Educação
| Bioorganic concepts involved in the determination of glucose, cholesterol and triglycerides in plasma using the enzymatic colorimetric method |
|
Fabrício G. Menezes; Ana C. O. Neves; Djalan F. de Lima; Sheeza D. Lourenço; Lilian C. da Silva; Kássio M. G. de Lima*
Instituto de Química, Universidade Federal do Rio Grande do Norte, 59072-970 Natal - RN, Brasil Recebido em 13/08/2014 *e-mail: kassiolima@gmail.com Bioorganic and biological chemistry have been found to be highly motivating to undergraduate students and in this context, biochemical blood parameter analysis emerges as highly attractive content. In this proposal, several aspects related to analyses of glucose, cholesterol and triglycerides using the enzymatic colorimetric method were involved, and the findings have at least two relevant implications: i) introducing students to connections between organic chemistry and biology based on enzymatic processes, including reactivity and mechanistic aspects; ii) performing a micro scale bioassay analysis. The proposal requires two theoretical classes (2 h per class) and one practical class (4 h). INTRODUCTION Bioorganic has been pointed to as a driving force of stimulation for students of different areas to learn chemistry.1-3 In this context, the subject of "blood" which is minimally approached in undergraduate courses, promisingly emerges to be implemented as programmatic content. Blood is an aqueous solution, basically consisting of a liquid part (plasma) and figured elements (cells and fragments). Several organic and inorganic compounds in varied structural complexity are present in the blood, such as proteins, sugars, lipids and salts, among others, which must be at adequate levels for good health. Some of the most common biological parameters found in blood are glucose, cholesterol and triglycerides, and their periodic control is highly relevant to diagnostics, prevention and treatment of several pathologies.4,5 The gold standard for analysis of these three parameters is called the enzymatic colorimetric method (ECM), and the principle of the method as the name suggests is based on reactions that lead to chromophore species, which may be quantified by molecular spectroscopy.5 From our point of view, organic and biological chemistry applied to routine biochemical analysis could fill a gap in chemistry teaching in a fairly attractive way, and in this work we describe a simple and multidisciplinary didactic proposal concerned with the theoretical and practical aspects of biochemical blood parameters analysis (BBPA). The strategy is easily executable in a conventional teaching laboratory and consists of two theoretical classes (2 h per class) and one practical class (4 h). Firstly it is proposed an intensive discussion about many classical and relevant theoretical aspects of organic chemistry based on enzymatic reactions behind the analysis of glucose, cholesterol and triglycerides in blood through ECM. In the second part, students may experience the analysis of these three biochemical parameters in real plasma samples by collecting experimental data, using analytical techniques, performing statistical treatment and working on interpreting the results. The background required for the present activity consists in dominating the basic concepts of: i) atomic and molecular structure; ii) functional groups, their reactions and mechanisms; iii) catalysis; and iv) UV-vis spectroscopy. To evaluate if the objectives were achieved, the students were asked to answer a questionnaire of the experiment performed in the laboratory.
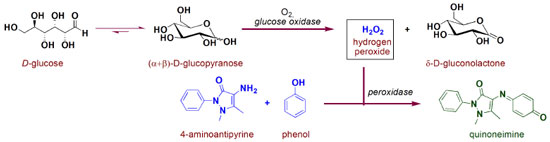
THEORY General aspects of biochemical blood parameters analysis In the first step of this proposal, a discussion of the reactions involved in the analysis of glucose, cholesterol and triglycerides in the blood plasma by the traditional ECM is suggested. This can be performed in two two-hour classes. Analysis of glucose Figure 1 presents the reactions involved in the quantification of glucose through ECM.
