Artigo
| Isolation and antitrichomonal activity of the chemical constituents of the leaves of Maytenus phyllanthoides Benth. (Celastraceae) |
|
Juan Alberto Moo-PucI; Zhelmy Martín-QuintalI; Gumersindo Mirón-LópezI; Rosa Esther Moo-PucII; Leovigildo QuijanoIII; Gonzalo J. Mena-RejónI, *
ILaboratorio de Química Farmacéutica. Facultad de Química. Universidad Autónoma de Yucatán. Calle. 41 No. 421 x 26 y 28, Col. Industrial. C.P. 97150, Mérida, Yuc., México Recebido em 13/06/2013 *e-mail: mrejon@uady.mx Cyclolignan (+)-lyoniresinol (1), veratric acid (2), vanillic acid (3), lupeol, oleanolic acid, 3β-hydroxy-urs-11-en-28,13β-lactone (4), the mixture of α- and β-amyrin, trans-polyisoprene, and β-sitosterol were isolated from the leaves of Maytenus phyllanthoides. The structures of the isolated compounds were established based on spectroscopic data, mainly 1H and 13C nuclear magnetic resonance (NMR). Compound 1, its acetate analog 1a, and compounds 2, 3, and 4 were tested against Trichomonas vaginalis. (+)-Lyoniresinol showed activity corresponding to IC50 17.57 µM. This is the first report on the occurrence of 3β-hydroxy-urs-11-en-28,13β-lactone (4) in the Celastraceous family and lyoniresinol in the Maytenus genus, and on the antitrichomonal activity of lyoniresinol. INTRODUCTION Infections caused by protozoa are the leading cause of morbidity and mortality among human parasitic infections.1Trichomonas vaginalis is a flagellated protist that causes trichomoniasis, a common sexually transmitted human infection, increasingly recognized as an important disease in both women and men.2 According to the WHO, approximately 180 million cases are reported annually worldwide.3 Studies indicate that trichomoniasis is associated with the predisposition to cervical cancer, pelvic inflammatory disease, infertility, increasing infections by human papillomavirus (HPV), birth outcomes, and human immunodeficiency virus (HIV).4 Moreover, the relationship between serum antibodies of T. vaginalis and prostate cancer has been recently established.5 Presently, the Food and Drug Administration (FDA) recommends metronidazole and tinidazole for the systemic treatment of trichomoniasis. However, several resistant clinical isolates to these drugs have been recently described. Therefore, the need to find novel trichomonacidal agents to improve T. vaginalis infection drug therapy is imperative.6 It is well known that natural products could be a source of potent and selective drugs.7 In this context, plants of the Maytenus genus (Celastraceae) are known to produce several bioactive compounds, including antiprotozoal compounds.8 This genus is widely distributed around the world mainly in the tropics and subtropics. In Africa, the areas of the major diversity of the genus are found in Ethiopia, South Africa, the Canary Islands, and the Northwest African mountains. The distribution of the genus in the new world is diverse and can be found throughout the continent, from southern United States to Tierra del Fuego in South America, as well as in the Caribbean.9 Maytenus phyllanthoides (sweet mangrove) is a Mexican native species widely distributed either at coastal areas of the Pacific Ocean or Gulf of Mexico, growing at dunes or marshes and at the margins of mangrove areas. The species is an evergreen, branched, creeping or prostrate large shrub or small tree that grows up to 12 ft. The leaves are leathery, always green, from oval to elliptical shaped (1-2 in), and the flowers white to green (0.75-1 in).10 According to ethnobotanical records, the leaves of this species are used in the northern states of México, for the treatment of rheumatism in Baja California Sur, and against dysentery and toothache in Sonora.11 In a previous antiprotozoal screening performed in our laboratories, the CH2Cl2 and methanol extracts of the leaves of Maytenus phyllanthoides Benth. showed antitrichomonal activity (4.6 and 37.96 mg/mL, respectively), which encouraged further phytochemical studied in order to find the bioactive compounds. This paper reports on the phytochemical investigation of the leaves of Maytenus phyllanthoides Benth. and on the evaluation of the antitrichomonal activity of the isolated compounds.
