Artigo
| Acidity of modified mordenites synthesized from rice husk silica and catalytic transformation of methylbutynol |
|
Sittichai KulawongI; Sanchai PrayoonpokarachI; Frank RoessnerII; Jatuporn WittayakunI,*
ISchool of Chemistry, Institute of Science, Suranaree University of Technology, Nakhon Ratchasima, Thailand Recebido em 04/06/2014 *e-mail: jatuporn@sut.ac.th Mordenite (MOR) was synthesized using rice husk silica and modified by base (B), acid (A) or acid-base (AB) and converted to H-form. The modification did not destroy the MOR structure but increased surface area and generated mesopores. Lewis acidity of the parent and modified MOR samples investigated by aluminum NMR and NH3-TPD showed a decrease in the following order: HMOR > BMOR > ABMOR > AMOR. For the catalytic transformation of methylbutynol, ABMOR provided the highest conversion and selectivity of products from acid sites. INTRODUCTION Mordenite (MOR) is a zeolite with interconnected pore channels between 12-membered ring and 8-membered ring.1 Its Si/Al ratio can be varied depending on composition of the synthetic batch.2 MOR has been used as a catalyst in various reactions including isomerization, cracking, alkylation, and oxidation.3-6 Like other zeolites, MOR has a problem in accessibility to active sites within the cavities and diffusion limitation of large molecules. The large pores can be incorporated to MOR by either post-synthetic methods such as steam treatment, desilication by base, dealumination by acid or templating methods with both organic and inorganic templates.7,8 Li et al. reported that mesopores were generated in HMOR by a sequential dealumination and desilication.9 The dealumination was done by leaching with 2 mol/L HNO3 to increase the Si/Al ratio from 15 to 25 and surface area from 384 to 455 m2/g without much change in the N2 adsorption isotherm. Further desilication of the acid-treated HMOR by 0.2 mol/L NaOH generated mesoporous mordenite with the Si/Al ratio of 21-23 and surface area of 524-530 m2/g. The modification enhanced the catalytic activity in benzene alkylation by benzyl alcohol. Previously, NaMOR was synthesized by using rice husk silica (RHS) and modified by acid (A), base (B) and both acid-base (AB) to change the Si/Al ratio and pore properties but the acidity was not yet investigated.10 Thus, the acidity of the modified MOR is elaborated in this work by 27Al magic angle spinning nuclear magnetic resonance (27Al MAS NMR) and temperature-programmed desorption of ammonia (NH3-TPD). The 27Al MAS NMR can differentiate framework and the non-framework (extraframework) aluminum. The NH3-TPD provides information related to amount and strength of acid sites. Furthermore, the modified MOR was tested for transformation of methylbutynol (2-methyl-3-butyn-2-ol or MBOH) for additional information about different surface centers.
EXPERIMENTAL Synthesis and modification of MOR MOR in sodium form (NaMOR) was synthesized with a procedure adapted from Kim and Ahn using RHS from acid leaching as a silica source.11,12 A silicate solution prepared from RHS and NaOH was mixed with a solution of NaAlO2 in a polypropylene bottle to give a gel with a molar ratio of 2.5Na2O:Al2O3:22SiO2:518H2O, kept under stirring for 24 h and crystalized in a Teflon-lined stainless steel autoclave at 170 ºC without agitation. The resulting NaMOR was filtered, washed thoroughly with deionized water, dried at 80 ºC overnight and calcined in a muffle furnace at 500 ºC for 3 h. The NaMOR was converted to proton form (HMOR) by ion-exchange with a 1.0 mol/L NH4NO3 solution at 80 ºC and calcination in air at 500 ºC for 3 h. The zeolite modification method was adapted from the literature.9 In a treatment by base (desilication) to produce BMOR, the parent NaMOR was refluxed in a 0.2 mol/L NaOH solution at 65 ºC for 30 min, filtered, washed with deionized water and ion-exchanged to proton form. In an acid treatment (dealumination) to produce AMOR, the HMOR was refluxed in a 2.0 mol/L HNO3 solution at 100 ºC for 4 h, washed with deionized water, dried at 80 ºC for 10 h, and calcined at 500 ºC for 3 h. To produce a sample from acid-base treatment (ABMOR), the AMOR was refluxed in a 0.2 mol/L NaOH solution at 65 ºC for 30 