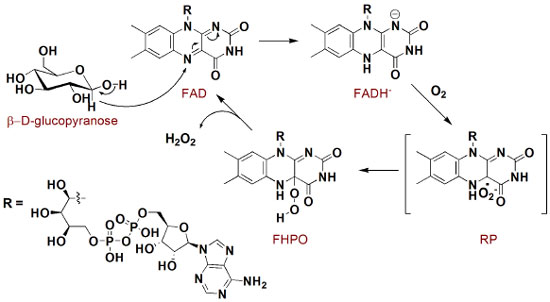
Through this schematic illustration, several interesting aspects of chemical stability and reactivity may be discussed. Glucose itself is an open-chain monosaccharide coexisting with α- and β-glucopyranose, and this equilibrium is called mutarotation. More specifically, in an aqueous solution glucose undergoes chemoselective intramolecular nucleophilic addition to afford two stable 6-membered cyclic hemiacetals, in which the only difference arises from the stereochemistry of the anomeric carbon. Since hemiacetal formation proceeds under thermodynamic control, it is expected that axial-OH substituent is itself due to the bonding interaction between the lone pair on oxygen (axial) and C-O antibonding molecular orbital (anomeric effect) as well as favorable dipole interactions.6 However, the proportion of α and β glucopyranose forming in water is about 2:1, which indicates that both stereoeletronic effects are not decisive. In fact, there is a solvent effect favoring equatorial hydroxyl group which is supported by theoretical studies in gas and aqueous phases.7 Oxidation of several organic compounds is usually found in organic text books, but not so deeply in mechanistic terms. This fact becomes even more pronounced when biological processes and enzymatic action are taken into account. In the formation of gluconolactone from glucopyranose, there is an enzyme-induced chemoselectivity factor presented, as only one carbon (the anomeric one) is oxidized facing the other six. Mechanistically, this reaction proceeds as illustrated in Figure 2.8-10 Initially, glucose and glucose oxidase rapidly form an enzyme-substrate complex, and the oxidation process takes place through a hydride transfer from the anomeric carbon of the carbohydrate to the FAD co-factor (presented in the glucose oxidase enzyme close to the active site) to generate FADH- . Sequentially, this anionic form reacts with molecular oxygen to form a radical pair (RP), which is converted into flavinhydroperoxide (FHPO) and then releases hydrogen peroxide in the biological media. Structural aspects presented in the system allow catalytic and selectivity of the process, such as hydrogen bond, non-covalent polar and hydrophobic interactions, which are illustrated in the work of Witt and colleagues.11
Figure 3 presents a literature-based overall view of peroxidase-catalyzed co-oxidation of phenol and 4-aminoantypirine to antipyrilquinoneimine dye.12-15 In this chemical transformation, an equivalent amount of phenol and 4-aminoantipyrine reacts in the presence of four equivalents of hydrogen peroxide and peroxidase, resulting in dye formation and four molecules of water (Figure 3A). The term peroxidase is related to a class of enzymes with potential to induce oxidative processes by using hydrogen peroxide, and being a hememoiety (prophirin-iron complex - Por-Fe(III)) in the active site. In the glucose quantification by ECM, two molecules of phenol are transformed in the respective phenoxy radicals, first by action of π-cation radical, which was generated from hydrogen peroxide and Por-Fe(III), and then in the regeneration of the native enzyme (Figure 3B). Subsequent reaction of the antipyril radical, generated by action of the phenoxy radical, reacts with another (resonance stabilized) phenoxy radical to afford a pyrazolone derivative, which is converted into quinoneimine dye by the action of hydrogen peroxide (Figure 3C).The generated antipyrilquinoneimine dye has maximum absorbance at approximately 500 nm.
