Nota Técnica
| Monitoring of organophosphorous pesticide resdues in samples of banana, papaya, and bell pepper |
|
Mariana F. Lemos; Mayara F. Lemos; Henrique P. Pacheco; Rodrigo Scherer*
Departamento de Farmácia, Universidade Vila Velha, Rua Comissário José Dantas de Melo, 21, 29102-770 Vitória - ES, Brasil Recebido em 11/07/2014 *e-mail: rodrigo.scherer@uvv.br The objective of this study was to monitor 11 organophosphorus pesticides in samples of papaya, bell pepper, and banana, commercialized in the metropolitan area of Vitória (ES, Brazil). The pesticides were determined by an optimized and validated method using high performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS). All three samples exhibited a matrix effect for most of the pesticides, mainly with signal suppression, and therefore the calibration curves were produced in matrices. Linearity revealed coefficients of determination (r2) greater than 0.9895 for all pesticides and recovery results ranged from between 76% and 118% with standard deviation no greater than 16%. Precision showed relative standard deviation values lower than 19% and HorRat values lower than 0.7, considering all pesticides. Limits of quantification were less than 0.01 mg/kg for all pesticides. Regarding analysis of the samples (50 of each), none of the pesticides exceeded the maximum residue limit determined by Brazilian legislation. INTRODUCTION With large, fertile lands and favorable weather for agriculture, Brazil is one of the world's main producers and suppliers of food, reaching third place in the global ranking of fruit production (41.5 million tons) in 2010, with significant banana and papaya crops.1 During the same year, the country was one of the largest exporters of fruits and vegetables of Latin America, thus contributing to the growth of Brazilian agribusiness, which was corroborated in 2012, when the export revenues raised to 910 million dollars.2 Among several fruits and vegetables commercialized in Brazil, papaya, banana, and bell pepper achieved great prominence in the country's economy. Carica papaya, commonly known as papaya, is produced in large scale in Brazil, which holds the second place in global production, only behind India.3 Moreover, the exports of this fruit accounted for 23 thousand tons, causing it to be the fifth most exported product in January/2013.4,5 Similarly, banana (Musa spp.) is one of the most produced and consumed fruits worldwide, mainly in Brazil, which has a crop area of 71,253 hectares and exports accounting for 82 thousand tons in 2013.4 It is a fruit known for its nutrients that contribute for elevated nutritional and energetic levels.6 Bell pepper (Capsicuum annuum), a vitamin C source and rich in minerals, stands among the top ten vegetables consumed in Brazil and around the world. In 2011 the Brazilian production was approximately 365.7 million tons and its sales accounted for near 1.5 billion reais.7 Fruits and vegetables are crucial for a healthy diet, due to the presence of significant amounts of nutrients and minerals. However, at the same time they may contain hazardous substances, such as pesticides.8 Agricultural procedures with them still the most common way in order to achieve food production in adequate quantities, as they are an efficient tool against pests that can jeopardize production and lead to food shortage.9 Between 2007 and 2012, the amount of pesticides used in crops was 346.6 thousand tons, making Brazil the world's leader in pesticide commerce in 2010 and the second largest market for pesticides in 2012, the first being the United States.10,11 Amid the classes of most toxic compounds and with greatest incidence in food in Brazil stand the insecticides, among which the organophosphorus compounds are prominent, accounting for more than 36% of the global market.12 With the rising of the organophosphorus, which are less persistent in the environment, organochlorine pesticides, though less hazardous, were substituted for being more resistant in the nature.13 In particular, organophosphorus are highly neurotoxic, presenting inhibitory function of cholinesterase, which controls the nervous system, leading to an elevation in levels of the neurotransmitter acetylcholine at nerve endings and causing neurobehavioral losses in humans.14,15 