Artigo
| Synthesis and evaluation of octocrylene-inspired compounds for UV-filter activity |
|
Hudson C. PoloniniI; Rosângela S. LopesII; Adilson BeatrizII; Roberto S. GomesII; Adriano O. SilvaII; Ricardo V. de LimaII; Gláucia. A. NunesII; Marcos Antônio F. BrandaoI; Nádia R. B. RaposoI; Dênis P. de LimaII,*
INúcleo de Pesquisa e Inovação em Ciências da Saúde, Universidade Federal de Juiz de Fora, 36036-900 Juiz de Fora - MG, Brasil Recebido em 25/10/2013 *e-mail: denis.lima@ufms.br Octocrylene (2-ethylhexyl 2-cyano-3,3-diphenyl-2-propenoate) is present in several sunscreens and is known to work synergistically with UV filters. We prepared eight octocrylene-related compounds to test their photoprotective activities by measuring diffuse transmittance. The compounds had varied photoprotection profiles, with Sun Protection Factors (SPF) ranging from 1 to 5 and UVA Protection Factors (UVAPF) ranging from 1 to 8. Compounds 4, 5, and 7 showed the best protection against UVB sunrays, while compounds 5, 6, and 7 presented the best results for protection from UVA, so compound 7 had the most balanced protection overall. Results for compounds 4, 8, and 9 are reported for the first time in the literature. INTRODUCTION Currently, both scientists and cosmetic companies are interested in developing new molecules with stable ultraviolet (UV) interactivities that can be incorporated into sunscreens.1 The motive is not just financial, as sunscreens play a major role in public health. Beginning in the 1980s, studies have demonstrated UV rays can cause skin cancer, and sunscreens have come to be understood as not only agents against sunburn, but as important elements in preventing chronic actinic damage, particularly in relation to the development of skin cancer.2 It is well known that skin cancer is the most common type of cancer worldwide, and sunscreen is an effective and low-cost measure that can prevent actinic keratoses,3 squamous cell carcinomas,4 and even some cases of melanomas.5 Sunscreens are products that contain molecules called UV filters, which have the ability to interact with incident radiation. These filters can be divided into organic and inorganic ones. Organic chemical filters absorb UV rays and convert them into IR, reducing UV rays' effects. Inorganic physical filters are usually metal oxides, and they offer protection by reflection of the incident radiation.6 UV radiation (UVR) from the sun is the main trigger of skin cancer, and it can be divided into UVA (315-400 nm), UVB (280-315 nm), and UVC (100-280 nm). Both UVA and UVB are linked to cancer pathogenesis,7,8 and it is estimated that, at minimum, 10% of all new cancer cases could be prevented if people made proper and continuous use of sunscreens with broad-spectrum protection.9 The efficacy of sunscreens is dependent on their capacity to absorb radiant energy, and broad-spectrum protection requires a combination of UVA and UVB filters.10 The goal of this study was to synthetize new candidates for innovative sunscreens and to determine their Sun Protection Factor (SPF), UVA Protection Factor (UVAPF), and spectrum of protection, using the Critical Wavelength Value (λc) and the UVA/UVB ratio. We opted for compounds with chemical structures similar to the cinnamate known as octocrylene (2-ethylhexyl 2-cyano-3,3-diphenyl-2-propenoate), since octocrylenes are present in several commercial sunscreens (e.g., Neo Heliopan® 303, Escalol® 597, Eusolex® OCR, and Parsol® 340),11 and it has been reported that other new analogues of octocrylene may be potential antitumor and antimicrobial agents.12 We designed and prepared our analogues of octocrylene using low-cost, easily accessible starting materials. (Octocrylene is effective and stable; however, cases of contact dermatitis and photo allergies in adults and children have been reported,13,14 and due to its high lipophilicity (logP = 6.9) and low biodegradability, it tends to accumulate in aquatic biota.15,16 Considering these facts, it should be noted that any compound of octocrylene being considered commercially for use as a UV filter should have its toxicity and environmental impact evaluated.)
