Artigo
| Validation of an HPLC method for the determination of 1,7-dihydroxy-2,3-methylenedioxyxanthone in extracts obtained from Polygala cyparissias A. St. Hil. & Moq. (Polygalaceae) and evaluation of the influence of its content on the gastroprotective activity |
|
Luiz Carlos Klein-Júnior; Sérgio Faloni de Andrade; Valdir Cechinel-Filho; Tania Mari Bellé Bresolin*
Núcleo de Investigações Químico-Farmacêuticas, Universidade do Vale do Itajaí, 88302-202 Itajaí - SC, Brasil Recebido em 24/06/2013 *e-mail: tbresolin@univali.br Polygala cyparissias is a plant widespread in Southern Latin America. Recently, we demonstrated the gastroprotective activity of the extract, as well as for one of the isolated metabolites-1,7-dihydroxy-2,3-methylenedioxyxanthone (MDX). In this study, a HPLC method for the quantification of MDX was validated. The HPLC method was linear (0.5-24 µg mL-1 of MDX) with good accuracy, precision and robustness. The content of MDX in the extracts from whole and different parts of the plant ranged from 0 to 5.4 mg g-1 and the gastroprotective index ranged from 72.1 to 99.1%. Thus, the method might be used for the standardization of the extracts based on the MDX marker. INTRODUCTION Polygala cyparissias A. St. Hil. & Moq. (Polygalaceae), known in Brazil as "pinheiro da praia", "avenca da praia", or "gelol" is widely used for its analgesic properties, particularly due to the presence of high concentrations of methyl salicylate, a counterirritant compound, in the roots.1,2 In the flowers, bornyl acetate and 1,8-cineol were found as the main constitutents.3 The oleoresin extracted by CO2 showed the presence of several different compounds, mainly p-benzoquinone, hexadecanoic acid, tetradecane, pentadecane and heptadecane.4 Furthermore, chemical studies of the plant organic extract have led to the isolation of several xanthones, including 1,3-dihydroxy-7-methoxyxanthone, 1,3,6-trihydroxy-2,7-dimethoxyxanthone, 1,7-dihydroxy-2,7-dimethoxyxanthone, 1,3,6,8-tetrahydroxy-2,7-dimethoxyxanthone, 1,3,7-trihydroxy-2-methoxyxanthone, and 1,7-dihydroxy-2,3-methylenedioxyxanthone.5 We have recently reported the gastroprotective action of the acetonic extract and methanolic fraction of P. cyparissias against ethanol- and indomethacin-induced gastric lesions. Moreover, we have observed that 1,7-dihydroxy-2,3-methylenedioxyxanthone (MDX) (Figure 1)-one of the compounds responsible for the gastroprotective effect of the extract-exhibits the capacity to inhibit the formation of gastric lesions.6 Also, the relevant antihyperalgesic activity of the methanolic extract of P. cyparissias was demonstrated and the two isolated xanthones (1,3-dihydroxy-7-methoxyxanthone and MDX) seemed to be responsible, at least in part, for this effect.7 In the same study, we developed a HPLC method for the quantification of MDX in P. cyparissias extract, however, for a more robust standardization of the method, the chromatographic parameters for the separation of the selected marker, MDX, must be optimized and validated, and MDX from different samples independently analyzed. Therefore, this study aims to validate a HPLC-PDA method to separate and quantify MDX in extracts obtained from whole plants collected in different seasons or from different parts of the plant and also to correlate the amount of MDX present in the extracts with their gastroprotective activity.
