Artigo
| Application of sulfonic acid functionalized nanoporous silica (SBA-Pr-SO3H) for the preparation of 4,6-diarylpyrimidin-2(1H)-ones |
|
Ghodsi Mohammadi ZiaraniI,*; Masoumeh AziziI; Parvin HajiabbasiI; Alireza BadieiII
IDepartment of Chemistry, Alzahra University, Vanak Square, P.O. Box 1993893973, Tehran, Iran Recebido em 09/05/2014 *e-mail: gmziarani@hotmail.com In this work, we report the Biginelli-type reaction between various aldehydes, acetophenones and urea systems in the presence of sulfonic acid functionalized silica (SBA-Pr-SO3H) under solvent-free conditions, which led to 4,6-diarylpyrimidin-2(1H)-ones derivatives. SBA-Pr-SO3H with a pore size of 6 nm was found to be an efficient heterogeneous solid acid catalyst for this reaction which led to high product yields, was environmentally benign with short reaction times and easy handling. INTRODUCTION The multi-functionalized dihydropyrimidinones (DHPMs) and dihydropyrimidines synthesis in a Biginelli or Biginelli-like three-component-coupling reaction are of considerable interest.1-9 In addition, it also provides an efficient access to the corresponding pyrimidines which are found in a broad variety of biologically active molecules and shown biological and therapeutic activities10,11 such as antitumoral agents12-17 and HIV inhibitors.18,19 Some of biological active dihydropyrimidinone compounds were represented in the Scheme 1, for example, flucytosine is an antibacterial and antifungal active compound, monastrol is a drug which inhibit vitamin Eg5 formation, nifedipine is a Ca regulator drug utilized for blood pressure treatments, and batzclladine B is a dihydropyrimidinone core containing natural product derived from marines which is anti HIV virus drug.20-23
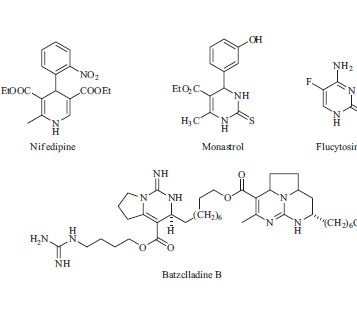 Scheme 1. Representative examples of biological active dihydropyrimidinone compounds
Solid acid catalysts such as ordered mesoporous materials24,25 have outstanding properties such as their environmental compatibility in terms of less toxicity of waste, reusability, simplicity in handling, non-corrosiveness and ease of isolation of the products in terms of heterogeneity. Thus, the surface of SBA-15 was modified by acidic functional groups (e.g., -SO3H) to prepare nano-solid acid catalyst that was gratefully used in organic synthesis.26 As mentioned in our recent studies in the application of SBA-Pr-SO3H,27-30 among different catalysts used for these Biginelli-type reactions such as Bi(TFA)3,31 H2NSO3H,32 I2,33 nanocomposites ZrO2-Al2O3-Fe3O4,34 iron,35,36 and manganese-containing periodic mesoporous organosilica37 we used sulfonic acid functionalized silica (SBA-Pr-SO3H) as an efficient heterogeneous catalyst to prepare 4,6-diarylpyrimidin-2(1H)-ones derivatives in excellent yields.
EXPERIMENTAL All chemicals were obtained commercially and utilized without further purification. IR spectra were recorded from KBr disk using a FT-IR Bruker Tensor 27 instrument. Melting points were measured by using the capillary tube method with an electro thermal 9200 apparatus. The 1H NMR (250 MHz) was recorded on a Bruker DPX at 250 MHz using TMS as an internal standard. Gas chromatography-mass spectrometry (GC-MS) analysis was achieved on an Agilent 6890-5973 GC/MS detector. Also, SEM analysis was performed on a Philips XL-30 field-emission scanning electron microscope operated at 16 kV while Transmission electron microscopy (TEM) analysis was performed on a Tecnai G2 F30 at 300 kV. Preparation of SBA-15-Pr-SO3H The modified SBA-Pr-SO3H used as a solid acid catalyst in the following reaction was synthesized and functionalized according to our previous report.38 General procedure for the preparation of 4,6-diarylpyrimidin-2(1H)-ones derivatives (4a-4i): The SBA-Pr-SO3H (0.05 g) was activated in vacuum at 100 ºC and then cooled to room temperature. The aromatic aldehydes 1 (1 mmol), acetophenones 2 (1 mmol) and urea 3 (1 mmol), and SBA-Pr-SO3H (0.05 g) were heated at 110 ºC for the appropriated time, as mentioned in Table 2. The completion of the reaction was monitored by TLC. The mixture was cooled to room temperature, the crude product was dissolved in hot ethanol and then the catalyst was removed by filtration. Ultimately, pure crystals of compounds 4a-4i were obtained from filtrates.