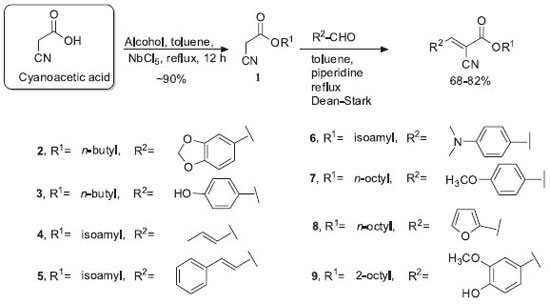
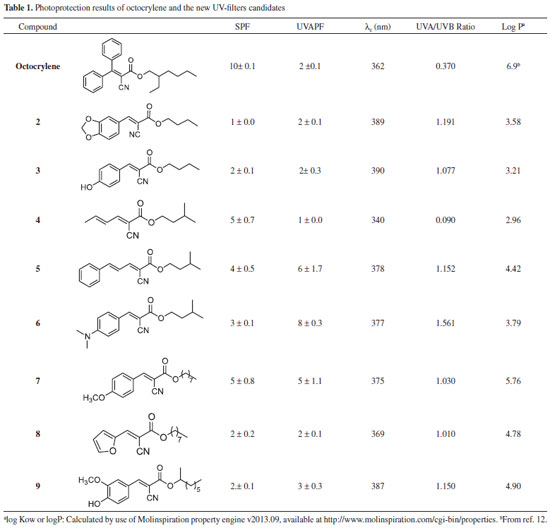
EXPERIMENTAL Materials Analytical-grade glycerin and ultrapure water (18.2 MΩ cm) obtained from an aquaMAXTM - Ultra 370 Series water purification system (YoungLin, Korea) were used throughout the analysis. Square-shaped (50 × 50 mm) polymethyl methacrylate (PMMA) HelioplateTM HD6 plates (HelioScreen, France) with roughened surfaces on one side (Sa ≈ 6 µm) were used as substrates for the determination of photoprotection activity by diffuse transmittance spectrophotometry. Octocrylene was purchased from Fluka (pharmaceutical secondary standard). Purification of the synthesized compounds was carried out by column chromatography using silica gel 60 (0.035-0.075 mm) and hexane and ethyl acetate mixtures as eluent. Thin layer chromatography was used to monitor the progress of the reactions. All solvents used in reactions and chromatographic purifications were previously treated and dried, and they were all of analytical grade. Equipment In vitro photoprotection experiments were conducted in a UV-2000S Ultraviolet Transmittance Analyzer (Labsphere, USA), composed of two photodiode array spectrographs and equipped with an integrating sphere and a xenon flash lamp, which emits a continuous spectrum of radiation with no peaks and supplies energy in the 290-450 nm spectral range with a wavelength increment of 1 nm and low irradiance, such that the photostability of the product is not unduly challenged. A long-arc xenon SUNTEST CPS+ exposure system (Atlas, Germany), with its original UV short cut-off filter combined with a special UV glass filter limiting radiation at approximately 290 nm, provided a VIS/UVA/UVB spectrum and was used as the artificial UV source for irradiation of the sunscreen samples. PMMA plates were supported firmly throughout the irradiation by a SunTray holder, which also provided dark background behind each plate to reduce the risk of any back exposure. An electronic analytical balance AY220 (Shimadzu, Japan) and a positive-displacement manual pipette (Mettler Toledo, USA) were used for the preparation of the samples on the PMMA plates. All compounds were characterized by Nuclear Magnetic Resonance (NMR) spectrometry, mass spectrometry (MS), and infrared (IR) spectrometry. 1H (300 MHz) and 13C (75 MHz) spectra were recorded on a Bruker DPX-300 spectrometer (Bruker Analytic GmbH, Germany) and calibrated with residual non-deuterated solvent as an internal reference. Chemical shifts are reported in ppm using tetramethylsilane as the internal standard (δ= 0 ppm), and the coupling constant (J) is expressed in Hertz. The liquid chromatography (LC)/MS analysis was performed using a micrOTOF hybrid quadrupole time-of-flight esquire 3000 plus mass spectrometer (Bruker Daltonics, USA) equipped with an Apollo II electrospray ion source in positive ion mode. For acquisition of mass spectra, ions were selected using an isolation width of ±4 Da and fragmented using nitrogen as the collision gas with collision energies in the range of 10-30 eV. The eluted compounds in methanol/ water 1:1 (v/v) were analyzed in positive ion mode with the following instrument settings: nebulizer gas, 1.6 bar; dry gas, nitrogen, 4 L min-1, 180 ºC; capillary, -5500 V; end plate offset, -500 V; funnel 1 RF, 200 Vpp; funnel 2 RF, 200 Vpp; insource CID energy, 0 V; hexapole RF, 100 Vpp; quadrupole ion energy, 5 eV; collision gas, argon; collision energy, 10 eV; collision RF 200/400 Vpp (timing 50/50); transfer time, 70 µs; prepulse storage, 5 µs; pulser frequency, 10 kHz. The calibration was performed using a Cole Palmer syringe pump connected to the interface, passing a solution of sodium acetate cluster containing 5 mM sodium hydroxide in a sheath liquid of 0.2% acetic acid in water/isopropanol 1:1 (v/v). Infrared spectra were registered on a Bomem FT-IR-M100 spectrometer. Melting points were determined using a Quimis Q340S23 instrument. Samples Eight octocrylene analogues (compounds 2-9) were synthesized, adapting procedures in the literature for esterification and acrylation reactions (Scheme 1). The lipophilic characteristics of compounds 2-9, based on their respective logP, were calculated using the Molinspirations web tool (http://www.molinspiration.com/cgi-bin/properties ) and are presented in Table 1.