Besides, pesticides are connected to other chronic health issues, such as cancer and adverse reproductive effects, dermatitis, respiratory problems, and can also be mutagenic and teratogenic.16,17 In Brazil there are several monitoring programs focusing on evaluating food quality and on implementing controlling actions for pesticides residues, as an endeavor to minimize the exposure of the population to these contaminants.18,19 The Program for Analysis of Pesticides Residues in Food (PARA), coordinated by the National Sanitary Surveillance Agency (ANVISA), and the National Program for Residues and Contaminants Control (PNCRC), ran by the Department of Agriculture, Livestock, and Supply (MAPA), are responsible for the monitoring of pesticides residues and for instituting maximum residue limits (MRLs) in food. In recent years, pesticides residues were found in about 65% of analyzed samples, revealing bell pepper to be the vegetable with largest percentage of irregular samples, 90%, and papaya with 20% of unsatisfactory samples.20-22 It is relevant to keep in sight that, in the same year, the most abundant chemical group found in crops was the organophosphorus one, with 38% nonconformities.20-22 In spite of the existence of the above-mentioned programs, they only monitor small quantities of samples at each region, and that is why they do not present the thorough reality of the incidence of pesticides in food. Therefore, the objective of this paper is to validate a method using sample preparation by QuEChERS method23 and high performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS) to monitor organophosphorus residues in samples of banana, papaya, and bell pepper from ten different market from at Vitória and Vila Velha cities (Espírito Santo, Brazil), during a three-month period.
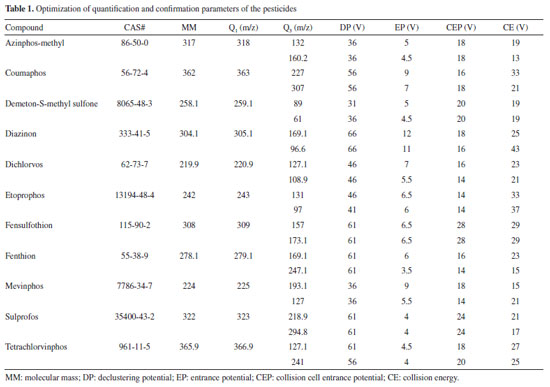
EXPERIMENTAL Chemicals and reagents HPLC grade methanol and acetonitrile were purchased from Tedia (Rio de Janeiro, Brazil) and J.T. Baker (Phillipsburg, USA), respectively. Ultrapure water was obtained from a Direct-Q 3 UV system (Millipore, France). The analyzed organophosphorus standards were bought from Absolute Standards in ampoules with 200 µg mL-1 of each pesticide (azinphos-methyl, mevinphos, sulprofos, demeton-S-methyl sulfone, diazinon, etoprophos, fensulfothion, fenthion, coumaphos, dichlorvos, and tetrachlorvinphos). Formic acid was purchased from Synth (Brazil), whereas acetic acid was obtained from Cromoline (Brazil). For the QuEChERS method, anhydrous magnesium sulfate, primary secondary amine (PSA), and sodium acetate (NaOAc), bought from VETEC (Brazil), Sigma Aldrich (USA), and Panreac (EU), respectively. Samples From September to November, 2013, 50 samples of Cavendish bananas, 50 of papaya, and 50 of green bell pepper were purchased from markets and open markets at the metropolitan area of Vitória (ES, Brazil). Samples were collected every fifteen days in polyethylene bags to store 1.0 kg of each sample (randomly chosen) and conducted immediately to the laboratory for the analyses. Extraction Samples of banana, papaya, and bell pepper were extracted according to the QuEChERS method described by AOAC Official Method 2007.01.24 Each unit of the studied food, from its respective sample, was cut in four pieces. Two were discarded and the remaining ones were homogenized in a food processor. Part of the ground sample was stored under refrigeration for retest. A 15 g portion was inserted into a 50 mL Falcon tube and then 15 mL of acetonitrile with 1% acetic acid, 6 g of anhydrous MgSO4, and 1.5 g of NaOAc were added to the system. Next the system was homogenized in an automatic agitator for 1 min and then centrifuged (Laborline Omega P.I.C microprocessor system centrifuge) at 3000 rpm for 1 min. An aliquot of 6 mL of the supernatant was transferred to a 15 mL Falcon tube that previously contained 150 mg of anhydrous MgSO4 and 50 mg of PSA. Afterwards the system was once again homogenized in an automatic agitator and centrifuged at 3000 rpm for 1 min. The final extract was filtered through a membrane (0.45 µm pore, 13 mm, non-sterile) to a vial and finally injected into the chromatography. Extractions were conducted in duplicates and the samples were stored at low temperatures (-10 ºC to -30 ºC range) prior