Artigo
| Physicochemical properties of lecithin-based nanoemulsions obtained by spontaneous emulsification or high-pressure homogenization |
|
Roselena S. Schuh#; Fernanda Bruxel#; Helder F. Teixeira*,#
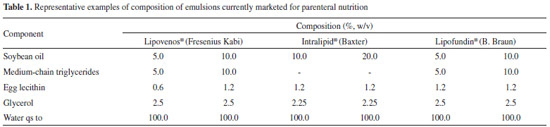
Faculdade de Farmácia, Universidade Federal do Rio Grande do Sul, 90610-000 Porto Alegre - RS, Brasil Recebido em 03/02/2014 * e-mail: helder.teixeira@ufrgs.br Nanoemulsions composed of a medium-chain triglyceride oil core stabilized by rapeseed or sunflower lecithins were prepared by spontaneous emulsification and high-pressure homogenization. These nanoemulsions are compared with formulations stabilized by egg lecithin. Nanoemulsions obtained by high-pressure homogenization display larger droplet size (230 to 440 nm) compared with those obtained by spontaneous emulsification (190 to 310 nm). The zeta potentials of the emulsions were negative and below -25 mV. Zeta potential inversion occurred between pH 3.0 and 4.0. The results demonstrate the feasibility of preparing lipid emulsions comprising rapeseed or sunflower lecithins by spontaneous emulsification and high-pressure homogenization. INTRODUCTION A parenteral nutrition regimen is basically composed of macronutrients (amino acids, carbohydrates, and lipids) and micronutrients (vitamins, electrolytes, and microelements), under the prescription of a physician depending on the condition, age, and weight of the patient.1 The lipid macronutrients, administered as emulsions, are energy donors, essential fatty acid suppliers, and fat-soluble vitamin carriers. The fatty acids contained in these formulations have major metabolic importance, since they are cell membrane components and play specific roles in hormonal signalization and transportation. Moreover, they are precursors of prostaglandins, leukotrienes, thromboxanes, and prostacyclins, which modulate inflammatory processes, renal function, and platelet aggregation.2 An essential fatty acid deficiency in preterm infants during brain development results in learning problems and visual function impairment, which may be irreversible, even if an adequate fatty acid-containing diet is provided later in development.2 Parenteral lipid emulsions are heterogeneous systems, consisting of an oily phase homogeneously dispersed in an aqueous phase (dispersant), by the presence of an emulsifier. A small droplet size, usually between 200 and 500 nm, characterizes the formulations, because of the risk of embolism due to use of larger particles. The emulsions must also display a physiologically compatible pH (around 7), isotonicity, low viscosity, and a high zeta potential (in modulus), to prevent the occurrence of instability phenomena.3 Lipid nanoemulsions are commonly employed in total parenteral nutrition admixtures, known as 3-in-1 systems, in which all macronutrients and micronutrients are added to an ethylvinylacetate (EVA) bag. However, these mixtures experience some physical instability related to the presence of electrolytes and other components, which may precipitate or interact with the emulsion droplets. Calcium and phosphate precipitation are widely reported in literature. Furthermore, divalent ions (such as calcium and magnesium) may interfere with the zeta potential of the emulsion and induce aggregation/flocculation of lipid droplets, followed by coalescence. This phenomenon is very serious, because any droplet above 5 µm in diameter that enters the bloodstream may cause a fat embolism.4,5 The physical characteristics and consequent stability of lipid emulsions are strongly related to their production method and composition.3,6 Production methods are diverse and may require more than one step to produce an emulsion with a reduced droplet size. A high-speed homogenizer (Ultraturrax®) may first create a coarse emulsion, for example. Droplet size reduction may then be achieved by high-pressure homogenization, microfluidization, or ultrasonication.7-9 Among methods that do not require pre-treatment is spontaneous emulsification, primarily used in formulation studies and easily performed on a laboratory scale, because it is not necessary to use sophisticated equipment.10 Table 1 shows the composition of typical commercially available intravenous lipid emulsions. Besides the components described, the formulations must meet the requirements for injectable products.11