General procedure for esterification of cyanoacetic acid The preparation of the cyanoacetates (1) was adapted from the literature,17 with the substitution of the catalyst zirconium oxychloride (ZrOCl2.8H2O) for niobium pentachloride (NbCl5). Cyanoacetic acid (5 mmol) and the selected alcohol (10 mmol) were added to dry toluene (5 mL), followed by addition of NbCl5 (0.025 mmol). The reaction mixture was stirred under reflux for 12 hours. The mixture was transferred to a separatory funnel, and ethyl acetate (EtOAc) (2 × 2 mL) was used for extraction. The organic layer was dried over magnesium sulfate (MgSO4). After filtration, the organic solvent was removed using a rotary evaporator. The products were purified by flash chromatography, using hexane/EtOAc as the eluent to produce the corresponding cyanoacetate with good yields (~90%). General procedure for acrylation The cyanoacetate (1) underwent Knoevenagel condensation using a methodology adapted from the literature18 but with a different type of base. The cyanoacetate (1) (5 mmol) was put in a 50 mL flask along with dry toluene (5 mL), and then aldehyde (3 mmol) and piperidine (2 drops) were added. A reflux condenser was attached to the flask with a Dean-Stark trap. The reaction mixture was refluxed until the removal of the condensation water. Extraction of the compound was performed with ethyl acetate (2 × 3 mL). The organic layer was dried over anhydrous MgSO4. After filtration, the organic solvent was removed using a rotary evaporator. The products were purified by flash chromatography, using hexane/EtOAc as the eluent to produce the corresponding 2-cyano enoate (68-82% yield). Characterization of compounds 2-9are available as Supplementary Material. Photoprotection assay The analogues were incorporated into an anionic Lanette lotion (Fagron, Brazil) at 10% (m/m). These samples were accurately and quickly weighed (to reduce product evaporation and dryness) to satisfy the application rate of 1.3 mg cm-2 on each PMMA plate, as set by the Cosmetics Europe guidelines.16 The quantity applied, 32.5 mg, was determined by weighing the plates before and immediately after applying the products. The samples were applied as a large number of small droplets of approximately equal mass distributed in an even manner on the roughened surface of the plate. Then, the droplets were spread over the whole surface with a fingertip covered with a vinyl glove that was pre-saturated with the product to prevent possible absorption of the weighed amount. The spreading was achieved in two steps: (i) quick distribution of the product without pressure (20-30 seconds); and (ii) rubbing it into the rough surface using pressure (also 20-30 seconds). For each product, three plates were prepared and were kept protected from light exposure in a dark chamber at room temperature (≈ 20 ºC) for 15 minutes, in order to facilitate the formation of a standard stabilized sunscreen film. After this period, the plates containing the product were placed in the light path of the transmittance analyzer. The transmission of UV radiation through each sample was measured from 290 to 450 nm at 1 nm intervals on 9 different sites of each plate (total measurement area = 2.0 cm2). The blank was prepared using a HD6 plate covered with 15 µL of glycerin, because of its non-fluorescence and UV transparency. Using the generated data, SPFin vitro was calculated using Eq. 1:
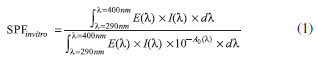
where E(λ) is the erythema action spectrum,19 I(λ) is the spectral irradiance of the UV source, A0(λ) is the mean monochromatic absorbance measurements per plate of the test product layer before UV exposure, and dλ is the wavelength step (1 nm). In order to generate the UVAPF value, the coefficient of adjustment "C" was calculated according to Eq. 2 using the SPF label value generated by the UV-2000's software.