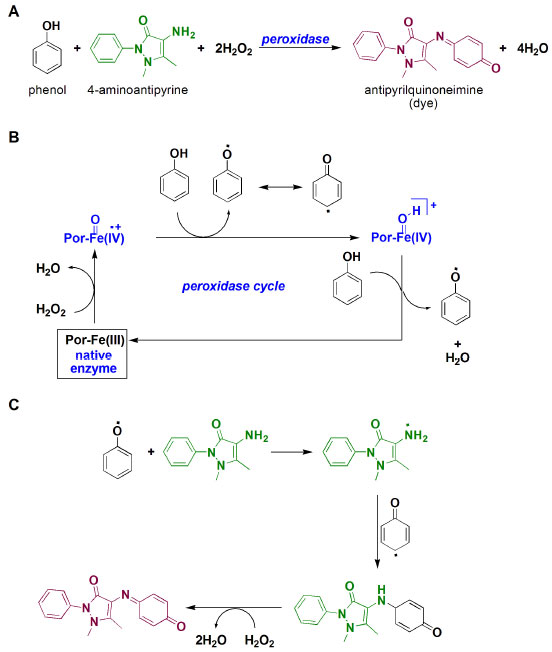 Figure 3. A) Overall quinoneimine dye formation; B) peroxidase cycle mechanism; C) mechanism associated to quinoneimine formation
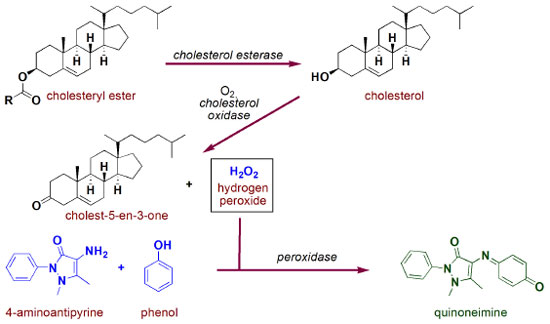
Analysis of cholesterol Figure 4 presents a schematic illustration of the reactions involved in the total cholesterol quantification by the ECM.
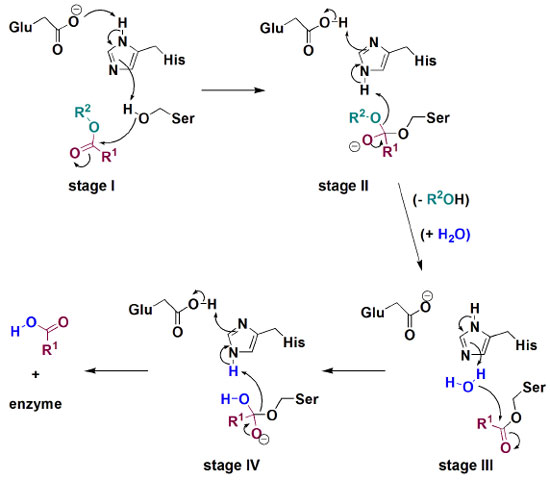
Most of this lipid is found flowing in the blood as the cholesterol ester, which is enzymatically converted into free cholesterol in the first step of the protocol. Hydrolyses of esters are found in many biological processes and from an academic point of view, they are one of the most contributing reactions to mechanistic studies involving nucleophilic substitution at the carbonyl carbon, with special emphasis on Hammet relationship studies.16-18 In the chemical route presented in Figure 4, cholesterol hydrolysis is performed by action of cholesterol esterase, an enzyme which is also capable of inversely catalyzing esterification reactions. Enzymatic hydrolyses of esters are chemical processes which require high synergy of the active site components. To exemplify this cooperation, Figure 5 presents a schematic illustration of the mechanism involved in the transformation of a general ester into carboxylic acid and alcohol by carboxylesterase action through a process governed by a catalytic triad formed by Glu, His and Ser aminoacids.19 Initially, a hydroxyl group of Ser side chain attacks the carbonyl carbon of the substrate, through a Glu-His cooperative general base catalysis type (stage I), leading to a tetrahedral intermediate which is decomposed by cationic His assistance leaving group departure (stage II). Then, similarly to stages I and II, hydrolysis of O-acylated serine by water proceeds as demonstrated in stages III and IV, respectively.
