. Carey, F. A.; Química Orgânica, 7ª ed.; Amgh: Virginia, 2011. 33. Clayden, J.; Greeves, N.; Warren, S.; Wothers, P.; Organic Chemistry; Oxford University Press: Oxford, 2001. 34. Thompson, M.; Rios, E. P.; Spinelli, W.; Reis, H.; Sant'anna, B.; Novais, V. L. D.; Antunes, M. T.; Conexões: Ciências da Natureza e suas Tecnologias; Moderna: São Paulo, 2020. 35. Santos, K. C.; Diálogo: Ciências da Natureza e suas Tecnologias; Moderna: São Paulo, 2020. 36. Lopez, S.; Rosso, S.; Ciências da Natureza Lopes & Rosso; Moderna: São Paulo, 2020. 37. Amabis, J. M.; Martho, G. R.; Ferraro, N. G.; Penteado, P. C. M.; Torres, C. M. A.; Soares, J.; Canto, E. L.; Leite, L. C. C.; Moderna Plus: Ciências da Natureza e suas Tecnologias; Moderna: São Paulo, 2020. 38. Mortimer, E.; Horta, A.; Mateus, A.; Panzera, A.; Munford, D.; Garcia, E.; Franco, L.; Matos, S.; Pimenta, M.; Matéria, Energia e Vida - Uma Abordagem Interdisciplinar, 1ª ed.; Scipione: São Paulo, 2020. 39. Fukui, A.; Nery, A. L. P.; Carvalho, E. G.; Aguilar, J. B.; Liegel, R. M.; Nahas, T.; Oliveira, V. S.; Ser Protagonista: Ciências da Natureza e suas Tecnologias; SM Educação: São Paulo, 2020. 40. Godoy, L.; Agnolo, R. M. D.; Melo, W. C.; Multiversos: Ciências da Natureza; FTD: São Paulo, 2020. 41. Mullins, J. J.; J. Chem. Educ. 2008, 85, 83. [Crossref] 42. Karakoyun, G. Ö.; Asiltürk, E.; Acta Chim. Slov. 2021, 68, 3. [Crossref] 43. Lima, J. A.; Sampaio, C. G.; Barroso, M. C. S.; Vasconcelos, A. K. P.; Saraiva, F. A.; Revista Thema 2017, 14, 37. [Link] accessed in August 2025 44. Tumay, H.; Sci. Educ. 2016, 25, 21. [Crossref] 45. Jensen, W. B. In Inorganic Chemistry, 3rd ed.; Miessler, G. L.; Tarr, D. A., eds.; Pearson: Upper Saddle River, 2004. 46. Rodrigues, S. B. V.; Silva, D. C.; Quadros, A. L.; Quim. Nova 2011, 34, 1840. [Crossref] 47. Kleinman, R. W.; Griffin, H. C.; Kerner, N. K.; J. Chem. Educ. 1987, 64, 766. [Crossref] 48. Olimpo, J. T.; Kumi, B. C.; Wroblewski, R.; Dixon, B. L.; Chem. Educ. Res. Pract. 2015, 16, 143. [Crossref] 49. Arellano, D. C. R.; Towns, M. H.; Chem. Educ. Res. Pract. 2014, 15, 501. [Crossref] 50. Grove, N. P.; Cooper, M. M.; Cox, E. L.; J. Chem. Educ. 2012, 89, 850. [Crossref] 51. Fernandez, C.; Marcondes, M. E. R.; Quim. Nova Esc. 2006, 24, 20. [Link] accessed in August 2025 52. Nunes, A. O.: Possibilidades de Enfoque CTS para o Ensino Superior de Química: Proposta de uma Abordagem para Ácidos e Bases; Tese de Doutorado, Universidade Federal do Rio Grande do Norte, Natal, Brasil, 2014. [Link] accessed in August 2025. 53. Chaves, E. J. C.; Guerrero, J. L.; Campos, M. V.; Martínez, A. G.; Memórias CIIEC 2006, 1, 60. [Link] accessed in August 2025 54. Oversby, J.; Educar em Revista 1998, 14, 7. [Link] accessed in August 2025 55. Vergnaud, G.; Recherches en Didactique des Mathématiques 1990, 10, 133. [Link] accessed in August 2025 56. Gabel, D. L.; J. Chem. Educ. 1993, 70, 193. [Link] accessed in August 2025
Associate Editor handled this article: Nyuara A. S. Mesquita |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access