Using the "C" value, the initial UVAPF was calculated using Eq. 3, and the dose "D" of UV irradiation was determined by Eq. 4.
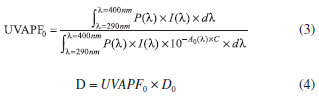
where P(λ) is the persistent pigment darkening (PPD) action spectrum,19 and D0 = 1.2 J cm-2. The plates were inserted into the UV irradiation source (temperature was kept below 40 ºC) and were then exposed to the calculated UV dose D. After that, new transmission measurements of the sunscreen samples were conducted for acquisition of the second UV spectrum. The final UVAPF was calculated according to Eq. 5.
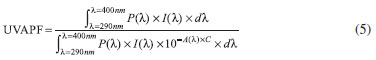
where A(λ) is the mean monochromatic absorbance of the test product layer after UV exposure. If the coefficient of variation (CV) between the calculated UVAPF values of the individual plates exceeded 20%, then further plates were measured until the CV threshold was achieved. For calculation of the Critical Wavelength Value (λc), a series of absorbance values were calculated for each of the three separate plates to which each sample was applied. Absorbance at each wavelength increment A(λ) was calculated using Eq. 6, and λc was calculated using Eq. 7.
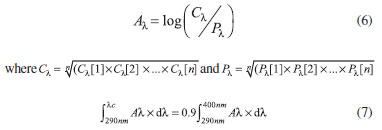
Finally, the UVA/UVB ratio was calculated as the ratio between the final UVAPF and the SPF label. Verification of the validity of the experimental setup was obtained by testing Cosmetics Europe Reference Sunscreen S2 (determined SPF = 18 ± 1.2, UVAPF = 12 ± 0.9, λc = 381 nm, and UVA/UVB Ratio = 0.88). All results were expressed as the mean of 27 determinations (3 plates, 9 readings on each at different sites) for lotions containing 10% of each molecule in isolation.
RESULTS AND DISCUSSION We focused our attention on organic UV-filter analogues of octocrylene and determined their photoprotection activity using diffuse transmittance, an official technique of the Cosmetics Europe agency.19 Our group previously used this technique with good results for determining the same activity for resveratrol analogues.10 In fact, we consider that in vitro photoprotection studies are of utmost importance in measuring the efficacy of candidates for active sunscreen ingredients or final products against UVR. They also can be used for in-house lot-to-lot quality control of the products, which enables real-time verification of consistency among different lots during production.20 The structure-activity relationship of sunscreen candidates for UVA and UVB protection is closely related to stereoelectronic effects, which can be affected by many factors, including molecular geometry, substituents that alter the electron density at the carbonyl group, steric hindrance, and out-of-plane rotation of bonds.21 For the compounds synthetized in this work, the photoprotection varied greatly, with SPFs ranging from 1 (no protection) to 5, as shown in Table 1. Octocrylene (2-ethylhexyl 2-cyano-3,3-diphenyl-2-propenoate) (Figure 1) belongs to the class of cinnamates. Compounds 2-9 are cyanoacrylates with alkyl ester chains of 4, 5, and 8 carbon atoms. The double bonds were generated (acrylation) through Knoevenagel reaction of esters with different type of aldehydes at the α-position of the carbonyl.