to the assays. Analysis Chromatographic conditions (HPLC-MS/MS) The analyses were conducted in an Agilent Technologies 1200 Series chromatography, with automatic sampler, quaternary pump, degassing system, and reverse phase C18 column (4.6 mm x 150 mm, 5 µm i.d., Agilent Eclipse XDB), kept at 35 ºC, following AOAC Offical Method 2007.1 with modifications.24 The compounds were separated using as mobile phase Milli-Q water with 0.1% (v/v) formic acid (phase A) and methanol with 0.1% (v/v) formic acid (phase B). The elution gradient started at 40% B, staying at this level for 2 min, followed by linear growth up to 70% B in 5 min and up to 90% B in 8 min, and then kept constant for another 5 min. Reequilibration time was 2 min. Injected sample volume was 20 µL and the flow rate was constant at 0.8 mL min-1. The chromatography was coupled to a mass spectrometer with triple quadrupole (API3200, Applied Biosystems), operating in positive (+5500 V) ionization mode. Ion source temperature was kept at 600 ºC and nitrogen was used as collision gas. Data were collected by multiple reaction monitoring (MRM) and processed by the AnalystTM 5.0 software. HPLC-MS/MS parameters optimization Due to its sensitivity and selectivity for trace analysis in complex matrixes, liquid chromatography is a widespread technique to determine pesticides in large scale using MRM. As a way to obtain maximum sensitivity to identify and quantify the target compounds, the optimization of all mass spectrometer parameters was performed for each analyte in a 0.8 µg mL-1 acetonitrile solution with 0.1% (v/v) acetic acid. Optimization of the parameters is displayed in Table 1.
Method validation Method performance was evaluated according to the reference document DOQ-CGCRE-008, by the National Institute of Metrology, Normalization, and Industrial Quality.25 Evaluated analytical parameters were: selectivity (matrix effect), linearity, precision (repeatability and intermediate precision), accuracy (recovery), and limits of detection (LOD) and quantification (LOQ). The validation process was developed using spiked samples and pesticide-free samples as blank (with previously confirmed absence of pesticides in samples of banana, papaya, and bell pepper). Selectivity and matrix effect Selectivity assesses the studied substance in presence of other interfering compounds in the sample. Matrix effect is observed by the increase or decrease of the detector response of an analyte in the matrix extract when compared to the same substance analyzed in an organic solvent. This evaluation was done by comparing detector responses (peak areas), analyzing a standard solution of pesticides in solvent (acetonitrile 0.1% (v/v) acetic acid) and in extracts of each matrix (banana, papaya, and bell pepper) at three spike levels (0.05, 0.025, and 0.0125 mg L-1), with seven repetitions each. The effect was evaluated by the statistical test ANOVA. Groups with p<0.05 were considered statistically different. Matrix effect was quantified by comparison of the slope of the curve done in matrix extract and in solvent, estimated by the following equation.19 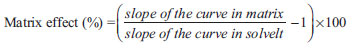 Linearity Linearity corresponds to the ability of a method to present results directly proportional to the concentration of the analyte within a determined range. Linearity was assessed by coefficient of determination (r2), obtained by linear regression. In order to determine the linear range, the statistical method of least squares was applied and points with average residuals smaller than 15% were approved. For the smallest concentration, however, this criterion was raised up to 20%. After evaluation of the linearity, the analytical curve was plotted with at least 5 points by external standard. Precision Precision was tested by repeatability and intermediate precision parameters in three concentration levels (0.05, 0.025, and 0.0125 mg L-1) added to each matrix extract. For the repeatability assays, seven consecutive repetitions in each concentration level for each matrix were performed. For intermediate precision, 21 tests in three different days (7 per day) were done. In order to validate the acceptability of precision, HorRat values were calculated by the following expression: HorRat = RSD/PRSD, where RSD is the