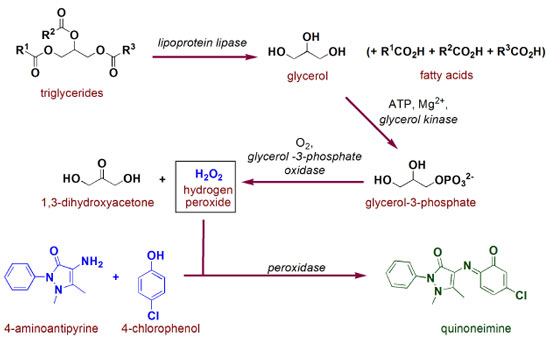
After being released in its free form, cholesterol is then oxidized to cholest-5-en-3-one by action of cholesterol oxidase. This process is very similar to those presented in Figure 2b for glucose (except for the nature/specificity of the substrate/enzyme), i.e. first a hydride is transferred to FAD to be able to afford FADH2, which is then oxidized by molecular oxygen.20 In this reaction, some interesting mechanistic insights are discussed in literature, such as key acid/base catalysis role and stabilization interaction of aminic hydrogen of the side chain aspargine amino acid residue (Asn-485) and FAD π-system (pyrimidine ring), which greatly contributes to this redox process.21,22 Since hydrogen peroxide is also produced in this latter reaction, it will be acting directly in the formation of spectroscopically measurable quinoneimine chromophore (as presented in Figure 3). Analysis of triglycerides An illustration of the reactions involved in the protocol for quantification of triglycerides based on the ECM is presented in Figure 6. Initially, triglycerides are hydrolyzed by lipoprotein lipase, an enzyme containing a site for interaction with the lipids as well as a catalytic domain formed by Ser132, Asp156 and His241 .23,24
In the second stage, glycerol from triglycerides hydrolyses is converted into glycerol-3-phosphate by reaction with ATP mediated by glycerol kinase and magnesium cation. Among the different complexes which could be formed in this reaction, Figure 7 shows a mechanistic illustration based on X-ray crystallography work reported by Mao and collaborators.25 After complexation, nucleophilic attack of the 1-OH from glycerol to magnesium-activated ATP is suggested to proceed assisted by Asp245 through general base catalysis. In the transition state, Asp10 residue also complexes to Mg2+ ion and helps in the orientation and stabilization of the γ-phosphoryl group transfer, resulting in the reaction products. In fact, several metalloenzymes are present in biological processes, including those associated to cleavage of P-O bonds in organophosphorous compounds, playing the role of the specific metallic cation in each system.26
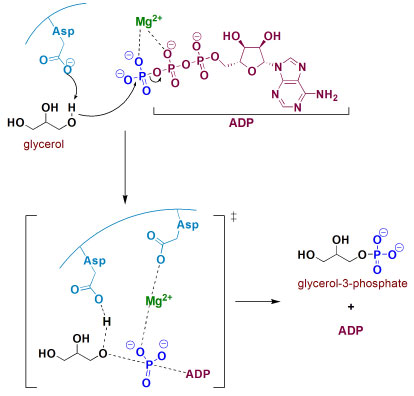 Figure 7. Phosphorylation of glycerol by ATP, mediated by glycerol kinase and Mg2+
The second last step in triglycerides analysis by ECM consists of an oxidative process involving FAD, molecular oxygen and glycerol 3-phosphate oxidase. The oxidation of C2 from glycerol 3-phosphate was proposed by Yeh and colleagues to proceed through a catalyzed general base (by Arg317 action) hydride transfer from C2-H to FAD,27 as briefly presented in Figure 8. In this study, the active site was modeled based on experimental and theoretical data, and a highly complex system was found with at least fourteen amino acid residues interacting in the system.27 In this oxidation process, hydrogen peroxide is generated and then used in quinoneimine chromophore formation step, exactly as mentioned in the glucose and cholesterol analysis and presented in Figure 3.