4,6-Diphenyl-pyrimidin-2(1H)-one (4a): IR (KBr, vmax): 3420, 3285, 3084, 3005, 2894, 2856, 2741, 1618, 1577, 1495, 1455, 1397, 1338, 1069, 823, 681 cm-1. 1H NMR (250 MHz, CDCl3): δ 7.50-7.61 (m, 7H, ArH& H-5), 8.13-8.16 (m, 4H, ArH), 12.07 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δ 164.9, 157.0, 133.2, 132.3, 128.9, 128.0, 100.8, 56.0.39 EI-MS: 248 (M+), 171, 94, 77. 4-(4-Chlorophenyl)-6-phenyl-pyrimidin-2(1H)-one (4b): IR (KBr, vmax): 3423, 3385, 3104, 3058, 3002, 2892, 2743, 1614, 1568, 1490, 1437, 1391, 1333, 1089, 990, 821, 765, 683 cm-1. 1H NMR (250 MHz, CDCl3): δ 7.49 (d, J = 7.5 Hz, 2H, ArH), 7.52-7.62 (m, 4H, ArH & H-5), 8.12 (d, J = 7.5 Hz, 2H, ArH), 8.18 (d, J = 7.5 Hz, 2H, ArH), 12.17 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6): δ 160.2, 144.9, 135.6, 129.0, 127.7, 109.6.39 EI-MS: 282 (M+), 171, 111, 94, 77. 4-(4-Methylphenyl)-6-phenyl-pyrimidin-2(1H)-one (4c):39 IR (KBr, vmax): 3410, 2794, 1674, 1592, 848, 773 cm-1. 1H NMR (400 MHz, DMSO-d6): δ 8.11-8.20 (m, 4H, ArH), 7.49-7.60 (m, 6H, ArH), 2.36 (s, 3H, CH). 13C NMR (100 MHz, DMSO-d6): δ 142.1, 131.9, 129.9, 129.3, 128.0, 128.0, 21.5;. EI-MS: 262 (M+), 247, 171, 94, 92, 77. 4-(3-Methylphenyl)-6-phenyl-pyrimidin-2(1H)-one (4d): IR (KBr, vmax): 3423, 3293, 3093, 3005, 2897, 1618, 1491, 1339, 1202, 1154, 1070, 991, 808, 774, 692 cm-1. 1H NMR (250 MHz, CDCl3): δ 2.39 (s, 3H, CH3), 7.49 (d, J = 7.5, 2H, ArH), 7.53-7.58 (m, 4H, ArH & H-5), 7.91-8.12 (t, 2H, ArH), 8.14 (d, J = 5.75 Hz, 2H, ArH), 12 (s, 1H, NH). EI-MS: 262 (M+), 247, 171, 94, 77. 4-(4-Hydroxyphenyl)-6-phenyl-pyrimidin-2(1H)-one (4e):39 IR (KBr, vmax): 3331, 3300, 2928, 1659, 1616, 779 cm-1. 1H NMR (400 MHz, DMSO-d6): δ 11.88 (hr, s, lH, NH), 10.21 (s, lH, OH), 8.08 (m, 4H, ArH), 7.56 (d, J= 7.2 Hz, 3H, ArH), 7.42 (s, lH, ArH), 6.91 (d, J = 8.8 Hz, 2H, ArH) 13C NMR (100 MHz, DMSO-d6): δ 161.0, 131.5, 129.7, 128.9, 127.6, 115.7. EI-MS: 264 (M+), 247, 171, 94, 77. 4-(4-Methoxy-phenyl)-6-phenyl-pyrimidin-2(1H)-one (4f): IR (KBr, vmax): 3320, 3096, 1615, 1513, 809, 744 cm-1. 1H NMR (400 MHz, DMSO-d6): δ 8.09 (tri, 2H,ArH), 7.99 (d, J=7.6 Hz, 2H, ArH), 7.59 (d, J= 5.2 Hz, 3H, ArH), 7.39 (d, J= 8.0 Hz, 2H, ArH), 7.14 (m, lH, ArH), 2.48 (s, 3H, CH). 13C NMR (100 MHz, DMSO-d6): δ 162.3, 131.5, 129.5, 128.9, 127.7, 117.4, 55.6. EI-MS: 278 (M+), 247, 171, 108, 94, 77. 4-(Phenyl)-6-(4-Methylphenyl)-pyrimidin-2(1H)-one (4g): IR (KBr, vmax): 3436, 3280, 3096, 3005, 2901, 2745, 1616, 1512, 1459, 1338, 1180, 919, 808, 774, 691 cm-1. 1H NMR (250 MHz, CDCl3): δ 2.38 (s, 3H, CH3), 7.35 (d, J = 7.5 Hz, 2H, ArH), 7.51-7.58 (m, 4H, ArH & H-5), 8.05 (d, J = 7.5 Hz, 2H, ArH), 8.13 (d, J = 7.5 Hz, 2H, ArH), 12 (s, 1H, NH). EI-MS: 262 (M+), 247, 171, 94, 77. 