relative experimental standard deviation and PRSD is the predicted relative experimental standard deviation, given by Horwitz equation: PRSD = 2(1-0.5logC), where C is the concentration. Precision was considered adequate when the HorRat value remained below 2.0. Limits of detection and quantification LOD reveals the smallest concentration of the studied substance that can be detected, but not necessarily quantified. LOQ, on the other hand, is the lowest concentration by which the analyte can be measured with a certain confidence level. Determination of LOD and LOQ was done by the signal-to-noise ratio method, using proportions of 3:1 and 6:1, respectively, obtained from seven sample blank injections (pesticide-free matrixes). Practical quantification limit (PQL) was defined as the lowest concentration of the linear working range. Recovery Recovery is defined as the proportion of the quantity of the target substance present in or added to the samples from which it is extracted and able to be quantified. Recovery of pesticides was estimated by analysis of spiked samples in three different concentration levels, with seven repetitions each. Each test utilized different concentrations according to the matrix's correspondent linear range: banana (0.009, 0.025, and 0.01 mg kg-1), papaya (0.009, 0.125, and 0.025 mg kg-1), and bell pepper (0.00625, 0.0125, and 0.1 mg kg-1). Recovery was determined by the arithmetic mean of the obtained values from the following equation: 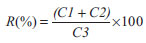 where C1 is the concentration of the analyte in the spiked sample, C2 is the concentration of the analyte in the non-spiked sample, and C3 is the concentration of the analyte added to the spiked sample. Statistical analysis All results are displayed as arithmetic mean ± standard deviation. Data were analyzed by ANOVA, followed by a post-hoc Tukey test and significance was accepted when p<0.05. Analyses were conducted by StatisticaTM 6.0 Statsoft, Inc.
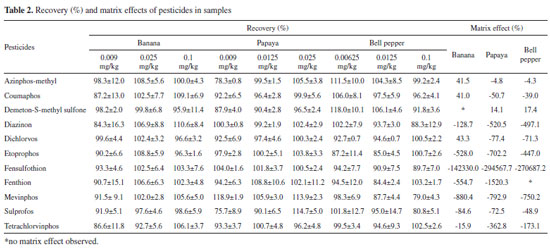
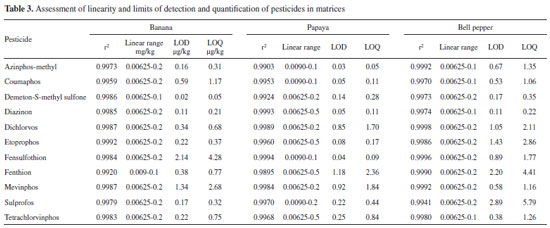
RESULTS AND DISCUSSION All 11 studied pesticides (azinphos-methyl, mevinphos, sulprofos, demeton-S-methyl sulfone, diazinon, fensulfothion, fenthion, etoprophos, coumaphos, dichlorvos, and tetrachlorvinphos) belong to the class of organophosphorus compounds, the major group of pest controllers used in agricultural production. They are organic compounds that contain phosphorous and generally present an ester in their structure.26,27 The pesticides selected for this study are not authorized for banana, papaya, and bell pepper crops;20 consequently, the employed analytical methodology has minimum required performance limit (MRPL) of 0.01 mg kg-1.20 But the fact that these pesticides are still authorized for other crops makes their improper use persistent, bringing along serious consequences for the environment and for human health, as they are classified as highly or moderately toxic. Matrix effect It is known that matrix interferents can increase or decrease the signal of the analyte in chromatography tandem mass spectrometry, generating the so-called matrix effect. Both matrix components and pesticide structure can affect the analyte signal. Therefore, as an alternative, the calibration curve in matrix extracts was used, as it is recommended when matrix effect occurs.28 Results revealed that more than 78% of the analytes presented matrix effect in all different concentrations for all samples, except for demeton-S-methyl sulfone, which did not display matrix effect in banana extracts, and for fensulfothion, in bell pepper. It was also observed that the lowest concentration, 12.5 mg L-1, did not present significant difference when comparing the curves in solvents and in matrixes in the majority of the analyses. Furthermore, from the three examined matrixes, papaya showed more intense matrix effect than banana and bell pepper. Curbelo et al. analyzed matrix effect in banana leaves and proved that all tested analytes (seven organophosphorus and one tiadiazinone) exhibited relevant matrix effect when assessed in samples.6 In other study, matrix effect was evaluated by comparison of the detector response for standards of seven pesticides prepared in solvent with those prepared in matrixes of banana and mango, and the