min, filtered, washed with deionized water and ion-exchanged to proton form. Characterization of MOR samples All MOR samples were characterized by XRD (Bruker AXS diffractometer D5005) using Cu Kα radiation to confirm the MOR structure and by nitrogen adsorption-desorption analysis (Micromeritics ASAP 2010) to determine their surface area, pore volumes and pore sizes. Their Si/Al ratios were determined by inductively coupled plasma-mass spectrometry (ICP-MS, Agilent 7500CE). The nature of Al atoms in the MOR samples were studied by 27Al MAS NMR (500 MHz Varian INOVA) using a 4 mm probe with resonance frequency of 130.32 MHz. The spectra were obtained with small flip angles of approximately 9º and with a recycle delay of 1 s. The 27Al chemical shift was referenced to 1 mol/L Al(NO3)3 in H2O. The accessible acidity were determined by NH3-TPD in a commercial unit (Raczek Analysentechnik, Germany) equipped with a thermal conductivity detector. A sample amount of 300 mg was packed in a tubular U-shaped quartz cell, and degassed at 500 ºC with a heating rate of 10 ºC/min for 1 h in a He flow (49.8 mL/min), and cooled down to 70 ºC. Then, the sample was exposed to a stream of 5 vol. % NH3/Ar gas mixture at flow rate of 50 mL/min for 30 min. Then, the sample was heated to 130 ºC with a heating rate of 10 ºC/min and held for 17 h to remove physisorbed species. The NH3-TPD measurement was performed from 130 ºC to 650 ºC with a heating rate of 20 ºC/min with He as a carrier gas. Catalytic transformation of MBOH The catalytic transformation of MBOH was carried out in a fixed-bed reactor similar to the previous work.13 The MOR samples were pressed and sieved to 200 - 315 µm mesh size. One hundred milligrams of sample were packed in the center of a quartz tubular reactor, activated under air flow by heating to 400 ºC with a rate of 8 ºC/min and holding for 4 h to remove water and CO2 adsorbed on the surface. Then it was kept under N2 flow at this temperature for 4 h. After the activation, the reactor was cooled to the reaction temperature (180 ºC). At a permanent pressure of N2 over the storage vessel containing a mixture of MBOH and toluene (95:5 v/v) a constant pulsation free liquid flow was passed over the evaporator. Toluene was used as an internal standard. The reaction products were analyzed by a gas chromatograph (Hewlett Packard HP 5890 Series II).
RESULTS AND DISCUSSION Characterization by XRD, nitrogen adsorption-desorption and ICP XRD patterns of all samples (Figure 1S in Supplementary Materials) showed characteristic peaks of zeolite MOR.10,11,14 The modification did not destroy the zeolite structure. The nitrogen adsorption-desorption isotherm of the NaMOR, HMOR, BMOR, AMOR, and ABMOR are shown in Figure 2S in Supplementary Materials. Their BET surface areas were 414, 531, 545, 612 and 632 m2/g, respectively. The modified samples had higher surface area. The formation of large pores were suggested by the hysteresis loop which was caused when the adsorption and desorption lines were not superimposed. Their Si/Al ratios determined from ICP were 11.0, 10.5, 9.5, 35.5 and 27.8 respectively. The decrease and increase of Si/Al ratio indicated desilication and dealumination, respectively. Both changes caused formation of pores larger than the micropores of the zeolite. In AMOR, the acid treatment could open the side pocket of HMOR resulted in an increase of micropore surface area.3,5 The sample with the most open structure was ABMOR which had the highest surface area and adsorption isotherm type IV with narrow slit-like mesopores.15 Characterization by 27Al MAS NMR 27Al MAS NMR profiles of the parent NaMOR and modified MOR are shown in Figure 1(a). Comparisons of intensities of the position at 56 and 1 ppm are provided in Figure 1 (b) and (c), respectively. The parent NaMOR showed only one peak at 56 ppm which is a characteristic of framework tetrahedral coordinated AlO4 (AlF-td) in the zeolite.16 In HMOR and modified MOR, the intensity of AlF-td decreased in the following order: NaMOR > HMOR > BMOR > ABMOR > AMOR. The peak at 1 ppm, characteristic of extraframework octahedral coordinated AlO6 (AlEF-oh) was also observed. The intensity was also in the same order, namely, HMOR > BMOR > ABMOR > AMOR.