The oily phase of parenteral emulsions is composed of long-chain triglycerides (LCT), which may be combined with medium-chain trigylcerides (MCT), as shown in Table 1. LCT comprise a wide variety of oils, such as sunflower, castor, olive, or, more commonly, soybean oil. These oils all contain fatty-acid chains longer than 12 carbons. MCT are obtained by esterification of coconut oil fatty acids. The emulsifiers of choice for injectable emulsion stabilization are lecithins, since they are biocompatible and biodegradable. Lecithins are natural mixtures of polar and neutral phospholipids, obtained from animal or vegetable sources.12 The phospholipid composition of lecithins from vegetable sources can be variable due to extraction, crop, and other processing conditions.13 They mainly contain amphoteric phospholipids, such as phosphatidylcholine and phosphatidylethanolamine, but negative phospholipids may also be present. Commercially available lipid emulsions for parenteral nutrition are most often composed of egg-yolk lecithin or, rarely, soybean lecithin (Solipid® E&S). Despite the numerous benefits of fat supplementation, there are reports of adverse clinical effects related to long-term supplementation, due to metabolic limitations and immune reactions in critically ill patients.14,15 Adverse reactions to parenteral lipid emulsions are reported to be related to the presence of soybean and egg-yolk lecithins.16-18 Drug-food allergy interactions can lead to a range of adverse responses, from gastrointestinal upset to anaphylaxis.19 In this context, the search continues for alternative raw materials to find hypoallergenic substitutes that are safer for parenteral administration in patients. This work prioritizes the search for different lecithins with the purpose of finding new alternatives for lipid emulsions intended for parenteral nutrition, or even as drug carriers, to provide the safest options for patients (especially preterm infants) with hypersensitivity to egg- or soybean-based emulsifiers. We sought to develop parenteral lipid nanoemulsions stabilized by rapeseed or sunflower lecithins, and compared these with egg lecithin-containing nanoemulsions. Furthermore, preparation by spontaneous emulsification is compared with high-pressure homogenization, commonly used for the industrial production of parenteral lipid emulsions.
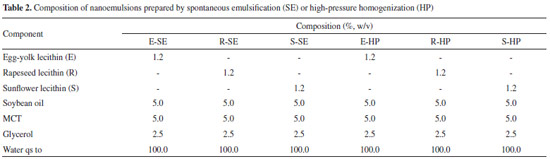
MATERIALS AND METHODS Chemicals and reagents MCT, soybean oil, and egg-yolk (Lipoid E80®), rapeseed (Lipoid R20®), and sunflower (Lipoid H100®) lecithins were obtained from Lipoid GmbH (Ludwigshafen, Germany), who kindly donated the rapeseed and sunflower lecithins. Glycerol and ethanol were obtained from Merck (Brazil) and Sigma-Aldrich (St. Louis, MO, USA), respectively. Ultrapure water was obtained from a Milli-Q® apparatus (Millipore, Billerica, USA). Preparation of nanoemulsions Lipid emulsions were prepared in triplicate by two methods: spontaneous emulsification and high-pressure homogenization. The formulations obtained by spontaneous emulsification were prepared according to a previously described procedure.10,20 Briefly, soybean oil was mixed with MCT, lecithin, and ethanol. Glycerol was dissolved in water, into which the ethanolic phase was slowly added under moderate magnetic stirring for 30 min. The solvent was then removed by distillation under reduced pressure in a rotatory evaporator. The formulations obtained by high-pressure homogenization were prepared as previously described.21 Firstly, lecithin was dispersed in water containing glycerol and mixed under magnetic stirring at 40 ºC, until a homogeneous aqueous phase was obtained. The oil phase consisted of soybean oil and MCT. Both oil and water phases were mixed under magnetic stirring (15 min, at room temperature) to obtain a coarse emulsion. The coarse emulsions were then mixed at 9500 rpm for 2 min using an IKA® Ultra-Turrax T8 mixer (IKA® Works Inc., NC, USA) to form crude pre-emulsions, that were individually subjected to high-pressure homogenization (EmulsiFlex-C3®, Avestin, Canada) at 750 bar (10 000 psi) for 10 cycles to produce the final emulsion. The pH value of all formulations was adjusted to 8.0 with 0.01 mol L-1 NaOH solution. The emulsions were stored at 4 ºC. The formulations and their constituents are given in Table 2.