RESULTS AND DISCUSSION As part of our search for biologically active metabolites from the Celastraceae species growing in the Yucatan Peninsula in Mexico, we have studied the leaves of Maytenus phyllanthoides. As a result, we have isolated the cyclolignan of the tetralin type (+)-lyoniresinol (1). Moreover, two other phenolic compounds, veratric (2) and vanillic (3) acids were identified, as well as five pentacyclic triterpenes [i.e., lupeol, oleanolic acid, 3-β-hydroxy-urs-11-en-28,13β-lactone (4) (Figure 1)], and a mixture of α- and β-amyrin] together with trans-polyisoprene and the ubiquitous β-sitosterol. The isolated compounds were identified by spectroscopic methods, mainly by 1H and 13C nuclear magnetic resonance (NMR), including 1D and 2D, homo- and heteronuclear experiments [i.e., distortionless enhancement by polarization transfer (DEPT), correlation spectroscopy (COSY), heteronuclear single-quantum correlation (HSQC), and heteronuclear multiple bond correlation (HMBC)], and by the comparison with the data reported in the literature.
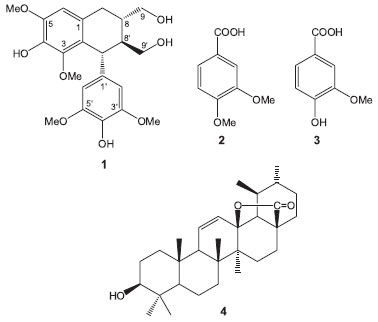 Figure 1. Some of the compounds isolated from leaves of Maytenus phyllanthoides
Compound (1) was isolated from the methanol extract as an amorphous powder. The mass spectrum obtained by electrospray ionization-mass spectrometry (ESI-MS) showed a pseudo-molecular ion pick at m/z 443, corresponding to the sodiated ion [M+Na]+, in agreement with the molecular formula C22H28O8, and the 1H and 13C NMR data for compound 1. The 1H NMR spectrum of 1, showed two singlets in the aromatic region at δ 6.58 (1H, s) and 6.37 (2H, s) for one and two equivalent aromatic protons, respectively. Three sharp singlets indicated the presence of four methoxyl groups in the molecule, two equivalent at δ 3.73 (6H) and two non-equivalent at δ 3.37 (3H) and 3.85 (3H). The 13C NMR spectrum together with the DEPT and HSQC experiments confirmed the above assumptions. Accordingly, in the 13C NMR spectrum, according to the DEPT experiments19 signals due to 22 carbon atoms corresponding to six methines (3 aromatic, including 2 equivalent at δC 106.8), three methylenes, four methoxyl groups (2 equivalent at δc 56.8) and nine fully substituted aromatic carbons (2 equivalent at δC 149.0) were observed. The comparison of the NMR data with those described in the literature allowed the identification of compound 1 as (+)-lyoniresinol.12 The nuclear Overhauser spectroscopy (NOESY) spectrum of compound 1 showed interactions that are consistent with the proposed structure and relative configuration at C-7', C-8, and C-8'. Lyoniresinol is a tetralin-type lignan that has been isolated free and as glycoside from several species of different genera and families of plants; however, to our knowledge this is the first report describing its presence in the genus Maytenus and the second in the family Celastraceae because it has been previously isolated from Salacia chinensis.13 The optical rotation value ([α]D +3.0º) of lyoniresinol (1) isolated from M. phyllanthoides, was lower than those reported for (+)-lyoniresinol isolated from Vitex negundo (Lamiaceae) ([α]D +68º) and Cinnamomum cassia (Lauraceae) ([α]D +58º);14,15 however, it was similar but opposite to the optical rotatory activity reported for lyoniresinol isolated from Betula maximowicziana ([α]D - 3.6º), which was described as a racemic mixture of (7'R, 8'S, 8S)- and (7'S, 8'R, 8R)-lyoniresinol, where the first enantiomer was dominant.16 These facts suggest that compound 1 isolated from M. phyllanthoides is an enantiomeric mixture in which the (+)-lyoniresinol enantiomer (with relative configuration 7'S, 8'R, 8R) is predominant. Similarly, other authors have also reported low optical rotation values [α]D for (+)-lyoniresinol isolated from other species such as Quercus petraea (Fagaceae),17Dendrobium chrysanthum (Orchidaceae),18 and Caesalpinia sappan (Fabaceae)19 ([α]D +13.3º, +11.0º and + 2.08º, respectively), indicating