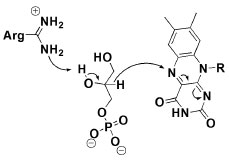 Figure 8. Hydride transfer by glycerol 3-phosphate to FAD in the active side of glycerol 3-phosphate oxidase
Some considerations In fact, there are several relevant chemical aspects involved in the protocols of quantification of glucose, cholesterol and triglycerides by the ECM, especially by the presence and effective action of enzymes. For example, mechanisms involving simple ester hydrolyses are often presented in text books proceeding with specific basic or acid catalysis, however as demonstrated herein for biochemical reactions, they are usually found proceeding with general catalysis in highly ordered processes mediated by enzymes. Nowadays, these reactions have also been adapted and applied as green processes for biodiesel production.28 Phosphoryl transfer, such as those presented in the triglycerides protocol are quite minimally approached in conventional organic chemistry material, even though being very relevant to several areas. In fact, these reactions are remarkably reported in preparative and mechanistic studies, including those related to molecular biology and new development of therapeutic agents, pesticides and chemical weapons degradation.29,30 Oxidative processes and their correlated reduction ones are carried out in biological media with an impressive chemical balance, including substrates, enzymes, metallic cations and reactive species such as molecular oxygen, hydrogen peroxide and hydride transfer. Some competitive aspects related to enzymatic reactions may be presented, and at this point enzyme promiscuity, related to capacity of enzymes catalyzing distinct chemical transformation (i.e. reactions involving different substrates) in different rates can also be discussed.31 A classic example arises from ascorbic acid, which can undergo fast oxidation in the reaction media due to its higher affinity for peroxidase when compared to phenolic compounds, impairing quinoneimine chromophore formation.32 In fact, ascorbic acid when present in high levels is treated as an interferent in biochemical analysis.33 Dopamine is another example of chemical species that produces negative interference in hydrogen peroxide/peroxidase-based biochemical analysis (especially those based on 4-aminophenazone), due to the possibility of catechol moiety oxidation to o-quinone, and further reactions, including alternative dyes with distinct absorptivity (Figure 9).34
 Figure 9. Conversion of dopamine into dopamine o-quinone by action of hydrogen peroxide and peroxidase
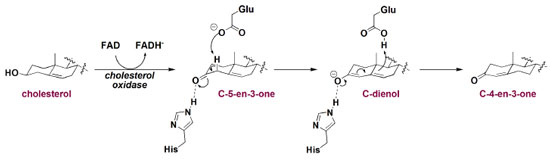
On the other hand, catalytic promiscuity is not always an adverse event, such as in the case of lipoprotein lipase and its hydrolylitc capacity toward triglycerides derived from distinct fatty acids along with di- and mono-acylglycerol.35 Another example of non-prejudicial catalytic promiscuity is the action of cholesterol oxidase. This enzyme also acts in the isomerization of cholest-5-en-3-one (C-5-en-3-one) to cholest-4-en-3-one (C-4-en-3-one), as presented in Figure 10.21,22 Mechanistically, after cholesterol is oxidized, Glu/His-assisted enolization of C-5-en-3-one leads to C-dienol which is then converted to C-4-en-3-one.