 Figure 1. Chemical structure of 1,7-dihydroxy-2,3-methylenedioxyxanthone (MDX)
EXPERIMENTAL Chemicals and reagents All solvents were HPLC grade (Tedia, Fairfield, Ohio, USA). The eluents were filtered through 0.45 µm membrane (Millipore, Massachusetts, USA) and degassed under reduced pressure and ultrasonic bath. As MDX is not commercially available, it was isolated from the acetone extract of P. cyparissias, as described previously.7 The purity of the standard was estimated by HPLC-PDA as 99%. Omeprazole was purchased from Sigma Aldrich (St. Louis, MO, USA). All the other reagents and solvents used were of analytical grade. Plant material The plant material was collected in February, May, August and November of 2010 at Navegantes, Santa Catarina, Brazil. Moreover, roots, branches, leaves and flowers were collected in May 2011. P. cyparissias was previously identified by Professor Leila da Graça Amaral and a voucher specimen was deposited at the FLOR Herbarium (UFSC) under number 22.744. The plant materials were dried in an oven with air circulation at 35 ºC for seven days and then powdered. Preparation of the methanol extract Eight different extracts of the plant, categorized either on the basis of the season of collection or by part of the plant, were evaluated. The whole dried plant material collected in summer (13.57 g), autumn (26.69 g), winter (13.67 g) and spring (14.06 g), as well as the roots (4.15 g), branches (10.24 g), leaves (5.58 g) and flowers (2.22 g) collected in May 2011 were separately submitted to exhaustive maceration at room temperature for five days with methanol at a plant material:solvent ratio of 1:10 (w:v). The extracts were filtered (Whatmanr, O = 11 µm) and concentrated under reduced pressure. Chromatographic conditions A Shimadzu LC-20AT LC system (Shimadzu, Tokyo, Japan), consisting of a SPD-M20A photo diode array detector, SIL-20AHT auto-sampler, CTO-10AS VP column oven and software LC-Solution (Shimadzu, Tokyo, Japan) was used. For the development of the method, two different columns were tested: a Phenomenex Luna C18 Fusion RP 100 Å (250 x 4.6 mm; 5 µm) (Torrance, California, USA) and a Waters X-Bridge C8 (150 x 4.6 mm; 5 µm) (Tauton, Massachusetts, USA), conditioned at 30 or 35 ºC. Several solvent systems were tried, both in isocratic and gradient conditions, and the flow was varied from 0.8 to 1.2 mL min-1. The mobile phase, best suited for our purposes, consisted of water (pH 2.9, acetic acid) (A), acetonitrile (B) and methanol (C) (0 min, 75:10:15 (v/v/v); 5 min, 70:15:15; 7-20 min, 65:15:20; 22-30 min, 60:20:20,35 min,59:20:21; 40-45 min, 58:20:22; 48-52 min, 75:10:15) at a flow rate of 1.2 mL min-1. The column temperature was maintained at 30 ºC. A 20 µL aliquot of the sample was injected and the absorbance at 254 nm monitored. Eluted compounds were identified by chromatographic comparison with authentic standards and their UV spectra. Sample preparation The dried crude extracts were dissolved in a mixture of water and acetonitrile (1:1) at the desired concentration by sonication for 5 min. All the samples were filtered through a 0.45 µm cellulose regenerated membrane filter. Standard solution MDX (2.5 mg) was dissolved in 25 mL of acetonitrile (100 µg mL-1) by sonication. This solution was freshly prepared before each experiment and filtered through a 0.45 µm cellulose regenerated membrane filter. Method validation The method was validated according to the ICH guidelines.8 To assess the system's stability, the standard solution was injected at least six times before all the measurements. The stability of the standard solutions diluted at 15 µg mL-1 was analyzed at times 0,24 and 48 h, and showed little deviation in the response (< 0.1%). Linearity Three analytical curves were obtained. First, seven different concentrations of the extract, ranging from 2 to 5 mg mL-1, were analyzed. Then, to obtain the curve for MDX, seven concentrations (0.5-24 µg mL-1), prepared by diluting the standard solution, were analyzed. Finally, the last curve was obtained by co-injecting varying quantities of MDX (0.5-24 µg mL-1) along with a fixed concentration of the sample solution (0.5 mg mL-1). Each solution was prepared in triplicate and injected in duplicate. The areas of the analyte peaks were plotted against the corresponding concentrations of the sample and linearity assessed by linear regression analysis using Excel 5.0 software. Limit of detection (LOD) and limit of quantification (LOQ) determination LOD and LOQ were calculated based on the standard derivation of the y-intercepts of regression lines. LOD = 3σb/a and LOQ = 10σb/a, where a is the slope of the analytical curve, b is the intercept and σb is the standard deviation associated with the intercept. Accuracy Accuracy was evaluated by means of analyte recovery assay. MDX, at concentrations of 5, 10 and 15 µg mL-1, was added to the methanolic extract (0.5 mg mL-1). All the solutions were prepared in triplicate and analyzed in duplicate, and the mean recovery was calculated. Precision For the repeatability (intra-day precision), six different samples of the methanolic extract (2 mg mL-1) were