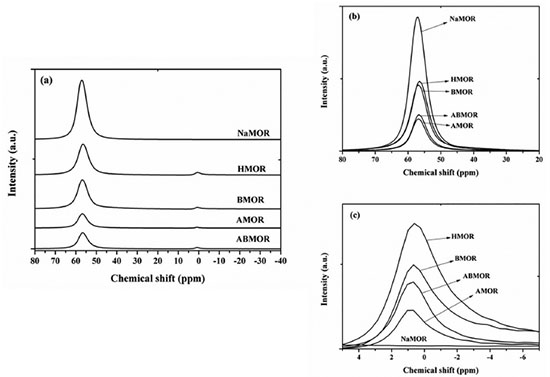 Figure 1. (a) 27Al MAS NMR spectra of NaMOR, HMOR, BMOR, AMOR, and ABMOR, (b) comparison of the chemical shift peaks at 56 ppm, and (c) comparison of the chemical shift peaks at 1 ppm When converted to HMOR, some AlF-td atoms were converted to AlEF-oh.17 In BMOR, the intensity of AlF-td peak was slightly lower than that of HMOR indicating that some Al atoms in the framework were removed during desilication. This could open up the zeolite structure and enhance the accessibility to the active sites. From the work of van Laak et al. on a commercial MOR with Si/Al ratio of 5.7, the peak intensity of AlF-td decreased and a new peak corresponding to AlEF-oh was observed after NaMOR was transformed to HMOR.16 Both Al peaks were observed after the HMOR was desilicated in 1.0 M NaOH for 15 min and transformed to proton form. In AMOR, the intensity of AlF-td and AlEF-oh peaks significantly decreased compared to those of HMOR. It was previously reported that AlEF-oh which are Lewis acid sites could be removed from the lattice by dealumination.16 An increase in Si/Al ratio of HMOR from 15 to 25 and decrease in the intensity of AlF-td peak by dealumination with a condition similar to this work was reported.9 In ABMOR which was obtained by desilication of AMOR, peaks of AlF-td and AlEF-oh were higher than those of AMOR. The results suggested that further desilication of the AMOR removed some Si atoms resulting in higher Si/Al ratio and surface area than AMOR. Acid-base properties characterized by NH3-TPD NH3-TPD profiles of the parent NaMOR, HMOR and modified MOR from are shown in Figure 2. All samples showed desorption in a low temperature range (LT, lower than 400 ºC) and a high temperature range (HT, higher than 400 ºC) corresponding to weakly and strongly adsorbed NH3, respectively. If the samples were treated at higher temperatures, the LT band would become smaller.18
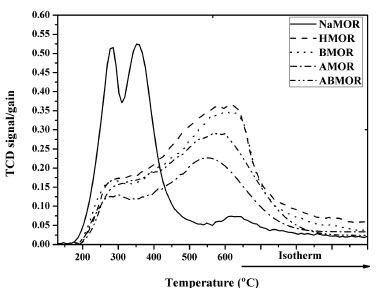 Figure 2. NH3-TPD profiles of (a) NaMOR, (b) HMOR, (c) BMOR, (d) AMOR, and (e) ABMOR
The NH3-TPD profile of NaMOR consisted of two LT peaks at 270 and 330 ºC corresponding to the adsorption on Na+ ions on isolated silanol groups (weak Brönsted acid sites) and on NH4+ formed by prior adsorption of ammonia on the Brönsted acid sites.18 Moradi et al. observed a single, broad LT peak on commercial NaMOR with Si/Al ratio ~ 8 in the temperature range of 180 - 450 ºC.19 In addition, the HT band at around 550 ºC corresponds to the adsorption on extraframework Al which were Lewis acid sites. The trace amount of AlEF-oh in NaMOR could correspond to the low intensity of the HT band. Compared to NaMOR, the intensity of LT band from HMOR decreased and that of the HT bands increased corresponding to the adsorption on protonic sites. The TPD profile of other samples which were in proton form