that lyoniresinol is commonly isolated as a racemic mixture. The acetylation of compound 1 produced the tetra-acetate 1a, whose 1H NMR spectrum showed signals and chemical shifts consistent with the presence of the acetate groups in the molecule. Accordingly, four sharp singlets due to the acetate moieties at C-9 and C-9' (δ 2.05 and 2.08, respectively) and to two acetate groups on the aromatic ring at δ 2.29 and δ 2.30 were observed, besides the downfield shifts of the C-9 and C-9' methylene proton signals. Thus, the signal of the H-9 protons are observed at δ 4.12 (dd, J = 11.2, 5.6) and 4.23 (dd, J = 11.2, 4.0) and those of the H-9' protons at δ 4.03 (dd, J = 11.6, 4.0) and 4.32 (dd, J = 11.6, 2.8). All these data were consistent with those reported in the literature.20 The anomalous chemical shifts of the methoxyl group at C-3 in compound 1 (δ 3.37) and the acetate analogous 1a (δ 3.18) suggest that this group may be shielded by the phenyl group at C-7'. From the methanol extract, two phenolic-type compounds derived from benzoic acid, veriatric acid (2), and vanillic acid (3) were also isolated. The pentacyclic triterpenes α- and β-amyrin, lupeol, oleanolic acid, and 3β-hydroxy-urs-11-en-28,13β-olide (4), besides the trans-polyisoprene and the ubiquitous β-sitosterol, were isolated from the dichloromethane extract. Compound 4 was isolated as an amorphous white powder. Its 1H NMR spectrum showed the presence of seven signals between δ 0.7 and 1.15, corresponding to five tertiary and two secondary methyl groups. A doublet of doublets at δ 3.22 corresponding to a methine with an oxygen function, a doublet of doublets at δ 5.53 (J = 10.4, 3.2 Hz), and a broad doublet at 5.95 (J = 10.4 Hz) were also identified. The above NMR signals suggested the presence of an ursane skeleton with a double bond. The 13C NMR DEPT spectrum confirmed the presence of 30 carbon atoms due to seven methyl groups, eight methylenes, eight methines, and seven quaternary carbons including an ester carbonyl group (δ 179.9). The ESI-MS spectrum showed a molecular ion peak at m/z 454 corresponding to the molecular formula C30H46O3, in agreement with the 13C NMR DEPT experiment. These data, together with infra-red (IR) absorption at 1770 cm-1,21 indicated the presence of a g-lactone moiety in the molecule. All the spectroscopic data were in good agreement with those available in the literature for 3-β-hydroxy-urs-11-en-28,13β-lactone (4) previously isolated from several species, mainly belonging to the genera Eucalyptus (Myrtaceae) and Ilex (Aquifoliaceae).22 This work describes, for the first time, the isolation of 3-β-hydroxy-urs-11-en-28,13β-lactone (4) from a Celastraceous species. The in vitro antiprotozoal activity of the tested compounds was assessed against Trichomonas vaginalis by using the subculture method of Cedillo-Rivera et al.23 According to Cos et al.,24 the results showed that only (+)-lyoniresinol (1) exhibited a relevant antitrichomonal activity with an IC50 value of 17.57 µM, while the other compounds showed IC50 greater than 25 µM (Table 1). Lyoniresinol belongs to lignans, a group of phenolic natural products that have mostly been tested against Trypanosoma cruzi, Leishmania donovani, and Plasmodium falciparum, but their antiprotozoal properties have not yet been evaluated against Trichomonas vaginalis.25
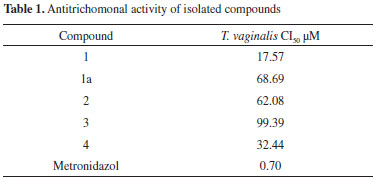
The antitrichomonal activity of lyoniresinol peracetate (1a) decreased remarkably by a 4-fold factor in comparison to the parent compound. Therefore, the antitrichomonal activity of lyoniresinol could be associated to its hydrophilic character due the presence of four hydroxyl groups in its structure. Finally, the high antitrichomonal activity of the CH2Cl2 extract of Maytenus phyllanthoides leaves contrasted with the poor activity of the isolates of this extract. A synergistic action of the isolated compounds could explain the observed differences in the bioactivity levels.