4-(4-Chlorophenyl)-6-(4-nitrophenyl)-pyrimidin-2(1H)-one (4h): IR (KBr, nmax): 3412, 3192, 2921, 1612, 1548, 1515, 1456, 1348 cm-1. 1H NMR (300 MHz, DMSO-d6): δ 6.90-7.83 (m, 9H, ArH & H-5), 9.98 (s, 1H, NH). EI-MS: 327 (M+), 247, 171, 77. 4-(Chlorophenyl)-6-(4-Methoxyphenyl)-pyrimidin-2(1H)-one (4i):31 IR (KBr) 3429, 3 100, 289 0, 1617, 1582, 810, 600 cm-1; 1H NMR (CDCl3, 200 M Hz) d 3.8 (s, 3H, OCH3 ), 7.09 (d, J =8.4 Hz, 2H, Ar), 7.53 (s, 1H, =CH-), 7.61 (d, J = 8.09 Hz, 2H, Ar), 8.17 (m, 4H, Ar), 1 2.0 (br, 1H, NH); 13C NMR (CDCl3, 50 MHz) d 100.4, 115.1, 115.4, 128.8, 129.7, 129 .9, 130.2, 130.4, 130.7, 131.2, 137.1, 160.7, 163.0.
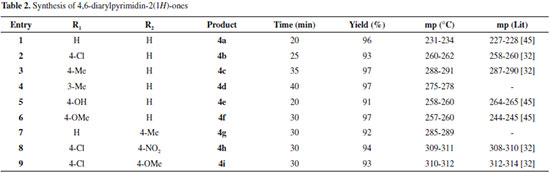
RESULTS AND DISCUSSION In continuation of our interest in the developing of green methodologies and the synthesis of diverse heterocyclic compounds of pharmaceutical importance, we report herein the synthesis of some 4,6-diarylpyrimidin-2(1H)-ones 4a-4i derivatives under neat condition. Thus, a three-component reaction of aromatic aldehydes 1, acetophenones 2 and urea 3, in the presence of sulfonic acid functionalized silica (SBA-Pr-SO3H) as catalyst at 110 ºC, has been shown to give 4,6-diarylpyrimidin-2(1H)-ones derivatives 4a-4i in excellent yields (Scheme 2). However, 3,4-dihydropyrimidin-2(1H)-ones has been prepared40 from the reaction of aromatic aldehydes, aromatic ketones, and urea using a Lewis acid (FeCl3•6H2O-TMSCl) as catalyst; but we did not find 3,4-dihydropyrimidin-2(1H)-ones in our investigation. In addition, the crucial role of catalysis, solvent effects, mechanisms, kinetics and etc. in the Biginelli reactions are discussed in the recently published articles.41-45
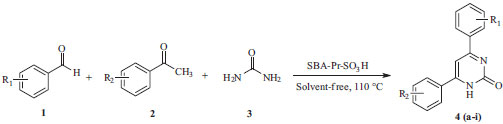 Scheme 2. Synthesis of 4,6-diarylpyrimidin-2(1H)-ones using SBA-Pr-SO3H as catalyst
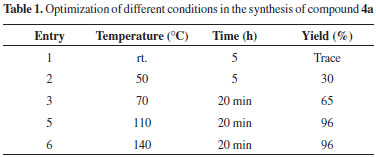
As can be seen in Table 1, we found that the yield of 4a improved and the reaction time was shortened when the reaction would proceed at 110 ºC under solvent free condition (entries 1-5). After optimization of the reaction condition, the generality of this method was demonstrated with respect to six different aromatic aldehydes and four acetophenones, and the results were summarized in Table 2. On the base of our investigation, we also find out that the nature of substitute groups in the aldehydes or ketones has no significant effect in this reaction. Completion of the reaction was monitored by TLC, the crude product was dissolved in hot ethanol, the heterogeneous solid catalyst was removed by simple filtration, and the pure crystals of the desired products (4a-4i) were obtained and characterize by IR, mass spectrometric analysis and 1H & 13C NMR spectroscopic analyses. The acid catalyst can also be reused without significant loss of activity by simple washing subsequently with diluted acid