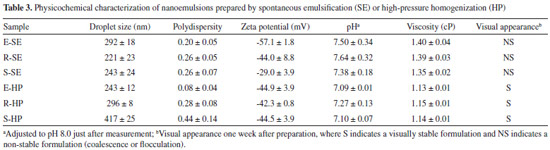
Physicochemical characterization of nanoemulsions The pH values of the formulations were determined directly in the samples just after preparation, using a calibrated potentiometer (Digimed, São Paulo, Brazil) at room temperature. The mean droplet size and polydispersity index were measured by photon correlation spectroscopy (PCS) and zeta potential was determined by electrophoretic mobility, using a Malvern Zetasizer Nano ZS (Malvern Instrument, UK) at 25 ºC. For these measurements, the nanoemulsions were diluted in 1 mmol L-1 NaCl solution in the pH range from 2.0 to 8.0 units. The viscosity was evaluated by capillary viscometry at 25 ºC (viscometer constant, k = 0.0212), at 25 ± 0.1 ºC. The time was recorded, in seconds, for the liquid to flow from the upper to the lower mark in a capillary tube. All formulations were analyzed in triplicate. Morphological analysis The morphologic examination was conducted by transmission electron microscopy (TEM). One drop of the nanoemulsion was placed on a carbon-coated copper grid (200 mesh), negative stained with a 2.0 % uranyl acetate solution, and left to dry for 24 hours before examination. A JEM-1200 EXII instrument (JEOL, Tokio, Japan), operating at 80 kV, was used.
RESULTS AND DISCUSSION In the present study, we develop lipid emulsions intended for parenteral nutrition or drug carrying, stabilized by two lecithins obtained from vegetable sources (rapeseed (R) and sunflower (S)), as alternatives to egg-yolk lecithin (E), a traditional stabilizer for parenteral emulsions. To compare the new formulations with traditional ones, all other emulsion components were kept at concentrations similar to those of the commercial lipid emulsion products. This work also compares two different production methods: spontaneous emulsification and high-pressure homogenization. Table 3 presents the physicochemical properties of the resultant nanoemulsions. The formulations obtained by spontaneous emulsification display a mean droplet size from 220 to 300 nm, as determined by PCS. In theory, this is a range of high emulsion stability.11,22,23 As the droplet size is reduced, the rate of self-diffusion increases to a point where very small droplets may be kept from creaming by diffusional mixing.7,23 Nanoemulsions containing rapeseed or sunflower lecithin exhibit a smaller mean droplet size compared with those containing egg-yolk lecithin. Similar results have been described for nanoemulsions obtained by the same method, solely composed of MCT, as the oil phase, and stabilized by 2 % (m/m) egg-lecithin.24 Based on these data, one could conclude that 1.2 % concentration would be sufficient to emulsify the soybean oil, MCT, and water mixture. However, although a small droplet size and low polydispersity index were obtained, the emulsions did not remain physically stable for more than one week after preparation, following which phase separation (coalescence) could be visually observed. The coalescence process is an irreversible instability phenomenon, since oil droplets lose their interfaces and fuse into larger droplets.25
The qualitative and quantitative composition of nanoemulsions, in addition to the type of emulsifier and method of emulsification, can directly influence the droplet size.7,23 A second method was therefore tested for nanoemulsion preparation. High-pressure homogenization is commonly used in the pharmaceutical industry for the production of such formulations, although on an industrial scale. Among the various methods available for emulsification, this method is preferred due to its efficient droplet disruption. This is a high-energy method, where size reduction is achieved by forcing a coarse emulsion under high pressure through a homogenizing valve, thereby deforming and reducing the droplet size.26 Spontaneous emulsification is a low cost, easy, and reliable method, and it is usually used in experimental studies instead of a high-pressure homogenizer, which is much more complex and expensive. As demonstrated in Table 3, high-pressure homogenization produced larger droplet sizes in nanoemulsions comprising rapeseed (296 ± 18 nm) or sunflower lecithin (417 ± 25 nm), in comparison with the previous method, and relative to the control egg-lecithin emulsions (243 ± 12 nm). Nevertheless, it must be pointed out that, even if the high-pressure homogenization was less efficient in droplet disruption, it conferred more stability on the formulations. Contrary to emulsions obtained by spontaneous emulsification, these were visually stable for at least 30 days. These results confirm the importance of the preparation method in imparting emulsion stability. Considering intravenous applications, the size distribution of lipid emulsion droplets may be even more important than the average droplet size. A small population of large oil droplets may be sufficient to cause a fat embolism in patients.4,5 The droplet size distributions of the prepared formulations are presented in Figure 1.