effect was also observed.29 As matrix effect was confirmed for most analytes, it was quantified by the comparison of the slope of the curves done in matrixes of banana, papaya, and bell pepper and the slope of the curve done in pure solvent for each pesticide. Negative or positive values from the equation mean matrix-induced suppression or enhancement of the signal, respectively. Depending on the results, distinct matrix effects can be observed: it was considered low signal suppression when the result was between -20% and 0%; low signal increase when between 0% and 20%; moderated effect when between -50% and -20% or 20% and 50%; and strong signal suppression or signal enhancement when the values were below -50% or above 50%, respectively.19 In this study, most of the pesticides displayed a decrease in signal, that is, from the 31 samples of papaya, banana, and bell pepper, 26 had negative matrix effect results. In a HPLC-MS system, signal suppression occurs more often, since the matrix components decrease the efficiency of spray formation and/or decrease the amount of generated ions.30 Three cases revealed mainly strong signal suppression, but papaya was significantly stronger when compared to banana and bell pepper. Signal suppression or signal enhancement responses for the three matrixes altogether were: low in 16.13% of the samples; moderated in 19.35%; and strong in the 64.52% remaining. These results are shown in Table 2. Linearity As matrix effect was noticed in the majority of the analyzed matrixes, calibration cures were done in matrixes of banana, papaya, and bell pepper for the eleven pesticides in concentrations ranging from 0.2 to 0.00625 mg kg-1, mainly due to matrix effect. Results exhibited that the coefficient of determination for all pesticides were above 0.9903, except for fenthion, which had r2 of 0.9895 in papaya samples (Table 3). Residuals found in the three samples remained below 19.7% for the PQL and below 14.9% for the remaining points, which is under the limits established by ANVISA (lower than 15% for regular points and lower than 20% for the lowermost concentration). The coefficient of determination of the curve done in banana samples were between the 0.9920 and 0.9992 range, which agrees with studies done in Spain, which certified that the detector response for the banana matrix was linear for the tested organophosphorus pesticides tested with coefficients above 0.992.6,31 For papaya samples, on the other hand, r2 varied between 0.9895 and 0.9994. In a study done by Navickiene et al., linearity was also determined by papaya sample blank and the results revealed linear response with r2 ranging from 0.962 to 0.999.28 For bell pepper samples, coefficients of determination were between 0.9940 and 0.9998. As the matrix effect was significant, quantification was developed using the calibration curves obtained by the samples of banana, papaya, and bell pepper. Precision After the conclusion of the 90 analyses, it was observed that all organophosphorus in the three studied samples and in the three concentrations presented RSD below 20%, corroborating other authors who assessed precision in banana and papaya samples.28,32,33 Values of RSD (%) in the repeatability studies varied from 2.8 to 18.4 for banana; from 3.1 to 13.8 for papaya; and from 4.2 to 14.3 for bell pepper, considering the concentration levels mentioned in section 2.5.3. Values of intermediate precision ranged from 4.6 to 19.0 for banana; from 3.8 to 19.0 for papaya; and from 5.0 to 18.2 for bell pepper at the same concentrations. In the same way, HorRat values were smaller than 0.7 considering all performed analyses. Results showed that the majority of the pesticides displayed RSD no greater than 10%, agreeing with Carneiro et al., who validated 128 pesticides in bananas and demonstrated that most of their RSD results were also below 10%.32 Accuracy Method accuracy was estimated by recovery assays that were conducted for the pesticides at three spike levels. For the recovery done in the banana matrix, the concentrations were 0.1, 0.025, and 0.009 mg kg-1; for papaya, 0.025, 0.0125, and 0.009 mg kg-1; and for bell pepper, 0.1, 0.0125, and 0.00625 mg kg-1, with seven replicates for each level in the three samples. These results are displayed in Table 2.