consisted of a broad HT band with intensity decreased in the following order: HMOR > BMOR > ABMOR > AMOR. This order conformed to the decrease of the AlEF-oh peak from the NMR result. These results indicated that modification of MOR by acid and base lowered the zeolite acidity. Groen et al. reported similar results but from a different NH3-TPD procedure that the HT bands were not enhanced significantly after the alkaline treatment.5 The decrease of strong acid sites was consistent with the Si/Al ratio from ICP. When the AMOR was treated with NaOH, the desilication opened up the structure and accessibility to the Lewis acid sites. Consequently, the HT band of ABMOR was higher than that of AMOR. Catalytic transformation of MBOH The catalytic transformation of MBOH is sensitive to reactive sites and can be applied to several materials.11,13,20 The reaction scheme can be found in Figure 3S in the Supplementary Materials. The conversion of MBOH on acid, base and defect sites produces 2-methyl-1-butene-3-yne (MBYNE), 3-methyl-2-butenal (prenal); acetone and acetylene; and 3-hydroxyl-3methyl-2-butanone (HMB) and 3-methyl-2-butyne-2-one (MIKP), respectively. MBOH conversion and product selectivity from all zeolites are shown in Figure 3. The MBOH conversions (Figure 3a) were in the following order: ABMOR > BMOR > AMOR > HMOR ≅ NaMOR. The highest conversion from ABMOR could be attributed to the presence of mesopores which improve diffusion of reactants to active sites. All samples showed a decrease in MBOH conversion but this is typical for amorphous silica-alumina which is an acidic material. Alsawalha and Roessner reported that the MBOH conversion on silica-alumina at 120 ºC decreased with more Al content attributing to strong adsorption of products.21 Less decrease was observed at higher reaction temperature. In addition, Huang and Kaliaguine reported the decrease of MBOH conversion on NaMOR (Si/Al = 5.7) in a continuous flow reactor operated at atmospheric pressure at 180 ºC with different activation procedure and amount of sample.22 The more decrease was observed from the sample with higher Si/Al ratio. The decrease in MBOH conversion without change in selectivity was also reported on silica-alumina (Si/Al =18) in a pulse reactor, possibly due to strong product adsorption or secondary reactions leading to coke production on acidic sites.23
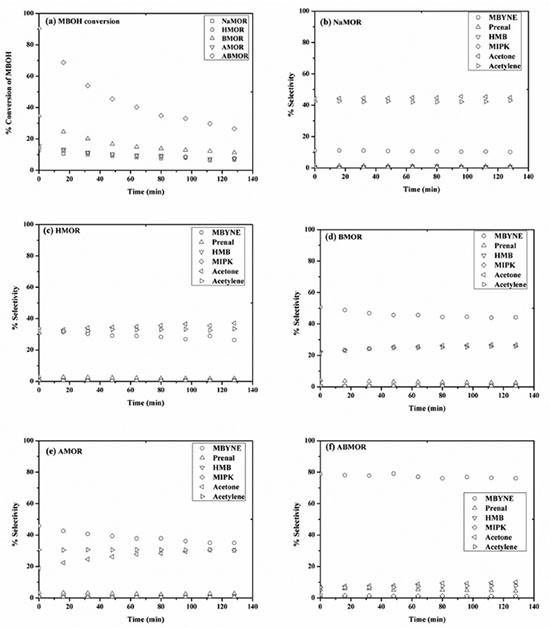 Figure 3. (a) MBOH conversion on MOR zeolites, (b) - (f) product selectivity on NaMOR, HMOR, BMOR, AMOR, and ABMOR