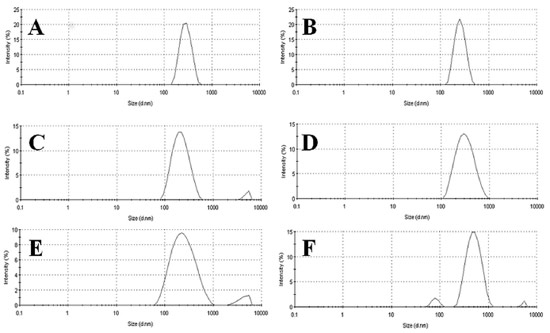 Figure 1. Comparison of formulation particle size distribution (nm) by intensity (%): (A) E-SE, (B) E-HP, (C) R-SE, (D) R-HP, (E) S-SE, (F) S-HP. (SE: spontaneous emulsification; HP: high-pressure homogenization)
In Figure 1, two populations are observed in formulations composed of rapeseed lecithin (obtained by spontaneous emulsification, Figure 1C) and sunflower lecithin (obtained either by spontaneous emulsification or by high-pressure homogenization, Figures 1E and 1F). As a result, a polydispersity index higher than 0.20 is obtained for these formulations. The stability of emulsions may be correlated with the composition and properties of their interfacial film (lecithin), since this determines the zeta potential of the formulations and the repulsion between droplets, which is one of the mechanisms for emulsion stabilization.27 Lecithin is a heterogeneous mixture of phospholipids; its heterogeneity is extremely beneficial because of the fluidity of the interfacial film, when compared with that of a pure phospholipid.28 The main phospholipids of lecithin mixtures are phosphatidylcholine and phosphatidylethanolamine, which are uncharged at physiological pH (7.4). Smaller quantities of acidic lipids, such as phosphatidylinositol, phosphatidylserine, and phosphatidylglycerol, may also be present. These lipids are ionized at pH 7.0 and induce a negative surface charge on emulsion droplets, which contributes to their stability. Any added substance that interferes with this charge is likely to alter the stability of the system.29 Even if the parenteral grade lecithin is highly purified, it still contains a small amount of other phospholipids, as shown in Table 4, which describes the composition of the three lecithin raw materials used in this study.
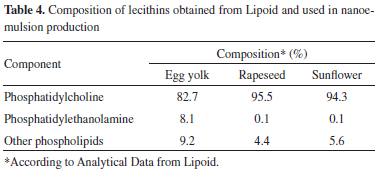
As demonstrated in Table 3, a smaller zeta potential (module value) is observed for nanoemulsions composed of rapeseed or sunflower lecithin and obtained by spontaneous emulsification. However, no differences are observed in zeta potentials of nanoemulsions produced by high-pressure homogenization. These results indicate that the main factor affecting zeta potential is the preparation method. Although our group has optimized both methods, the experimental conditions must usually be adjusted, taking into account the composition of formulations. Parameters such as the number of cycles and pressure may be modified to obtain desired physicochemical properties of the final formulations.30,31 Zeta potential of nanoemulsions also depends on ionization of the emulsifier. A reduction in the resulting charge (in modulus) from 40 mV to less than 25 mV can increase the flocculation and coagulation rates.32 The zeta potential and mean droplet size of nanoemulsions composed of different lecithins and produced by different emulsification methods were evaluated at in the pH range from 2.0 to 8.0. The results are presented in Figure 2.
 Figure 2. Zeta potential values (A, C) and mean droplet size (B, D) of formulations obtained by spontaneous emulsification (A, B) and high-pressure homogenization (C, D) in 1 mM NaCl solution at various pH values. Key: E-SE and E-HP (triangle), R-SE and R-HP (square), S-SE and S-HP (circle)
The surface charge of all formulations declines to zero between pH 3.0 and 4.0, as previously observed for Intralipid®, an egg lecithin-stabilized triglyceride emulsion.25 Zeta potential depends on the pH, since H+ is a potential-determining ion on the phospholipid surfaces, with an isoelectric pH of 3.1.33 A reduction in pH results in a decreased (less negative) zeta potential and more rapid rate of flocculation.34 Mean droplet size shows a small increase at the pH of zeta potential inversion. From Figure 3, one can conclude that the pH of nanoemulsions should preferably be higher than 7.0, since a plateau is achieved at that pH value, where maximum repulsion between oil droplets is observed.