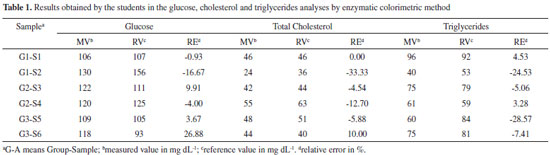
Since spectroscopically quantitative detectable quinoneimine dyes are generated in the ECM, the possibility of exploring molecular spectroscopy in class emerges, including its applications in biochemistry.36 In fact, the color of solutions containing quinoneimine is due to the presence a π-conjugated system, and this feature is made possible by the reaction of phenol and 4-aminoantipyrine which was recently pioneered and explored in the chemosensor area.37 For the quinoneimines involved in the herein presented analysis, energy is related to an absorption in the visible green region (λmax = 500-505 nm).38 Laboratory experiment: A suggested model for implementation One more goal for this proposal consists in the possibility of performing a practical activity involving biochemical blood analysis. More specifically, this experiment refers to analysis of glucose, cholesterol and triglycerides in rat blood plasma through ECM and can be performed in a conventional teaching laboratory. All real samples were provided by the Animal Department of Biophysics and Pharmacology of the Federal University of Rio Grande do Norte, however with the impossibility of using animal models, synthetic or isolated samples or even human blood can be applied in this study.39-45 Experimental details are presented in the supporting information. The activity was applied to a total of 9 undergraduate students of bioorganic chemistry (fifth semester) from the 2013-2014 course, working in teams of three, and guided by the instructor. Each group received two samples of rat blood plasma and then performed the quantification of glucose, total cholesterol and triglycerides by ECM. Table 1 shows the results achieved by the students for this experiment. To demonstrate the influence of interfering species, a spatula tip of ascorbic acid was added to one of the samples, which measured zero mg dL-1 of glucose after enzymatic colorimetric method.
After the practical activity, students were gathered to discuss the results, under supervision of the instructor. Firstly, working with real rat blood plasma samples was treated as a very motivating factor, since it is in fact unusually applied in undergraduate courses. The students proved to be quite enthusiastic about the experiment, especially due to the new view of organic chemistry and its reactions, and also due to the biological factor being far from the basic concepts usually presented in text books. Also, the possibility of applying the content in routine activities when associated to enzymatic processes and UV-vis spectroscopy became motivating. Further, they were able to formulate some hypotheses to explain those results that significantly differed from the values taken as reference. The first factor to be suggested was the time of reaction, followed by temperature of water bath. However, assuming that colorimetric reagents were in enough excess, these parameters could only explain lower measured values in relation to the reference ones. Possible operation errors were also taken into account, which enables an interesting discussion concerned about the reliability of clinical analysis. Another relevant aspect was related to when the blood was collected/stored and the relative stability of the analyzed biochemical parameters (degradation). In the discussion, problems due to contamination and validity of the reagents, as well as undesirable reactions were minimized. Finally, the students were required to answer a questionnaire (see supporting information) in an attempt to be able to evaluate the whole activity on many aspects, such as novelty, new view of organic chemistry relevance and its application in biological chemistry, multidisciplinarity, stimulus to learning, among others. The results were accepted as quite motivating for continuity of the activity.
CONCLUSIONS This manuscript describes a theoretical/experimental didactic proposal to be applied in biological chemistry disciplines for students of different courses. Classical organic chemistry reactions and the correlated enzymatic processes involved and BBPA of glucose, cholesterol and triglycerides were discussed in two theoretical classes and then during a practical activity students were allowed to directly quantify these biochemical parameters through gold standard ECM. The present proposal is coherent to the need for the development of educational materials exploring numerous chemical concepts associated to student's everyday themes in undergraduate courses.
SUPPLEMENTARY MATERIAL Student handout, experimental section and post-lab questionnaire are available in http://quimicanova.sbq.org.br, PDF format, with free access.
ACKNOWLEDGMENTS The authors would like to acknowledge the Brazilian entities CAPES, FAPERN and UFRN for financial and structural support, as well as all students who participated in the activity. Also, thanks to the editor and referees for substantial comments and suggestions for the improvement of the manuscript.