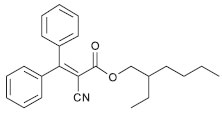 Figure 1. Structure of octocrylene
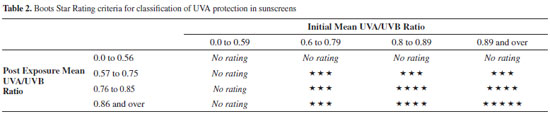
As seen in Table 1, octocrylene shows the best SPF, i.e., protection against UVB sunrays, which is related to its extended aromatic conjugation caused by its rigid geometry. The length and branched-type of the ester moiety might also be important, as suggested by the present study. However, its UVA protection is similar to most of the prepared compounds, with the exception of compounds 5-7. Some of the molecules exhibited significant photoprotection, considering they are a single UV-filter substance. Their SPFs were comparable, but the activities for compounds 4, 5, and 7 were greater than for the others. A comparison between the n-octyl alkyl esters 7 and 8 suggests that the electron donor p-methoxy group seems to be important for this type of photoprotection, leading compound 7 to have activity in both the UVB (5 ± 0.8) and UVA (5 ± 1.1) regions. Surprisingly, the non-aromatic conjugated diene 4 showed a reasonable level of photoprotection against UVB rays (5 ± 0.7), even though the majority of solar filters are substituted (ortho/para) aromatic molecules.22 This might be related to its unique molecular geometry. Its ester alkyl chain counterpart 5 is aromatic and showed a lower value for UVB protection (4 ± 0.5), perhaps due to a non-ideal electron density distribution in the conjugated system. In spite of different alkyl chains, it is notable that compound 6 had a lower value in comparison with 7. If we take into account only the groups near the aromatic regions of the molecules-the methoxy group for compound 7 and the N,N-dimethyl group for compound 6-they are both donating groups and activate the aromatic rings, though they have different electronegativities, which could explain the difference in UVB protection. Couteau et al.23 note that an ester analogue of 6, isoamyl 4-methoxy-phenyl cinnamate (IMC), has poor photostability when incorporated into an O/W emulsion. On the other hand, the butyl esters 2 and 3 do have oxygen-donating groups attached to the aromatic ring, which suggests that the length of the ester chain appears to be responsible for lower activity than compound 7. Regarding protection against UVA rays, again, the n-octyl ester 7 is a moderately active photoprotector agent (5 ± 1.1). However, the isoamyl esters 5 and 6 are the most active photoprotectors. The more conjugated compound 5 (UVAPF = 6 ± 1.7) is not as active as 6 (UVAPF = 8 ± 0.3). The effect of the electron donating group N,N-dimethyl in the para position of the aromatic ring seems to be the more important factor for UVA activity. All the remaining compounds had similar activities for UVA protection, and a rational to treat these results would be difficulty. As anticipated, the SAR results were somewhat intriguing and led us to realize that subtle dissimilarities in the length and level of chain branching of the alkyl ester or the stereoelectronic aspects of the attached 2-cyano-en portion are as important for UVA protection activity as for UVB protection activity. Another important aspect when evaluating a UV filter for this class of compounds is the breakdown of the compounds and E/Z photoisomerization, as reported in the case of octyl 4-methoxy-phenyl cinnamate (OMC).23-25 The protection against UVA rays is of utmost importance, as they are known to play an important role in skin cancers. Actually, both UVA and UVB cause damage to cellular DNA, alone or synergistically. The UVB radiation damage is due to the process of direct excitation of nucleotides, which leads to the formation of dimeric photoproducts. However, the effects of UVA involves photosensitization reactions, since the nucleotides weakly