prepared and analyzed. Similarly, for the intermediate precision (inter-day precision), the samples were freshly prepared and analyzed after two days. The concentrations of MDX in the samples were determined and the relative standard deviations (%RSDs) were calculated. Robustness The robustness was obtained by the analysis of the variations of the MDX in the sample when the method was subjected to small but deliberate deviations in oven temperature (29, 30 and 31 ºC) and flow rate (1.1, 1.2 and 1.3 mL min-1). In all the analyses, the extract (2 mg mL-1) and MDX (15 µg mL-1) were prepared in triplicate and injected in duplicate. The data were analyzed by single factor ANOVA (p < 0.05) using Excel 5.0 software. Animals Swiss mice (25-40 g) were provided by the Central Animal House of the University of Vale do Itajaí (UNIVALI), Itajaí - SC. The animals were housed in groups of six in standard cages at room temperature (25 ± 3 ºC), with 12 h dark/12 h light cycles, and food and water ad libitum. The animals used in the present study were housed and cared for in accordance with the Federal Government legislation on animal care. The experiments were also authorized by the Ethical Committee for Animal Care of the Universidade do Vale do Itajaí, Itajaí, Santa Catarina, Brazil, under number 414/09. Ethanol/HCl-induced ulcer After 12 h of fasting, the mice were divided into six groups of six animals each. The first group was given 1 mL of vehicle (1% Tween-80 aqueous solution), and the second group was treated with omeprazole (30 mg kg-1). The other four groups received 125 mg kg-1 (p.o.) of the methanolic extract of P. cyparissias. One hour after treatment, all the mice received 0.2 mL of an ethanol/HCl solution (60%/0.3 mol L-1) to induce gastric ulcer. One hour later, the animals were sacrificed by cervical dislocation, and the stomachs removed and opened along the greater curvature. The stomachs were gently rinsed with water to remove the gastric contents and blood clots and for subsequent scanning. The images obtained were analyzed using specific "EARP" software to measure each lesion point. The gastroprotective ratio (GR) was calculated based on the percentage of lesion area.9 The data were compared using one-way ANOVA, followed by Dunnett's pairwise test, with p values < 0.05 considered significant.
RESULTS AND DISCUSSION The principal class of secondary metabolites that chemically characterizes P. cyparissias is the xanthones. These are polyphenolic compounds that can be easily detected by their absorption in UV light and are used as markers for the quality control of extracts obtained from plants of the Polygalaceae family,10 as well as in others families.11 Chen et al.12 selected nine different xanthones as markers for the quality assessment of Polygala caudate. However, the proposed method was time-consuming (> 80 min until re-equilibration of the initial gradient) and did not clearly resolve all the xanthone peaks. In addition, the described method was developed specifically for P. caudata and is not suitable for all species of the genus. MDX is a tetraoxygenated xanthone that has already been isolated from P. cyparissias, P. fallax5,13,14 and P. caudate15 Considering that, until now, MDX is distributed in just two Polygala species and exerts a significant gastroprotective activity,6 this metabolite was evaluated as the chemical marker to validate a chromatographic method for the analysis of the methanolic extract of P. cyparissias. HPLC method development The methanolic extract of P. cyparissias collected in the spring (2 mg mL-1) was used initially in the optimization of the chromatographic parameters to obtain good separation between the main peaks and to establish a chromatographic fingerprint. This fingerprint development is important to obtain a global perspective of the constituents in the extract and to achieve a good resolution between the peaks that are the focus of the study. Since MDX does not contain easily ionizable groups (only weakly acidic phenol groups), the initial method development efforts considered mixtures of water and organic solvents and a C18 column. The water was acidified to pH 2.9 (trifluoracetic acid or acetic acid) and its combination with both methanol and acetonitrile (isocratic or gradient) independently were tested, while maintaining the flow (1 mL min-1) and oven temperature (35 ºC). Given that the run was prolonged with no good resolution for the main peaks, a C8 column was tested. Moreover, seeking to obtain a better separation, a triphasic mobile phase composed of water (pH 2.9 with acetic acid)/acetonitrile/methanol in a gradient elution was applied. With these changes, the run time was reduced from 95 min to 52 min, and an improved resolution was obtained. To obtain a more symmetric and sharpened MDX peak, oven column temperature and flow were changed to 30 ºC and 1.2 mL min-1, respectively, as described in section 2.4; this constitutes the optimized method. As observed in the chromatogram (Figure 2A), the final method provided a good selectivity for MDX (retention time, tR = ~27 min) and system repeatability (%RSD < 2.0).