CONCLUSIONS This is the first report on the presence of the lignane lyoniresinol in the Maytenus genus and its bioactivity against Trichomonas vaginalis. The leaves of Maytenus phyllanthoides Benth, are rich in pentacyclic triterpenes of the oleanane and ursane type. These leaves are also a source of phenolic compounds such as benzoic acid derivatives, as well as the lignan (+)-lyoniresinol. To the best of our knowledge, this is the first report on the chemical analysis of this species.
EXPERIMENTAL General The 1H and 13C NMR spectra were recorded on Bruker Avance III 400 and Jeol Eclipse 300 spectrometers, using CDCl3 or CD3OD as solvents. Tetramethylsilane (TMS) or the residual solvent signal was used as internal reference. High resolution mass spectrometry (HRMS) was performed on a Jeol JMS GC-Mate II spectrometer, using chemical ionization techniques, and on Jeol JMS AX-505 HA, using ESI technique. IR spectra were recorded on a Bruker Tensor 27 spectrometer, using an accessory for attenuated total reflectance. Optical rotations were measured in MeOH solutions on a Rudolph Research Autopol IV polarimeter. For column chromatography, silica gel 60 (63-210 mm, Fluka) was used. Flash chromatography was performed on silica gel 60 (37-74 mm, Fluka or 2 - 25 mm, Merck). For preparative thin layer chromatography (TLC), silica gel F254-coated plates (20 × 20 cm × 0.5 mm) were used. Plant material M. phyllanthoides (Celastraceae) was collected in September 2009 at the coastal dune area in Chicxulub Puerto, Yucatán (Mexico) (N 21º 17.9', W 89º 33.5') and authenticated by Dr. Juan Tun. A voucher specimen (J. T. 12350) was deposited in the herbarium "Alfredo Barrera Marin" of the Universidad Autónoma de Yucatán. Extraction and isolation Dried and ground leaves (3.7 kg) of M. phyllanthoides were successively subjected to extraction with CH2Cl2 and methanol in a Soxhlet apparatus. The resulting extracts were concentrated under vacuum yielding 150 g of residue from the CH2Cl2 extract and 300 g from the methanol extract. The methanol extract (300 g) was dissolved in water (1.5 L) and subjected to liquid-liquid partitioning using EtOAc and butanol, yielding 3.2 g and 12 g of residue, respectively. The EtOAc fraction residue (3.2 g) was separated by column chromatography on silica gel 60 (37-74 mm) obtaining 13 fractions (A-M). Fraction G was rechromatographed on silica gel 60 (37-74 mm) and eluted with CH2Cl2-EtOAc-MeOH (8:1:1); 15 fractions were collected and combined based on their TLC profiles to afford six combined fractions (1-6). Fraction G5 was purified by TLC using CH2Cl2-EtOAc-MeOH, 8:1:1 as eluent (2×), yielding 30 mg of (+)-lyoniresinol (1). A 15 mg sample of compound 1 was acetylated in pyridine and acetic anhydride at room temperature for 24 h. The reaction mixture was purified by preparative TLC to give 4 mg of the acetyl derivative 1a as a dark yellow powder, which was identified by 1H NMR as the tetra acetylated derivative of (+)-lyoniresinol (1a). Fraction A was purified by TLC using CH2Cl2-acetone (98:2) as eluent to give 80 mg of veratric acid (2). From fraction B, after column chromatography on silica gel 60 (37-74 mm) eluted with mixtures of CH2Cl2-EtOAc, 30 mg of vanillic acid (3) were obtained. The CH2Cl2 extract residue was dissolved again in CH2Cl2 and treated with methanol to obtain a precipitate that was filtered and identified spectroscopically as