All tested pesticides exhibited recoveries between 76 and 118% with RSD between 0.7 and 16%. Carneiro et al., who used a QuEChERS method along with UHPLC-MS/MS in order to quantify 128 pesticides in bananas, also reached good recovery results (70-120%), with RSD below 20% for most tested analytes.32 Navickiene et al. applied a method based on the dispersion of solid phase matrix and GC-MS to determine seven pesticides in papaya and mango, obtaining average recovery rates from 80 to 146%, with RSD between 1.0 and 28%.29 Limits of detection and quantification LOD and LOQ were evaluated by the injection of banana, papaya, and bell pepper matrix blanks and of the lowermost concentration of the calibration curve, respecting the linear working range of each analyte. LODs of all three matrixes were between 0.02 and 2.14 µg kg-1; 0.03 and 1.18 µg kg-1; and 0.11 and 2.89 µg kg-1, respectively (Table 3). For the LOQs, on the other hand, the results ranged between 0.05 and 4.28 µg kg-1 for banana; 0.05 and 2.36 µg kg-1 for papaya; and 0.22 and 5.79 µg kg-1 for bell pepper.
Sample monitoring Method development was used for monitoring and analyzing pesticides residues in samples of banana, papaya, and bell pepper that were bought during three months at ten markets and open markets. All the 150 samples were extracted by QuEChERS and then analyzed by HPLC-MS/MS. This extraction method, along with chromatography and spectrometry techniques, has been widely used for the analysis of pesticide residues in food, presenting itself as an excellent option for cleaning up complex samples. Peak identification was verified by comparison of retention times, by formation of precursor ion and product ion, and by injection of the spiked sample. No residue of the assessed pesticides was found in the studied samples. Only dichlorvos was detected in a single bell pepper sample, at the first fortnight of October, but in a level below the LOQ. The presence of pesticides in food is so worrying that ANVISA created PARA in 2001 as a way to monitor pesticide residues in several foods in Brazil. In 2010, this program tested 18 crops, including papaya and bell pepper, in 26 states. In Espírito Santo State, 6 papaya samples and 6 bell pepper samples were analyzed. Only one papaya sample was irregular, while all bell pepper samples presented nonconformities. The major pesticide class found in the analyses was the organophosphorus one, which present high toxicity and is not allowed for these crops, causing occupational risk to rural workers and other harmful effects to consumers.21 The report of the activities of the 2011/2022 biennium released by PARA monitored 67 foods in Espírito Santo and 22 irregularities were found, among which the ones related to papaya and bell pepper crops. From the eight analyzed samples of each food, one was illegal for papaya and 5 for bell pepper. In the same way as the previous year, the organophosphorus class was the major chemical group found in the study, accounting for 38% of the irregularities. Another governmental agency that works with pesticide residue monitoring is MAPA, which coordinates the PNCRC of products with vegetal origin. During the 2011/2012 crop year, PNCRC evaluated 23 crops, including banana, papaya, and bell pepper, revealing 100%, 91.3%, and 37.3% conformity rates, respectively. Papaya was the only analyzed sample from Espírito Santo, with 90.81% conformity, as 9 were irregular from the 98 tested.34 24 samples of papaya were also monitored in the state, during the 2012/2013 crop year, and 7 presented nonconformities.35 After the comparison between the data of this study with those of PARA and PNCRC, it can be concluded that the results were analogous. PARA did not find any residues of the pesticides analyzed by this study when testing the same crops. Likewise PNCRC revealed 100% conformity for all three samples regarding the organophosphorus pesticides analyzed by both studies (azinphos methyl, coumaphos, demeton, diazinon, dichlorvos, etoprophos, fenthion, mevinphos, and sulprofos). Therefore it can be noticed that the producers from Espírito Santo either are not using these pesticides or are utilizing them correctly; nevertheless, it is important to keep in sight that the pesticides assessed by this study are not allowed by Brazilian legislation for these crops.