absorb radiation below 320 nm.26 However, all three UV regions (UVA, UVB, and UVC) lead to the formation of direct products of photochemical reactions with DNA.27 Furthermore, of the solar UVR that reaches the earth's surface, 95% is UVA and only 5% is UVB (UVC rays are blocked by the ozone layer).28 In addition, UVA radiation is able to penetrate deeper within the layers of the epidermis, and a small portion reaches the subcutaneous tissue. Thus, upon reaching the basal layers responsible for the proliferation of epidermal cells, it contributes significantly to skin damage.8 Consequently, it is indeed imperative that sunscreens protect against both UVA and UVB rays. Critical Wavelength is another parameter to measure UVA protection and is the wavelength below which the integration of the area under the absorption spectrum of the sample is 90% of the total absorption from 290 to 400 nm.19 Thus, it provides a measure of the protection spectrum (sunscreens with λc values near 400 nm are considered broad spectrum). The classification of the spectrum, however, can be different according to the reference adopted. For instance, the US Food and Drug Administration (FDA)29 considers the broad spectrum test as a pass/fail test based on a minimum critical wavelength value of 370 nm. Under such classification, compounds 2, 3, 5, 6, 7, and 9 are considered broad spectrum filters. This is the same classification as the one created by Springsteen et al.30 The importance of a sunscreen being broad spectrum can be seen in the work of Fourtanier,31 who demonstrated the superiority of broad-spectrum sunscreens compared to non-broad-spectrum sunscreens in protecting against DNA damage and photocarcinogenesis. Broad-spectrum products delay tumor development. Therefore, we also determined the UVA/UVB ratio, which provides a good idea of which UV region is better blocked by the substances. The UVA/UVB ratio can also be used to provide the so-called Boots Star Rating,32 which classifies products into categories from 0 to 5 stars. Classification for our compounds is listed in Table 2.
The Boots Star Rating is important to determine the stability of the generated photoprotection values, since the components of the sunscreens may degrade. All the compounds are 5 stars, except compound 4 has no stars, because its protection is mostly limited to the UVB region. The lipophilicity of solar protecting filters is also a significant parameter that has to be taken into account in formulating sunscreens. The solar protecting agents must remain on the skin surface accumulating on corneous extract, creating a barrier against UV radiation without epidermal penetration. Unfortunately, several studies show that some solar protectors, including octocrylene, penetrate the epidermis. Octocrylene (logP = 6.9) is capable of forming deposits inside the lipid phase of the corneous extract.33 Comparatively, compounds 2-9 have lower lipophilicity than octocrylene, as shown in Table 1. Therefore, an ideal sunscreen has to be formulated using different UV filters to act synergistically, each one offering a different type of photoprotection, and all of them contributing to the quality of the final product.
CONCLUSION We verified that the octocrylene analogues 4, 5, and 7 showed the best protection against UVB sunrays, while compounds 5-7 presented the best results for protection from UVA, so compound 7 had the most balanced protection. Based on these results, the prepared compounds merit further attention as potential ingredients for sunscreen formulations.
SUPPLEMENTARY MATERIAL The data from compound characterization studies are available free of charge at http://quimicanova.sbq.org.br.