cis-polyisoprene (gutta-percha, 76 g). The solution was concentrated to dryness giving 73 g of residue that was chromatographed in column using silica gel (63-210 mm) and mixtures of hexane-ethyl acetate of increasing polarity as eluent. Fifty eluates were obtained and combined in 13 primary fractions (A-M) based on their chromatographic similarity determined by TLC. Fractions D, E, F, and G were chromatographed on Sephadex LH-20 using hexane-chloroform-methanol (2:1:1) as eluent. After several successive chromatographies and preparative TLCs, β-sitosterol (30 mg) was obtained from fraction D. Similarly, from fraction E, the mixture of α- and β-amyrin (5 mg) was obtained. The chromatography of fraction F provided 40 fractions that were combined in eight major fractions (1-8) based on the TLC analysis. Fraction F6 was rechromatographed on silica gel 60 (74-37 mm) using a mixture of CH2Cl2-ethyl acetate (95:5) affording 50 eluates that were combined into seven new fractions (a-g) according to their TLC profiles. Fraction F6d was separated by column chromatography using silica gel 60 (2-25 mm) and CH2Cl2-EtOAc (9:1), and seven (1-7) eluates were collected. The fraction F6d3 was dried under vacuum to afford compound 4 (3 mg). Chromatography of fraction G over Sephadex LH-20 afforded 36 fractions that, after a TLC analysis, were combined in eight fractions (1-8). Fraction G8 chromatographed on silica gel 60 (37-74 mm) eluted with a mixture of hexane-ethyl acetate of increasing polarity. Forty-eight fractions were collected, analyzed by TLC, and combined into 11 new fractions (a-k). Fraction G8g was further chromatographed on silica gel 60 (2-25 mm) using a mixture CHCl3-EtOAc (95:5). Fifty-six fractions were obtained and combined into 10 fractions. (1-10). Lupeol (6 mg) and oleanolic acid (3 mg) were obtained from fraction G8g7 after purification by TLC where they eluted with CHCl3-EtOAc (98:2). Antitrichomonal activity Parasites T. vaginalis strain GT3 was used in all experiments. Trophozoites of T. vaginalis were maintained in TYI-S-33 medium supplemented with 10% bovine serum. For the assay, trophozoites were axenically maintained and were employed in the log phase of growth. In vitro susceptibility assays The in vitro susceptibility assay was conducted according to the subculture method of Cedillo-Rivera et al.23 This is a highly stringent and sensitive method for assessing antiprotozoal effects ("gold standard") particularly in Giardia intestinalis, Entamoeba hystolitica, and T. vaginalis.26 The compounds were dissolved in 1 mL of dimethylsulfoxide (DMSO) and added to microtubes containing 1.5 mL of medium in order to reach concentrations of 1, 2, 10, and 20 µg/mL. The solutions were inoculated with T. vaginalis trophozoites to achieve an inoculum of 4 × 104 trophozoites/mL, and then were incubated for 48 h at 37 ºC. Each test included metronidazole as positive control, a control (culture medium plus trophozoites and DMSO), and a blank (culture medium). After incubation, the trophozoites were detached by chilling, and the samples of each tube were subcultured in fresh medium for another 48 h, without anti-protozoal drugs or compounds. The final number of parasites was determined with a haemocytometer and the 50% inhibitory concentration (IC50) was calculated by probit analysis (GraphPad Prim 4 software). The experiments were performed in duplicate and replicated.