CONCLUSION The developed and validated multiresidue method for the simultaneous determination of 11 organophosphorus in samples of banana, papaya, and bell pepper was efficient for this application. Significant matrix effect with signal suppression for most compounds was observed; thus, the analytical curves were done in the matrixes. None of the pesticides in the monitored samples exceeded the allowed limit, which indicates that these forbidden compounds are not being used by their producers in Espírito Santo.
ACKNOWLEDGMENTS This research was financed by FAPES. The authors thank Tommasi Analítica for their support and cooperation in the chromatographic analyses.
REFERENCES 1. http://www.agricultura.pr.gov.br/arquivos/File/deral/Prognosticos/fruticultura_2012_13.pdf, accessed in January, 2014. 2. http://www.agricultura.gov.br/arq_editor/projecoes%20-20versao%20atualizada.pdf, accessed in January, 2014. 3. http://faostat.fao.org/site/339/default.aspx, accessed in November, 2013. 4. http://www.cepea.esalq.usp.br/hfbrasil, accessed in January, 2014. 5. http://www.sebrae.com.br/setor/fruticultura/exportacoes-de-frutas-fevereiro-2013, accessed in December, 2013. 6. Curbelo, M. A. G.; Borges, J. H.; Pérez, L. M. R.; Delgado, M. A. R.; Food Chem. 2011, 1083, 125. DOI: http://dx.doi.org/10.1016/j.foodchem.2010.09.083 7. http://www.agricultura.gov.br/arq_editor/file/camaras_setoriais/Hortalicas/Dados_Economicos/ABCSEM%2011.pdf, accessed in November, 2013. 8. Knezevic, Z.; Serdar, M.; Food Control 2009, 419, 20. DOI: http://dx.doi.org/10.1016/j.foodcont.2008.07.014 9. Bhanti, M., & Taneja, A.; Chemosphere 2007, 63, 69. DOI: http://dx.doi.org/10.1016/j.chemosphere.2007.04.071 10. http://www.ibama.gov.br/phocadownload/Qualidade_Ambiental/pesticides_commercialized_in_brazil_2009.pdf, accessed in November, 2013. 11. http://www.sindag.com.br/noticia.php?News_ID=2337, accessed in January, 2014. 12. Ghosh, P. G.; Sawant N. A.; Patil, S. N.; Aglave, B. A.; International Journal of Biotechnology and Biochemistry 2010, 871, 6. 13. Rodrigues, F. M.; Mesquita, P. R. R.; Oliveira, L. S.; Oliveira, F. S.; Filho, A. M.; Pereira, P. A. P.; Andrade, J. B.; Microchem J. 2011, 56, 98. DOI: http://dx.doi.org/10.1016/j.microc.2010.11.002 14. Fenik, J.; Tankiewicz, M.; Biziuk, M.; Trends Anal. Chem. 2011, 814, 30. http://dx.doi.org/10.1016/j.trac.2011.02.008 15. Ahlbom, J.; Fredriksson, A.; Eriksson, P.; Brain Res. 1995, 13, 677. DOI: http://dx.doi.org/10.1016/0006-8993(95)00024-k 16. Quackenbush, R.; Hackley, B.; Dixon, J.; Journal of Midwifery & Women's Health 2006, 3, 51. DOI: http://dx.doi.org/10.1016/j.jmwh.2005.10.004 17. Nougadère, A.; Reninger, J.; Volatier, J.; Leblanc, J.; Food Chem. Toxicol. 