solution, water and acetone. According to proposed mechanism in Scheme 3, the condensation of aryl aldehyde 1 and urea 3 catalyzed by SBA-Pr-SO3H lead to the preparation of iminium intermediate 7 that followed by the nucleophilic addition of acetophenones 2 to give the intermediate 8, which consequently undergoes the ring closure by the nucleophilic attack of the amine onto the carbonyl group. Subsequently proton transfer, dehydration and oxidation results in the formation of adduct compound 4.
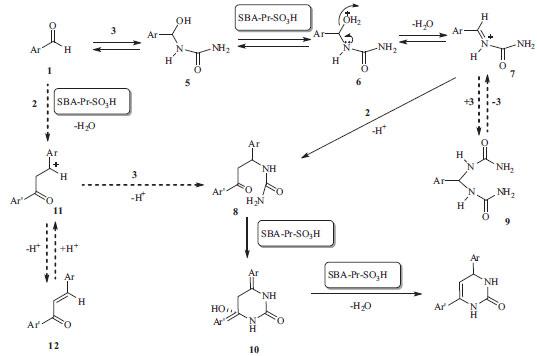 Scheme 3. Proposed mechanism for the synthesis of adduct compound 4
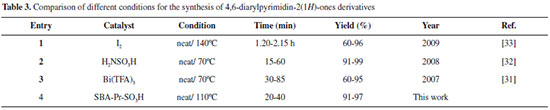
As illustrated in the Table 3, comparison of the effectiveness of various catalysts used in the synthesis of 4,6-diarylpyrimidin-2(1H)-ones derivatives is shown the advantages of current methodology. The simplicity, low reaction time and high yields of products under a green condition are resulted from the efficiency of sulfonic acid functionalized silica (SBA-Pr-SO3H) as a heterogeneous nano catalyst.
As preparation of SBA-Pr-SO3H is illustrated in Figure 1, the calcined SBA-15 silica was functionalized with (3-mercaptopropyl) trimethoxysilane (MPTS) and then, the thiol groups were oxidized to sulfonic acid by hydrogen peroxide. Different methods such as TGA, BET and CHN methods were employed to analyze the surface of the catalyst which was demonstrated that the organic groups (propyl sulfonic acid) were immobilized into the pores.29
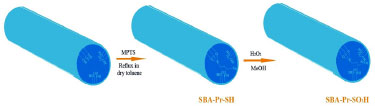 Figure 1. Preparation of SBA-Pr-SO3H
The SEM and TEM images of SBA-Pr-SO3H are illustrated in Figure 2. Uniform particles about 1 mm is shown in the SEM image (Figure 2a) as the same for SBA-15. It could be find out that morphology of solid was saved without change during the surface modification. Also, the TEM image (Figure 2b) shows the parallel channels as the pores configuration of SBA-15, indicating that the pore of modified catalyst was not collapsed. Nanopore size of SBA-Pr-SO3H act as a nano-reactor in the syntheses of 4,6-diarylpyrimidin-2(1H)-ones compounds.
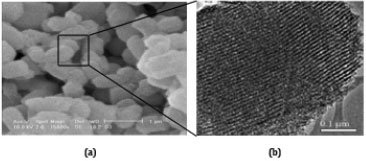 Figure 2. SEM image (a) and TEM image (b) of SBA-Pr-SO3H
CONCLUSION In conclusion, a mild and efficient protocol for the synthesis of 4,6-diarylpyrimidin-2(1H)-ones derivatives has been developed through a one-pot Biginelli reaction under neat condition according to green chemistry using SBA-Pr-SO3H as a nano reactor with the pore size of 6 nm. In addition, the results are shown that the reaction takes place easily in the nano-pores of catalyst.46 The attractive features of this simple procedure are short reaction time, excellent yields, simple workup, the reusability of catalyst, and non-chromatographic purification of products.