In terms of selectivity shown in Figure 3b-f, all zeolites gave products from both basic sites (acetone and acetylene) and acid sites (MBYNE). The highest selectivity of MBYNE was obtained from ABMOR (Figure 3f). The treatment by acid and base could open up the zeolite structure and enhance accessibility of large molecules like MBOH to the acid sites. The selectivity of MBYNE from BMOR (Figure 3d) was higher than that of acetone and acetylene suggesting that the acidity was enhanced. According to the ICP result, the Si/Al ratio of BMOR was lower than that of HMOR. The base treatment removed some silicate and generated more silanol groups which are Brönsted acid sites. For NaMOR, HMOR and AMOR which gave low MBOH conversion, products from both acid and basic sites were obtained. However, the major products from NaMOR were acetone and acetylene which are the products from basic sites. The results agreed with the work of Huang and Kaliaguine that the reaction on Na-exchanged zeolites only produced acetone and acetylene.22 The framework negative charge in basic zeolites like NaMOR is compensated by sodium cation which has low electronegativity and then the charge on framework oxygen creates basic properties.23 When NaMOR was transformed to HMOR, the selectivity of MBYNE increased indicating that HMOR is more acidic than NaMOR. These results were consistent with the results from NH3-TPD that the desorbed amount from strong acid sites of HMOR was significantly higher than that of NaMOR. From AMOR, the product selectivity from acid sites was higher than that from the basic sites. Dealumination removed some framework Al atoms which are Lewis acid sites resulting in a decrease of framework charge and, consequently, charge balancing cations which are basic sites. Thus, the basicity in BMOR was lower than NaMOR.
CONCLUSIONS Mordenite in sodium form (NaMOR) was synthesized using rice husk silica and transformed to proton form (HMOR). BMOR was produced by a treatment of NaMOR by base and transformation to proton form; AMOR was obtained from a treatment of HMOR in acid; ABMOR was obtained from a further treatment of AMOR in a base solution followed by a transformation to proton form. All treatment did not destroy the MOR structure but caused an increase in surface area due to formation large pores. From 27Al NMR The peak intensity of extraframework Al corresponding to Lewis acidity decreased in the following order: HMOR > BMOR > ABMOR > AMOR consistent with the result from NH3-TPD. In the catalytic transformation of methylbutynol, the highest conversion and highest selectivity to the product from acid sites was obtained from ABMOR attributing to openness of the structure which enhanced accessibility to acid sites.
SUPPLEMENTARY MATERIAL XRD patterns (Figure 1S); N2 adsorption isotherms of NaMOR, HMOR, BMOR, AMOR and ABMOR (Figure 2S); and a scheme of catalytic decomposition of MBOH (Figure 3S) are available on http://quimicanova.sbq.org.br in the form of a PDF file, with free access.
ACKNOWLEDGEMENTS Scholarship for S. Kulawong is from the Office of the Higher Education Commission, Thailand under the program Strategic Scholarships for Frontier Research Network.