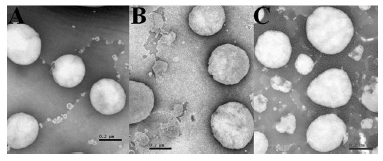 Figure 3. Morphology of oil droplets examined by TEM at 100 kV. Key: (A) E-HP, (B) R-HP, and (C) S-HP nanoemulsions
Finally, the morphology of the oil droplets of the nanoemulsions prepared by high-pressure homogenization was examined by TEM. Figure 3 reveals homogeneous and spherical particles, showing that emulsion droplets have a mean droplet size in the nanometer range. These results corroborate the previous droplet size analysis. Nanoemulsions are low viscosity systems with Newtonian behavior. Evaluation of emulsion viscosity is crucial, since the intravenous administration of high viscosity emulsions can be very painful to the patient.23,32 Nanoemulsions composed of different lecithins show similar viscosities. As expected, no relationship between mean droplet size and viscosity of nanoemulsions is observed, since all formulations contained only 10 % oil core.35 In contrast, some differences in viscosity are observed for formulations obtained by the different preparation methods: spontaneous emulsification produced slightly more viscous emulsions. It is worth mentioning that the compositions of the nanoemulsions studied in this work are based on those of commercial nanoemulsions using egg lecithin as the emulsifier. The use of a different emulsifier may require optimization of its concentration and/or emulsification conditions. Commercial injectable nanoemulsions composed of soybean lecithin (Solipid®) require 1.5 % concentration of the emulsifier, for example. Additional co-emulsifiers are sometimes used to stabilize the emulsions and promote less polydispersity and smaller droplets. However, their application is restricted to lipid emulsions as drug carriers, because small quantities of the formulations are administered for that purpose: co-emulsifiers are not frequently used in emulsions for parenteral nutrition, due to the high volumes of these formulations administered and safety problems, especially in the case of preterm infants. Sodium oleate is commonly used for stabilizing formulations of injectable lipid emulsions,36 acting as an anionic surfactant and solubilizing agent.27
CONCLUSIONS The results demonstrate the feasibility of preparing injectable lipid emulsions composed of rapeseed or sunflower lecithins by spontaneous emulsification and high-pressure homogenization, as alternatives to traditional egg-lecithin nanoemulsions for patients who are sensitive to egg derivatives. Further studies should be conducted to optimize the emulsification conditions to improve long-term stability of the formulations.
ACKNOWLEDGEMENTS The authors would like to thank the National Council for Scientific and Technological Development (CNPq) for financial support and Lipoid GmbH for the materials provided.