SUPPLEMENTARY MATERIAL The spectral data is available in .pdf format at http://quimicanova.sbq.org.br/ with free access.
ACKNOWLEDGEMENTS We gratefully acknowledge the financial support from the Research Council of Alzahra University and the University of Tehran.
REFERENCES 1. Kappe, C. O.; Acc. Chem. Res. 2000, 33, 879. DOI: http://dx.doi.org/10.1021/ar000048h PMID: 11123887 2. Bose, D. S.; Fatima, L.; Mereyala, H. B.; J. Org.Chem. 2003, 68, 587. DOI: http://dx.doi.org/10.1021/jo0205199 PMID: 12530887 3. Kappe, C. O.; Roschger, P.; J. Heterocycl. Chem. 1989, 26, 1555. DOI: http://dx.doi.org/10.1002/jhet.5570260609 4. Karimi, B.; Mobaraki, A.; Mirzaei, H. M.; Zareyee, D.; Vali, H.; ChemCatChem 2014, 6, 212. DOI: http://dx.doi.org/10.1002/cctc.201300739 5. Oliverio, M.; Costanzo, P.; Nardi, M.; Rivalta, I.; Procopio, A.; ACSSustainable Chem. Eng. 2014, 2, 1228. DOI: http://dx.doi.org/10.1021/sc5000682 6. Alvim, H. G. O.; de Lima, T. B.; de Oliveira, H. C. B.; Gozzo, F. C.; de Macedo, J. L.; Abdelnur, P. V.; Silva, W. A.; DaSilveira Neto, B. A.; ACS Catal. 2013, 3, 1420. DOI: http://dx.doi.org/10.1021/cs400291t 7. Sahu, P. K.; Sahu, P. K.; Gupta, S. K.; Agarwal, D. D.; Ind. Eng. Chem. Res. 2014, 53, 2085. DOI: http://dx.doi.org/10.1021/ie402037d 8. Pramanik, M.; Bhaumik, A.; ACS Appl. Mater. Interfaces 2014, 6, 933. DOI: http://dx.doi.org/10.1021/am404298a PMID: 24372168 9. Shen, Z. -L.; Xu, X. -P.; Ji, S. -J.; J. Org. Chem. 2010, 75, 1162. DOI: http://dx.doi.org/10.1021/jo902394y PMID: 20085235 10. Voronina, T. A.; Gordiichuk, G. N.; Andronati, S. A.; Garibova, T. L.; Zhilina, Z. I.; Khim.-Farm, Z.; Pharm. Chem. J. 1981, 15, 495. DOI: http://dx.doi.org/10.1007/BF00758535 11. Nagaraj, A.; Reddy, C. S.; J. Heterocycl.Chem. 2007, 44, 1181. DOI: http://dx.doi.org/10.1002/jhet.5570440534 12. Carraro, F.; Pucci, A.; Naldini, A.; Schenone, S.; Bruno, O.; Ranise, A.; Bondavalli, F.; Brullo, C.; Fossa, P.; Menozzi, G.; Mosti, L.; Manetti, F.; Botta, M.; J. Med. Chem. 2004, 47, 1595. DOI: http://dx.doi.org/10.1021/jm034257u PMID: 15027847 13. Manetti, F.; Santucci, A.; Locatelli, G. A.; Maga, G.; Spreafico, A.; Serchi, T.; Orlandini, M.; Bernardini, G.; Caradonna, N. P.; Spallarossa, A.; Brullo, C.; Schenone, S.; Bruno, O.; Ranise, A.; Bondavalli, F.; Hoffmann, O.; Bologna, M.; Angelucci, A.; Botta, M.; J. Med. Chem. 2007, 50, 5579. DOI: http://dx.doi.org/10.1021/jm061449r PMID: 17929792 14. Lacotte, P.; Buisson, D. -A.; Ambroise, Y.; Eur.J. Med. Chem. 2013, 62, 722. DOI: http://dx.doi.org/10.1016/j.ejmech.2013.01.043 PMID: 23454514 15. Lauro, G.; Strocchia, M.; Terracciano, S.; Bruno, I.; Fischer, K.; Pergola, C.; Werz, O.; Riccio, R.; Bifulco, G.; Eur. J. Med. Chem. 