2011, 1484, 49. DOI: http://dx.doi.org/10.1016/j.fct.2011.03.024 18. Jardim, A. N. O.; Caldas, E. D.; Food Control 2012, 607, 25. DOI: http://dx.doi.org/10.1016/j.foodcont.2011.11.001 19. Kmellár, B.; Fodor, P.; Pareja, L.; Ferrer, C.; Martinez-Uroz, M.A.; Valverde, A.; Fernandez-Alba, A.R.; J. Chromatogr. A 2008, 37, 1215. DOI: http://dx.doi.org/10.1016/j.chroma.2008.10.121 20. http://portal.anvisa.gov.br/wps/content/Anvisa+Portal/Anvisa/Inicio/Agrotoxicos+e+Toxicologia/Assuntos+de+Interesse/Monografias+de+Agrotoxicos, accessed in January, 2014. 21. http://portal.anvisa.gov.br/wps/wcm/connect/b380fe004965d38ab6abf74ed75891ae/Relat%C3%B3rio+PARA+2010+-+Vers%C3%A3o+Final.pdf?MOD=AJPERES, accessed in January, 2014. 22. http://portal.anvisa.gov.br/wps/wcm/connect/d480f50041ebb7a09db8bd3e2b7e7e4d/Relat%C3%B3rio%2BPARA%2B2011-12%2B-%2B30_10_13_1.pdf?MOD=AJPERESm, accessed in January, 2014. 23. Prestes, O. D.; Friggi, C. A.; Adaime, M. B., Zanella, R. Quim. Nova 2009, 32, 1620. DOI: http://dx.doi.org/10.1590/S0100-40422009000600046 24. http://www.weber.hu/PDFs/QuEChERS/AOAC_2007_01.pdf, accessed in September, 2013. 25. DOQ-CGCRE-008/INMETRO, 2011. 26. Jokanović, M.; Toxicology 2001, 139, 166. DOI: http://dx.doi.org/10.1016/s0300-483x(01)00463-2 27. Jokanović, M. Toxicol. Lett. 2009, 107, 190. DOI: http://dx.doi.org/10.1016/j.toxlet.2009.07.025 28. Kirchner, M.; Huskova, R.; Matisova, E.; Mocak, J.; Journal of Chromatography A 2008, 271, 1186. DOI: http://dx.doi.org/10.1016/j.chroma.2007.08.089 29. Navickiene, S.; Aquino, A.; Bezerra, D. S. S.; J. Chromatogr. Sci. 2010, 48. DOI: http://dx.doi.org/10.1093/chromsci/48.9.750 30. Gosetti, F.; Mazzucco, E.; Zampieri, D.; Gennaro, M. C.; Journal of Chromatography A 2010, 3929, 1217. DOI: http://dx.doi.org/10.1016/j.chroma.2009.11.060 31. Hernández-Borges, J.; Cabrera, J. C.; Rodríguez-Delgado, M. A.; Estrella, M.; Hernández-Suárez, E. M.; Saúco, V. G.; Food Chemistry 2009, 313, 113. DOI: http://dx.doi.org/10.1016/j.foodchem.2008.07.042 32. Carneiro, R. P.; Oliveira, F. A. S.; Madureira, F. D.; Silva, G.; Souza, W. R.; Lopes, R. P.; Food Control 2013, 413,33. DOI: http://dx.doi.org/10.1016/j.foodcont.2013.02.027 33. Plácido, A.; Paíga, P.; Lopes, D. H.; Correia, M.; Delerue-Matos, C.; J. Agric. Food Chem. 2013, 325, 61. DOI: http://dx.doi.org/10.1021/jf304027s 34. http://www.agricultura.gov.br/arq_editor/file/CRC/IN%20de%20Resultados%20ano%20safra%202011-2012%20.pdf, accessed in December, 2013. 35. http://pesquisa.in.gov.br/imprensa/servlet/INPDFViewer?jornal=1&pagina=6&data=02/09/2013&apm;captchafield= |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access