ACKNOWLEDGEMENTS This research was supported by CNPq, CAPES, FAPEMIG, PROPESQ/UFJF, PROPP/UFMS and, particularly, Clínica de Cirurgia Plástica João Ilgenfritz (Campo Grande, MS, Brazil).
REFERENCES 1. Guaratini, T.; Callejon, D. R.; Pires, D. C.; Lopes, J. N. C.; Lima, L. M.; Giannella Neto, D.; Sustovich, C.; Lopes, N. P.; Quim. Nova 2009, 32, 717. 2. Lowe, N. J.; Shaath, N. A.; Pathak, M. A.; Sunscreens. Development, evaluation, and regulatory aspects, 2nd ed., Marcel Dekker: New York, 1997. 3. Naylor, M. F.; Boyd, A.; Smith, D. W.; Cameron, G. S.; Hubbard, D.; Neldner, K.H.; Arch. Dermatol. 1995, 13, 170. 4. Green, A.; Williams, G.; Neale, R.; Hart, V.; Leslie, D.; Parsons, P.; Marks, G. C.; Gaffney, P.; Battistutta, D.; Frost, C.; Lang, C.; Russell, A.; Lancet 1999, 354, 723. 5. Rai, R.; Srinivas, C.; Indian J. Dermatol. Venereol. 2007, 73, 73. 6. Flor, J.; Davolos, M. R.; Correa, M. A.; Quim. Nova 2007, 30, 153. 7. Saladi, R. N.; Persaud, A. N.; Drugs Today 2005, 41, 37. 8. Delgado, J. A.; Quesada, I.; Montaño, L. M.; Anasagasti, L.; Rev. Mex. Fis. 2006, 52, 78. 9. Brasil, Ministério da Saúde. Secretaria de Atenção à Saúde. Instituto Nacional de Câncer. "Estimativa 2012 incidência de câncer no Brasil": Rio de Janeiro, 2011. 10. Polonini, H. C.; Lima, L. L.; Gonçalves, K. M.; Carmo, A. M. R.; da Silva, A. D.; Raposo, N. R. B.; Bioorg. Med. Chem. 2013, 21, 964. 11. Amine, H.; Gomez, E.; Halwani, J.; Casellas, C.; Fenet, H.; Mar. Pollut. Bull. 2012, 64, 2435. 12. Taher, A. T.; Life Sci. J. 2012, 9, 991. 13. Agustí-Mejias, A.; Messeguer, F.; de la Cuadra, J.; Martorell-Aragonés, A.; Actas Dermo-Sifiliogr. 2014, 105, 92. 14. Karlsson, I.; Vanden Broecke, K.; Martensson, J.; Goossens, A.; Borje, A.; Contact Dermatitis 2011, 64, 343. 15. Blüthgen, N.; Meili, N.; Chew, G.; Odermatt, A.; Fent, K.; Sci. Total Environ. 2014, 476-477, 207. 16. Buser, H. R.; Balmer, M. E.; Schmid, P.; Kohler, M.; Environ. Sci. Technol. 2006, 40, 1427. 17. Sun, H. B.; Hua, R.; Yin, Y.; Molecules 2006, 11, 263. 18. Yadav, J. S.; Reddy, B. V. S.; Basak, A. K.; Visali, B.; Narsaiah, A. V.; Nagaiah, K.; Eur. J. Org. Chem. 2004, 3, 546. 19. Cosmetics Europe. Method for in vitro determination of UVA protection, 2011. 20. Polonini, H. C.; Gomes, T. B. B.; Gonçalves, K. M.; Brandão, M. A. F.; Raposo, N. R. B. Lat. Am. J. Pharm. 2012, 31, 353. 21. Cawthray, J. F.; Ph.D. Thesis, The University of Adelaide at Adelaide, Australia, 2009. 22. Balogh, T. S.; Velasco, M. V. R.; Pedriali, C. A.; Baby, R. A.; Kaneko, T. M.; Baby, A. R. An. Bras. Dermatol. 2007, 86, 732. 23. Couteau, C.; Faure, A.; Fortin, J.; Paparis, E.; Coiffard, L. J. M. J. Pharm. Biomed. Anal. 2007, 44, 270. 24. Butt, S. T.; Christensen, T.; Radiat. Prot. Dosim. 2000, 91, 283. 25. Shaath, N. A.; Photochem. Photobiol. Sci. 2010, 9, 464. 26. Cadet, J.; Sage, E; Douki, T.; Mutat. Res., Fundam. Mol. Mech. Mutagen. 2005, 571, 3. 27. Jiang, Y.; Rabbi, M.; Kim, M.; Ke, C.; Lee, W.; Clark, R. L.; Mieczkowski, P. A.; Marszalek, P. E.; Biophys. J. 2009, 96, 1151. 28. El Ghissassi, F.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; Cogliano, V.; Lancet 2009, 10, 751. 29. Food and Drug Administration. 21 CFR Parts 201, 310, and 352. Sunscreen Drug Products for Over-the-CounterHuman Use; Final Rules and Proposed Rules, Food and Drug Administration, Silver Spring, 2011. 30. Springsteen, A.; Yurek, R.; Frazier, M.; Carr. K. F.; Anal. Chim. Acta. 1999, 380, 155. 31. Fourtanier, A.; Photochem. Photobiol. 1996, 64, 688. 32. Boots the Chemist Ltd. The revised guidelines to the practical measurement of UVA/UVB ratios according to the Boots star rating system, The Boots Co., PLC: Notthingham, 2008. 33. Vilela, F. M. P.; Fonseca, Y. M.; Vicentini, F. T. M. C.; Fonseca, M. J. V.; Quim. Nova 2011, 34, 879. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access