REFERENCES 1. Waitzberg, D. L.; Nutrição oral, enteral e parenteral na prática clínica, 1st ed., Atheneu: São Paulo, 2009. 2. Adolph, M.; Clin. Nutr. 2001, 20, 11. 3. Bruxel, F.; Laux, M.; Wild, L. B.; Fraga, M.; Koester, L. S.; Teixeira, H. F.; Quim. Nova 2012, 35, 1827. 4. Antunes, M.; Guedes, J.; Arq. Bras. Med. 1994, 68, 303. 5. Trissel, L. A.; Gilbert, D. L.; Martinez, J. F.; Baker, M. B.; Walter, W. V.; Mirtallo, J. M.; JPEN, J. Parenter. Enteral Nutr. 1999, 23, 67. 6. Almeida, M. E.; Teixeira, H. F.; Koester, L. S.; Lat. Am. J. Pharm. 2008, 27, 780-88. 7. Benita, S.; Levy, M. Y.; J. Pharm. Sci. 1993, 82, 1069. 8. Robin, O.; Blanchot, V.; Vuillemard, J. C.; Paquin, P.; Lait 1992, 72, 511. 9. Mbela, T. K. M. N.; Ludwig, A.; Landau, I.; Deharo, E.; Haemers, A.; Int. J. Pharm. 1994, 110, 89. 10. Bouchemal, K.; Briançon, S.; Perrier, E.; Fessi, H.; Int. J. Pharm. 2004, 280, 241. 11. Klang, S.; Benita, S. In Submicron emulsions in drug targeting and delivery; Benita, S., ed; Harwood Academic Publishers: Amsterdam, 1998, chap. 5. 12. Szuhaj, B. F.; Sipos, E. F. In Food Emulsifiers: Chemistry, Technology, Functional Properties and Application; Charalambous, G., Doxastakis, G., eds.; Elsevier Science Publishers B. V.: Amsterdam, 1989, chap. 10. 13. Schneider, M. In Lecithins: Sources, Manufacture, & Uses, American Oil Chemists' Society: Illinois, 1989. 14. Szuhaj, B. F. In Bailey's Industrial Oil and Fat Products; Shahidi, F., ed.; John Wiley and Sons: New Jersey, 2005, vol. 3, chap. 13. 15. Adan, D.; La Gamma, E. F.; Browne, L. E.; Crit. Care Clin. 1995, 11, 751. 16. Himaya, D. T.; Griggs, B.; Mittman, R. J.; JPEN, J. Parenter. Enteral Nutr. 1981, 13, 318. 17. Buchman, A. L.; Ament, M. E.; JPEN, J. Parenter. Enteral Nutr. 1991, 15, 345. 18. Kamath, K. R.; Berry A.; Cummins, G.; New Engl. J. Med. 1981, 304, 360. 19. Wiesner, A. M.; Romanelli, F.; Stratman, R. C.; Smith, K. M.; Orthopedics 2008, 31, 149. 20. Yu, W.; Tabosa Do Egito, E. S.; Barrat, G.; Fessi, J. P.; Devissaguet, J. P.; Puisieux, F.; Int. J. Pharm. 1993, 89, 139. 21. Floyd, A. G.; Pharm. Sci. Technol. 1999, 2, 134. 22. Driscoll, D. F.; Pharm. Res. 2006, 23, 1959. 23. Constantinides, P. P.; Tustian, A.; Kessler, D. R.; Adv. Drug Deliver. Rev. 2004, 56, 1243. 24. Martini, E.; Carvalho, E.; Leão, F.; de Oliveira, M. C.; Teixeira, H. F.; Quim. Nova 2007, 30, 930. 25. Washington, C.; Adv. Drug Deliver. Rev. 1996, 20(2-3), 131. 26. Walstra, P.; Smulders, P. E. A. In Modern Aspects of Emulsion Science; Binks, B. P., ed.; The Royal Society of Chemistry: Cambridge, 1998, chap. 12. 27. Lachman, L.; Liberman, H.; Kanig, L. J. Teoria e Prática na indústria farmacêutica; 3rd ed., Lea & Febiger: Philadelphia, 2001. 28. Lawrence, M. J.; Curr. Opin. Colloid Interface Sci. 1996, 1, 826. 29. Washington, C.; Int. J. Pharm. 1990a, 66, 1. 30. Dias, D. O.; Colombo, M.; Kelmann, R. G.; Souza, T. P. D.; Bassani, V. L.; Teixeira, H. F.; Anal. Chim. Acta 2012, 721, 79. 31. Fraga, M.; Laux, M.; Zandoná, B.; Santos, G. R.; Giuberti, C. S.; de Oliveira, M. C.; J. Drug Delivery Sci. Technol. 2008, 18, 398. 32. Jumaa, M.; Müller, B. M.; Int. J. Pharm. 1998, 163, 81; Roland, I.; Piel, G.; Delattre, L.; Evrard, B.; Int. J. Pharm. 2003, 263, 85. 33. Davis, S. S.; Galloway, M.; J. Pharm. Pharmacol. 1981, 33, 88. 34. Washington, C.; Int. J. Pharm. 1990a, 64, 67. 35. Silvander, M.; Hellstrom, A.; Warnheim, T.; Claesson, P.; Int. J. Pharm. 2003, 252, 123. 36. Formariz, T. P.; Sarmento, V. H. V.; Silva-júnior, A. A.; Scarpra, M. V.; Santilli, C. V.; Oliveira, A. G.; Colloids Surf., B 2006, 51, 54.
# Programa de Pós-Graduação em Ciências Farmacêuticas |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access