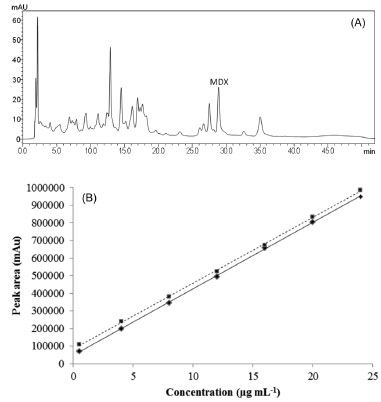 Figure 2. (A) Chromatogram of the methanol extract of Polygala cyparissias (whole plant, collected in spring), at the concentration of 2 mg mL-1; absorbance was monitored at 254 nm. (B) Analytical curves for the relationship between peak area (mAU) and concentration of 1,7-dihydroxy-2,3-methylenedioxyxanthone (MDX) (continuous line), ranging from 0.5 to 24 μg mL-1, and of the methanol extract of Polygala cyparissias (0.5 mg mL-1, fixed concentration) spiked with MDX (0.5-24 μg mL-1) (dotted line)
Method validation The method was validated according to the ICH guidelines.8 Analytical curves were obtained using a series of solutions of the extract (2-5 mg mL-1), MDX (0.5-24 µg mL-1) and extract (0.5 mg mL-1) spiked with MDX (0.5-24 µg mL-1). The MDX calibration curve proved to be linear over the proposed range, as shown by the linear regression coefficients (r2) > 0.999 for both the MDX and the standard addition in the extract matrix, demonstrating an acceptable data fit to the regression curve. Both curves proved to be parallel, as shown by the similar slopes, without interference of the matrix on the linearity of the method (Figure 2B and Table 1). The statistical analysis of the linear regressions obtained for both curves demonstrated the significance of the linearity (Table 1). Moreover, the sensitivity of the method was shown by LOD and LOQ values of 0.13 µg mL-1 and 0.44 µg mL-1, respectively. Results for the evaluation of accuracy and precision are presented in Table 1. In both cases, %RSD values were lower than 2.0%.
The final parameter evaluated for the validation of the method was robustness. This parameter was analyzed by making small variations in the column oven temperature (29, 30 and 31 ºC) and flow rate (1.1,1.2 and 1.3 mL min-1). The data (area, retention time and resolution of MDX peak as well as the content of this analyte in the extract) were evaluated by overall mean, standard deviation and %RSD for each variable. Although the Fcalc/Fcrit were higher than 1.0 (data not show), for the content of MDX the %RSD values were lower than 2.5% for both variables, demonstrating that the method can be used for the quantification of MDX with confidence.16 Application of the validated method in the analysis of the P. cyparissias extracts The validated method was applied for the determination of MDX content (mg g-1) in methanolic extracts of P. cyparissias collected either in different seasons or from different parts of the plant. The results are shown in Table 2. It can be observed that the MDX content in all extracts varied significantly (0-5.40 mg g-1). Considering this significant variation combined with the observation that MDX was one of the compounds that presented gastroprotective activity,4 it was decided to study the impact of the content of MDX in the different extracts on their gastroprotective activity.
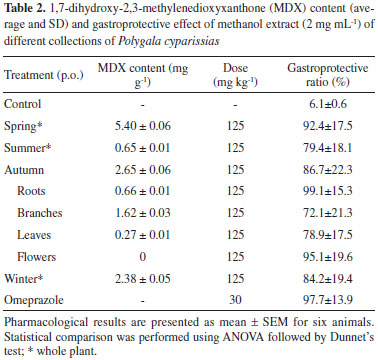
Evaluation of gastroprotective activity As observed in Table 2, all the extracts were able to demonstrate gastroprotective activity. However, it can be observed that no direct correlation between the MDX content and the gastroprotective activity can be established. As previously demonstrated,6 there are other secondary metabolites in P. cyparissias extracts that are also responsible for the gastroprotection. Moreover, it is well known that one of the main characteristics of extracts is to act in a synergistic way.