2014, 80, 407. DOI: http://dx.doi.org/10.1016/j.ejmech.2014.04.061 PMID: 24794772 16. Escande, V.; Garoux, L.; Grison, C.; Thillier, Y.; Debart, F.; Vasseur, J. -J.; Boulanger, C.; Grison, C.; Appl. Catal., B 2014, 146, 279. DOI: http://dx.doi.org/10.1016/j.apcatb.2013.04.011 17. Lacotte, P.; Puente, C.; Ambroise, Y.; ChemMedChem 2013, 8, 104. DOI: http://dx.doi.org/10.1002/cmdc.201200417 PMID: 23132843 18. Mugnaini, C.; Alongi, M.; Togninelli, A.; Gevariya, H.; Brizzi, A.; Manetti, F.; Bernardini, C.; Angeli, L.; Tafi, A.; Bellucci, L.; Corelli, F.; Massa, S.; Maga, G.; Samuele, A.; Facchini, M.; Clotet-Codina, I.; Armand-Ugón, M.; Esté, J. A.; Botta, M.; J. Med. Chem. 2007, 50, 6580. DOI: http://dx.doi.org/10.1021/jm0708230 PMID: 18052319 19. Mai, A.; Artico, M.; Rotili, D.; Tarantino, D.; Clotet-Codina, I.; Armand-Ugon, M.; Ragno, R.; Simeoni, S.; Sbardella, G.; Nawrozkij, M. B.; Samuele, A.; Maga, G.; Este, J. A.; J. Med. Chem. 2007, 50, 5412. DOI: http://dx.doi.org/10.1021/jm070811e PMID: 17910429 20. Pandiarajan, K.; Chitra, S.; Tetrahedron Lett. 2009, 50, 2222. DOI: http://dx.doi.org/10.1016/j.tetlet.2009.02.162 21. Mayer, T. U.; Kapoor, T. M.; Haggarty, S.; King, R. W.; Schreiber, S. L.; Mitchison, T. J.; Science 1999, 286, 971. DOI: http://dx.doi.org/10.1126/science.286.5441.971 PMID: 10542155 22. Boumoud, T.; Boumoud, B.; Rhouati, S.; Belfaitah, A.; Debachea, A.; Mosset, P.; Acta Chim. Slov. 2008, 55, 617. 23. Rovnyak, G. C.; Atwal, K. S.; Hedberg, A.; Kimball, S. D.; Moreland, S.; Gougoutas, J. Z.; Reilly, B. C. O.; Schwartz, J.; Malley, M. F.; J. Med. Chem. 1992, 35, 3254. DOI: http://dx.doi.org/10.1021/jm00095a023 PMID: 1387168 24. Díaz, I.; Márquez-Alvarez, C.; Mohino, F.; Pérez-Pariente, J.; Sastre, E.; J. Catal. 2000, 193, 283. DOI: http://dx.doi.org/10.1006/jcat.2000.2898 25. Maheswari, R.; Shanthi, K.; Sivakumar, T.; Narayanan, S.; Appl. Catal., A 2003, 248, 291. DOI: http://dx.doi.org/10.1016/S0926-860X(03)00182-0 26. Burri, D. R.; Jun, K. W.; Kim, Y. H.; Kim, J. M. Park, S. E.; Yoo; J. S.; Chem. Lett. , 2002, 212. DOI: http://dx.doi.org/10.1246/cl.2002.212 27. Mohammadi Ziarani, G.; Badiei, A.; Dashtianeh, Z.; Gholamzadeh, P.; Hosseini Mohtasham, N.; Res. Chem. Intermed. 2012, 39, 3157. DOI: http://dx.doi.org/10.1007/s11164-012-0828-y 28. Mohammadi Ziarani, G.; Badiei, A.; Khaniania, Y.; Haddadpour, M.; Iran J. Chem. Chem. Eng. 2010, 29, 1. 29. Lashgari, N.; Mohammadi Ziarani, G.; Badiei, A.; Gholamzadeh, P.; Eur. J. Chem. 2012, 3, 310. DOI: http://dx.doi.org/10.5155/eurjchem.3.3.310-313.659 30. Mohammadi Ziarani, G.; Badiei, A.; Shakiba Nahad, M.; Hassanzadeh, M.; Eur. J. Chem. 2012, 3, 433. DOI: http://dx.doi.org/10.5155/eurjchem.3.4.433-436.673 31. Khosropour, A. R.; Baltork, M. I.; Ghorbankhani, H.; Catal. Commun. 