SUPPLEMENTARY MATERIAL 1H and 13C NMR spectra of compounds 1, 1a, 3, 4, and 1H NMR spectrum of compound 2 are freely available at http://quimicanova.sbq.org.br in pdf.
ACKNOWLEDGEMENTS This work was funded by the grant 101265 provided by Consejo Nacional de Ciencia y Tecnología (CONACYT). J. A. Moo-Puc is grateful to the Programa para el Mejoramiento del Profesorado (PROMEP) for the fellowship. This paper was taken in part from the doctoral thesis of J. A. Moo-Puc.
REFERENCES 1. Ndjakou Lenta, B.; Vonthron-Sénécheau, C.; Fongang Sohd, R.; Tantangmo, F.; Ngouela, S.; Kaiser, M.; Tsamod, E.; Anton, R.; Weniger, B., J. Ethnopharmacol. 2007, 111, 8. 2. Ali, V.: Nozaki, T.; Clin. Microbiol. Rev. 2007, 20, 164. 3. Upcroft, P.; Upcroft, J. A.; Clin. Microbiol. Rev. 2001, 14, 150. 4. Grodstein, F.; Goldman, M. B.; Cramer, D. W.; Am. J. Epidemiol. 1993, 137, 577; Heine, P.; McGregor, J. A.; Clin. Obstet. Gynecol. 1993, 36, 137; Zhang, Z. F.; Begg, C. B.; Int. J. Epidemiol. 1994, 23, 682; Sorvillo, F.; Kerndt, P.; Lancet 1998, 351, 213; Tasca Cargnin, S.; Brum Vieira, P.; Cibulski, S.; Cassel, E.; Figueiró Vargas, R. M.; Montanha, J.; Roehe, P.; Tasca, T.; Lino von Poser, G.; Parasitol. Int. 2013, 62, 112. 5. Giordani, R. B.; De Almeida, M. V.; Fernandes, E.; da Costa, C. F.; De Carli, G. A.; Tasca, T.; Zuanazzi, J. A. S.; Biomed. Pharmacother. 2009, 63, 613. 6. Marrero-Ponce, Y.; Meneses-Marcel, A.; Rivera-Borroto, O. M.; García-Domenech, R.; De Julián-Ortiz, J. V.; Montero, A.; Escario, J. A.; Gómez Barrio, A.; Montero Pereira, D.; Nogal, J. J.; Grau, R.; Torrens, F.; Vogel, C.; Arán, V. J.; J. Comput.-Aided Mol. Des. 2008, 22, 523. 7. Butler, M. S.; J. Nat. Prod. 2004, 67, 2141. 8. González, A. G.; Bazzocchi, I. L.; Jiménez, I. A.; Moujir, L. In Studies in Natural Products Chemistry, Bioactive Natural Products (Part D); Atta-ur-Rahman, ed.; Elsevier Science Publisher: The Netherlands, 2000, chap 15; Mossi, A. J.; Cansian, R. L.; Leontiev-Orlov, O.; Treichel, H.; Mazutti, M.; Dariva, C.; Oliveira, J. V.; Echeverrigaray, S. In Recent Progress Medicinal Plants; Govil, J. N.; Singh, V. K.; Ahmad, K., eds; Studium Press: USA, 2006, chap 19. 9. Gentry, A. In Biological relationships between Africa and South America; Goldbatt, P., ed.; Yale University Press: New Haven, 1993, chap 17; Simmons, M. P. In The families and genera of vascular plants. Vol. VI. Flowering Plants Dycotiledons; Kubitzki, K., ed.; Springer: Berlin, 2004, chap 6. 