REFERENCES 1. Whitesides, G. M.; Deutch, J.; Nature 2011, 469, 21. DOI: http://dx.doi.org/10.1038/469021a PMID: 21209639 2. Reingold, I. D.; J. Chem. Educ. 2001, 78, 869. DOI: http://dx.doi.org/10.1021/ed078p869 3. Kirk, S. R.; Silverstein, T. P.; Willemsen, J. J.; J. Chem. Educ. 2006, 83, 1171. DOI: http://dx.doi.org/10.1021/ed083p1171 4. Selby, J. V; Krumholz, H. M.; Kuntz, R. E.; Collins, F. S.; Sci. Transl. Med. 2013, 5, 1. 5. Neves, A. C. O.; de Araújo, A. A.; Silva, B. L.; Valderrama, P.; Março, P. H.; de Lima, K. M. G.; J. Pharm. Biomed. Anal. 2012, 66, 252. DOI: http://dx.doi.org/10.1016/j.jpba.2012.03.023 PMID: 22483641 6. Gonthier, J. F.; Steinmann, S. N.; Wodrich, M. D.; Corminboeuf, C.; Chem. Soc. Rev. 2012, 41, 4671. DOI: http://dx.doi.org/10.1039/c2cs35037h PMID: 22660008 7. Ha, S.; Gao, J.; Tidor, B.; Brady, J. W.; Karplus, M.; J. Am. Chem. Soc. 1991, 113, 1553. DOI: http://dx.doi.org/10.1021/ja00005a015 8. Mattevi, A.; Trends Biochem. Sci. 2006, 31, 276. DOI: http://dx.doi.org/10.1016/j.tibs.2006.03.003 PMID: 16600599 9. Roth, J. P.; Klinman, J. P.; Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 62. DOI: http://dx.doi.org/10.1073/pnas.252644599 PMID: 12506204 10. Wilson, R.; Turner, A. P. F.; Biosensors and Bioeletronics 1992, 7, 165. DOI: http://dx.doi.org/10.1016/0956-5663(92)87013-F 11. Witt, S.; Wohlfahrt, G.; Schomburg, D.; Hecht, H.; Kalisz, H. M.; Biochem. J. 2000, 347, 553. DOI: http://dx.doi.org/10.1042/0264-6021:3470553 PMID: 10749686 12. Berglund, G. I.; Carlsson, G. H.; Smith, A. T.; Szöke, H.; Henriksen, A.; Hadju, J.; Nature 2002, 417, 463. DOI: http://dx.doi.org/10.1038/417463a PMID: 12024218 13. Uliana, M. P.; Vieira, Y. W.; Donatoni, M. C.; Corrêa, A. G.; Brockson, U.; Brockson, T. J.; J. Braz. Chem. Soc. 2008, 19, 1484. DOI: http://dx.doi.org/10.1590/S0103-50532008000800007 14. Nicell, J. A.; Wright, H.; Enzyme Microb. Technol. 1997, 21, 302. DOI: http://dx.doi.org/10.1016/S0141-0229(97)00001-X 15. Vojinović, V.; Azevedo, A. M.; Martins, V. C. B.; Cabral, J. M. S.; Gibson, T. D.; Fonseca, L. P.; J. Mol. Catal. B: Enzym. 2004, 28, 129. DOI: http://dx.doi.org/10.1016/j.molcatb.2004.02.003 16. Smith, M. B.; March, J.; Advanced Organic Chemistry: Reactions, Mechanisms and Structure; 6th ed.; John Wiley and Sons Inc.: New Jersey, 2007. 17. Carey, F. A.; Sundberg, R. J.; Sundberg, Advanced Organic Chemistry. Part A: Structure and Mechanisms; 5th ed.; Springer: New York, 2007; p. 1199. 18. Orth, E. S.; Medeiros, M.; Souza, B. S.; Caon, N. B.; Kirby, A. J.; Nome, F.; J. Phys. Org. Chem. 2012, 25, 939. DOI: http://dx.doi.org/10.1002/poc.2971 19. Montella, I. R.; Schama, R.; Valle, D.; Mem. Inst. Oswaldo Cruz 2012, 107, 437. DOI: http://dx.doi.org/10.1590/S0074-02762012000400001 PMID: 22666852 20. Kumari, L.; Kanwar, S. S.; Adv. Microbiol. 