REFERENCES 1. Baerlocher, Ch.; McCusker, L. B.; Database of Zeolite Structures: http://www.iza-structure.org/databases. 2. Roland, E.; Kleinschmit, P. In Ullmann's Encyclopedia of Industrial Chemistry; Bohnet, M; Brinker, C. J., eds.; 6th ed., Wiley: Weinheim, 2003. 3. Viswanadham, N.; Kumar, M.; Micropor. Mesopor. Mater.2006, 92, 31. DOI: http://dx.doi.org/10.1016/j.micromeso.2005.07.049 4. Katada, N.; Kanai, T.; Niwa, M.; Micropor. Mesopor. Mater.2004, 75, 61. DOI: http://dx.doi.org/10.1016/j.micromeso.2004.07.001 5. Groen, J. C.; Sano, T.; Moulijn, J. A.; Pérez-Ramírez, J.; J. Catal.2007, 251, 21. DOI: http://dx.doi.org/10.1016/j.jcat.2007.07.020 6. Preethi, M. E. L.; Revathi, S.; Sivakumar, T.; Manikandan, D.; Divakar, D.; Rupa, A. V.; Palanichami, M.; Catal. Lett.2008, 120, 56. DOI: http://dx.doi.org/10.1007/s10562-007-9249-8 7. González, M. D.; Cesteros, Y.; Salagre, P.; Medina, F.; Sueiras, J. E.; Micropor. Mesopor. Mater.2009, 118, 341. DOI: http://dx.doi.org/10.1016/j.micromeso.2008.09.005 8. Hong, Y.; Fripiat, J. J.; Micropor. Mater.1995, 4, 323. DOI: http://dx.doi.org/10.1016/0927-6513(95)00038-B 9. Li, X.; Prinsa, R.; van Bokhoven, J. A.; J. Catal.2009, 262, 257. DOI: http://dx.doi.org/10.1016/j.jcat.2009.01.001 10. Kulawong, S.; Prayoonpokarach, S.; Neramittagapong, A.; Wittayakun, J.; J. Ind. Eng. Chem.2011, 17, 346. DOI: http://dx.doi.org/10.1016/j.jiec.2011.02.037 11. Kim, G. J.; Ahn, W. S.; Zeolites1991, 11, 745. DOI: http://dx.doi.org/10.1016/0144-2449(91)80357-6 12. Khemtong, P.; Prayoonpokarach, S.; Wittayakun, J.; Suranaree Journal of Science and Technology2007, 14, 367. 13. Supamathanon, N.; Wittayakun, J.; Prayoonpokarach, S.; Supronowicz, W.; Roessner, F.; Quim. Nova2012, 35, 1719. DOI: http://dx.doi.org/10.1590/S0100-40422012000900003 14. Zhang, L.; van Laak, A. N. C.; de Jongh, P. E.; de Jong, K. P.; Micropor. Mesopor. Mater.2009, 126, 115. DOI: http://dx.doi.org/10.1016/j.micromeso.2009.05.034 15. Rouquerol, F.; Rouquerol, J., eds.; Adsorption by powders and porous solids: principles, methodology and application, 6th ed., Academic Press: San Diego, 1999. 16. van Laak, A. N. C.; Gosselink, R. W.; Sagala, S. L.; Meeldijk, J. D.; de Jongh, P. E.; de Jong, K. P.; Appl. Catal. A-Gen.2010, 382, 65. DOI: http://dx.doi.org/10.1016/j.apcata.2010.04.023 17. O'Donovan, A. W.; O'Connor, C. T.; Koch, K. R.; Micropor. Mater.1995, 5, 185. DOI: http://dx.doi.org/10.1016/0927-6513(95)00052-6 18. Lónyi, F.; Valyon, J.; Micropor. Mesopor. Mater.2010, 47, 293. DOI: http://dx.doi.org/10.1016/S1387-1811(01)00389-4 19. Moradi, G. R.; Yaripour, F.; Vale-Sheyda, P.; Fuel Process. Technol.2010, 91, 461. DOI: http://dx.doi.org/10.1016/j.fuproc.2009.12.005 20. Lauron-Pernot, H.; Catal. Rev.2006, 48, 315. DOI: http://dx.doi.org/10.1080/01614940600816634 21. Alsawalha, M.; Roessner, F.; React. Kinet. Catal. Lett.2008, 9, 63. DOI: http://dx.doi.org/10.1007/s11144-008-5249-y 22. Huang, M.; Kaliaguine, S.; Catal. Lett.1993, 18, 373. DOI: http://dx.doi.org/10.1007/BF00765284 23. Barthomeuf, D.; Catal. Rev. Sci. Eng.1996, 38, 512. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access