10. Guerrero-Ruiz, M. I.; Fernández-Nava, R.; Arreguín-Sánchez, M. L.; Polibotánica 2002, 14, 1; Clevinger, C.; Clevinger J. In Flora del valle de Tehuacán-Cuicatlán. Universidad Nacional Autónoma de México, ed. 2010, Fascículo 76. Celastraceae R.Br. 11. http://www.medicinatradicionalmexicana.unam.mx/monografia.php?l=3&t=Mangle _dulce&id=7605, accessed May 2013. 12. Ohashi, K.; Watanabe, H.; Okumura, Y.; Uji, T.; Kitagawa, I.; Chem. Pharm. Bull. 1994, 42, 1924. 13. Kishi, A.; Morikawa, T.; Matsuda, H.; Yoshikawa, M.; Chem. Pharm. Bull. 2003, 51, 1051. 14. Azhar-ul-Haq; Malik, A.; Khan, M. T. H.; Anwar-el-Haq; Khan, S.B.; Ahmad, A.; Choudhary, M. I.; Phytomedicine 2006, 13, 255. 15. Liu, C.; Zhong, S. M.; Chen, R. Y.; Wu, Y.; Zhu, X. J.; J. Asian Nat. Prod. Res. 2009, 11, 845. 16. Hanawa, F.; Shuro, M.; Hayashi, Y.; Phytochemistry 1997, 45, 589. 17. Nonier, M. F.; Vivas, N; de Gaulejac, N. V.; Fouquet, E.; C. R. Chim. 2009, 12, 291. 18. Ye, Q. H.; Zhao, W. M.; Qin, G. W.; J. Asian Nat. Prod. Res. 2004, 6, 39. 19. Fu, L; Huang, X.; Lai, Z. Hu, Y.; Liu, H.; Cai, X.; Molecules 2008, 13, 1923. 20. Miyamura, M.; Nohara, T.; Tomimatsu, T.; Nishioka, I.; Phytochemistry 1983, 22, 215. 21. Pretsch, E.; Clerc, T.; Seibl, J.; Simon, W.; Tables of Spectral Data for Structure Determination of Organic Compounds, 2nd ed., Springer-Verlag: New York, 1989. 22. Ahmad, V.; Rahman, A.; Handbook of Natural Products Data. Pentacyclic Triterpenoids. Vol. 2, Elsevier: The Netherlands, 1994; Qin, G.; Xu, R.; Huaxue Xuebao 1986, 44, 151; El-Domiaty, M. M.; El-Shafae, A. M.; Abdel-Aal, M. M.; Alex. J. Pharm. Sci. 1999, 13, 1; Siddiqui, B. S.; Sultana, I.; Begum, S.; Phytochemistry 2000, 54, 861; Nishimura, K.; Miyase, O.; Noguctii, H.; Chen, X. M.; J. Nat. Med.-Tokyo 2000, 54, 297; Pereira, S. I.; Freire, C. S. R.; Pascoal Neto, C.; Silvestre, A. J. D.; Silva, A. M. S.; Phytochem. Anal. 2005, 16, 364. Xie, G.; Zhou, S.; Lei, L.; Tu, P.; Zhongguo Zhongyao Zazhi 2007, 32, 1890; Sugimoto, S.; Nakamura, S.; Yamamoto, S.; Yamashita, C.; Oda, Y.; Matsuda, H.; Yoshikawa, M.; Chem. Pharm. Bull. 2009, 57, 257; Zhou, S.; Yao, Z.; Li, J.; Tu, P.; Zhongcaoyao 2012, 43, 444. 23. Cedillo-Rivera, R.; Chávez, B.; González-Robles, A.; Tapia-Contreras, A.; Yépez-Mulia, L.; J. Euk. Microbiol. 2002, 49, 201. 24. Cos, P.; Vlietinck, A. J.; Berghe, D. V.; Maes, L.; J. Ethnopharmacol. 2006, 106, 290. 25. Kayser, O.; Kiderlen, A. F.; Croft, S. L.; Parasitol. Res. 2003, 90, S55. 26. Gillin, F.; Diamond, L.; J. Antimicrob. Chemother. 1981, 8, 305; Argüello-García, R.; Cruz-Soto, M.; Romero-Montoya, L.; Ortega-Pierres, G.; J. Antimicrob. Chemother. 2004, 54, 711. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access