CONCLUSIONS Based on the results, it can be inferred that MDX, while not being independently responsible for the gastroprotective property of P. cyparissias extracts, is a good choice as a marker. The validated method is important for the future quality control of this medicinal plant and might be applied for the standardization of other similar derivative vegetables, as hydroethanolic extracts, aiming the future development of phytopharmaceuticals based in P. cyparissias.
ACKNOWLEDGEMENTS The authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenaçao de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundaçao de Apoio à Pesquisa Científica e Tecnológica do Estado de Santa Catarina (FAPESC), and Universidade do Vale do Itajaí (UNIVALI) for their financial support.
REFERENCES 1. De Campos, R. O. P.; Santos, A. R. S.; Vaz, Z. R.; Pinheiro, T. R.; Pizzolatti, M. G.; Cechinel-Filho, V.; Delle-Monache, F.; Yunes, R. A.; Calixto, J. B.; Life Sci. 1997, 61, 1619. 2. da Rocha, J. L. C.; Pastore, J. F. B.; Brandao, H. N.; Azeredo, A.; David, J. P.; dos Santos, E. O.; David, J. M.; Quim. Nova 2012, 35, 2263. 3. Pizzolatti, M. G.; Mendes, B. G.; Soldi, C.; Missau, F. C.; Bortoluzzi, J. H.; Carasek, E.; J. Essent. Oil Res. 2009, 21, 265. 4. Weinhold, T. S.; Bresciani, L. F. V.; Tridapalli, C. W.; Yunes, R. A.; Hense, H.; Ferreira, S. R. S.; Chem. Eng. Process. 2008, 47, 109. 5. Pinheiro, T. R.; Cechinel-Filho, V.; Santos, A. R. S.; Calixto, J. B.; Delle-Monache, F.; Pizzolatti, M. G.; Yunes, R. A.; Phytochemistry 1998, 48, 725. 6. Klein-Júnior, L. C.; Gandolfi, R. B;, Santin, J. R.; Lemos, M.; Cechinel-Filho, V.; Andrade, S. F.; Naunyn-Schmiedeberg's Arch. Pharmacol. 2010, 381, 121. 7. Klein-Júnior, L. C.; Meira, N. A.; Bresolin, T. M. B.; Cechinel-Filho, V.; Quintao, N. L. M.; Basic Clin. Pharmacol. Toxicol. 2012, 111, 145. 8. ICH Validation of Analytical Procedures: Text and Methodology - ICH Harmonized Tripartite Guideline, 2005. 9. Morimoto, Y.; Shimohara, K.; Oshima, S.; Sukamoro, T.; Jpn. J. Pharmacol. 1991, 57, 495. 10. Bo, T.; Liu, H.; J. Chromatogr. B 2004, 812, 165. 11. Peres, V.; Nagem, T. J.; Quim. Nova 1997, 20, 388. 12. Chen, S.-L.; Lin, L.-L.; Chen, S.-B.; J. Liq. Chromatogr. Related Technol. 2005, 28, 2953. 13. Ma, W.; Wei, X.; Ling, T.; Xie, H.; Zhou, W.; J. Nat. Prod. 2003, 66, 441. 14. Lin, L. L.; Huang, F.; Chen, S. B.; Yang, D. J.; Chen, S. L.; Yang, J. S.; Xiao, P. G.; Zhongguo Zhong Yao Za Zhi. 2005, 30, 827. 15. Lin, L. L.; Huang, F.; Chen, S. B.; Yang, D. J.; Chen, S. L.; Yang, J. S.; Xiao, P. G.; Planta Med. 2005, 71, 372. 16. Dhooghea, L.; Mesiaa, K.; Kohtala, E.; Tona, L.; Pieters, L;, Vlietinck, A. J.; Apers, S.; Talanta 2008, 76, 462. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access