2006, 7, 713. DOI: http://dx.doi.org/10.1016/j.catcom.2006.02.001 32. Heravi, M. M.; Ranjbar, L.; Derikvand, F.; Alimadadi, B.; Mol. Divers. 2008, 12, 191. DOI: http://dx.doi.org/10.1007/s11030-008-9089-5 PMID: 18830679 33. Ren, Y. R.; Cai, C.; Monatsh. Chem. 2009, 140, 49. DOI: http://dx.doi.org/10.1007/s00706-008-0011-8 34. Wang, A.; Liu, X., ; Sua, Z. Jing, H.; Catal. Sci. Technol. 2014, 4, 71. DOI: http://dx.doi.org/10.1039/C3CY00165B 35. Zhang, L.; Zhang, Z.; Liu, Q.; Liu, T.; Zhang, G.; J. Org. Chem. 2014, 79, 2281. DOI: http://dx.doi.org/10.1021/jo4023588 PMID: 24517724 36. Ramos, L. M.; Guido, B. C.; Nobrega, C.; Corrêa, J. R.; Silva, R. G.; de Oliveira, H. C. B.; Gomes, A. F.; Gozzo, F. C.; DaSilveira Neto, B. A.; Chem. Eur. J. 2013, 19, 4156. DOI: http://dx.doi.org/10.1002/chem.201204314 37. Elhamifar, D.; Shábani, A.; Chem. Eur. J. 2014, 20, 3212. DOI: http://dx.doi.org/10.1002/chem.201304349 38. Kidwai, S.; Saxena, S.; Khalilur, M.; Thukral, S. S.; Eur. J. Med. Chem. 2005, 40, 816. DOI: http://dx.doi.org/10.1016/j.ejmech.2005.02.009 PMID: 16122583 39. Hui, W.; Xiu-mei, C.; Yu, W.; Ling, Y.; Hai-qiang, X.; Hua-hong, X.; Li-ling, P.; Rui, M.; Cai-hui, Y.; J. Chem. Res. 2008, 12, 711. 40. Wang, Z. T.; Xu, L. W.; Xia, C. G.; Wang, H. Q.; Tetrahedron Lett. 2004, 45, 7951. DOI: http://dx.doi.org/10.1016/j.tetlet.2004.08.107 41. Alvim, H. G. O.; Lima, T. B.; de Oliveira, A. L.; de Oliveira, H. C. B.; Silva, F. M.; Gozzo, F. C.; Souza, R. Y.; da Silva, W. A.; DaSilveira Neto, B. A.; J. Org. Chem. 2014, 79, 3383. DOI: http://dx.doi.org/10.1021/jo5001498 PMID: 24665975 42. Clark, J. H.; Macquarrie, D. J. Sherwood J.; Chem. Eur. J. 2013, 19, 5174. DOI: http://dx.doi.org/10.1002/chem.201204396 43. Medeiros, G. A.; da Silva, W. A.; Bataglion, G. A.; Ferreira, D. A. C.; de Oliveira, H. C. B.; Eberlin, M. N.; DaSilveira Neto, B. A.; Chem. Commun. 2014, 50, 338. DOI: http://dx.doi.org/10.1039/C3CC47156J 44. Souza, R. O. M. A.; da Penha, E. T.; Milagre, H. M. S.; Garden, S. J.; Esteves, P. M.; Eberlin, M. N.; Antunes, O. A. C.; Chem. Eur. J. 2009, 15, 9799. DOI: http://dx.doi.org/10.1002/chem.200900470 45. Ramos, L. M.; Tobio, A. Y.; dos Santos, M. R.; de Oliveira, H. C. B.; Gomes, A. F.; Gozzo, F. C.; de Oliveira, A. L.; DaSilveira Neto, B. A.; J. Org. Chem. 2012, 77, 10184. DOI: http://dx.doi.org/10.1021/jo301806n PMID: 23101501 46. Mohammadi Ziarani, G.; Lashgari, N.; Badiei, A.; J. Mol. Catal. A 2015, 397, 166. DOI: http://dx.doi.org/10.1016/j.molcata.2014.10.009 |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access