2012, 2, 49. DOI: http://dx.doi.org/10.4236/aim.2012.22007 21. Yin, Y.; Sampson, N. S.; Vrielink, A.; Lario, P. I.; Biochemistry 2001, 40, 13779. DOI: http://dx.doi.org/10.1021/bi010843i PMID: 11705367 22. Sampson, N. S.; Vrielink, A.; Acc. Chem. Res. 2003, 36, 713. DOI: http://dx.doi.org/10.1021/ar9800587 PMID: 12974654 23. Emmerich, J.; Beg, O. U.; Peterson, J.; Previato, L.; Brunzell, J. D.; Brewer, H. B.; Santamarina-Fojo, S.; J. Biol. Chem. 1992, 267, 4161. PMID: 1371284 24. van Ilbeurgh, H.; Roussel, A.; Lalouel, J.; Cambillau, C.; J. Biol. Chem. 1994, 269, 4626. 25. Mao, C.; Ozer, Z.; Zhou, M.; Uckun, F. M.; Biochem. Biophys. Res. Commun. 1999, 259, 640. DOI: http://dx.doi.org/10.1006/bbrc.1999.0816 PMID: 10364471 26. Domingos, J. B.; Longhinotti, E.; Machado, V. G.; Nome, F.; Quim. Nova 2003, 26, 745. DOI: http://dx.doi.org/10.1590/S0100-40422003000500019 27. Yeh, J. I.; Chinte, U.; Du, S.; Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 3280. DOI: http://dx.doi.org/10.1073/pnas.0712331105 PMID: 18296637 28. Straathof, A. J. J.; Chem. Rev. 2014, 114, 1871. DOI: http://dx.doi.org/10.1021/cr400309c PMID: 23987659 29. Orth, E. S.; Medeiros, M.; Bortolotto, T.; Terenzi, H.; Kirby, A. J.; Nome, F.; J. Org. Chem. 2011, 76, 10345. DOI: http://dx.doi.org/10.1021/jo202074y PMID: 22049907 30. Medeiros, M.; Orth, E. S.; Manfredi, A. M.; Pavez, P.; Micke, G. A.; Kirby, A. J.; Nome, F.; J. Org. Chem. 2012, 77, 10907. DOI: http://dx.doi.org/10.1021/jo302374q PMID: 23167539 31. Hult, K.; Berglund, P.; Trends Biotechnol. 2007, 25, 231. DOI: http://dx.doi.org/10.1016/j.tibtech.2007.03.002 PMID: 17379338 32. Martinello, F.; da Silva, E. L.; Clin. Chim. Acta 2006, 373, 108. DOI: http://dx.doi.org/10.1016/j.cca.2006.05.012 PMID: 16806141 33. Vasilarou, A. G.; Georgiou, C. A.; J. Chem. Educ. 2000, 77, 1327. DOI: http://dx.doi.org/10.1021/ed077p1327 34. Karon, B. S.; Daly, T. M.; Scott, M. G.; Clin. Chem. 1998, 44, 155. PMID: 9550573 35. Kapoor, M.; Gupta, M. N.; Process Biochem. 2012, 47, 555. DOI: http://dx.doi.org/10.1016/j.procbio.2012.01.011 36. Penzer, G. R.; J. Chem. Educ. 1968, 45, 692. DOI: http://dx.doi.org/10.1021/ed045p692 PMID: 5681634 37. Kim, H. Y.; Lee, H. J.; Chenag, S.; Talanta, 2014, in press. DOI: http://dx.doi.org/10.1016/j.talanta.2014.05.020 38. Penzer, G. R.; J. Chem. Educ. 1968, 45, 692. DOI: http://dx.doi.org/10.1021/ed045p692 PMID: 5681634 39. Erikson, J. M.; Biggs, H. G.; J. Chem. Educ. 1973, 50, 631. DOI: http://dx.doi.org/10.1021/ed050p631 PMID: 4743026 40. Daines, T. L.; Morse, K. W.; J. Chem. Educ. 1976, 53, 126. DOI: http://dx.doi.org/10.1021/ed053p126 PMID: 1249125 41. Taylor, R. P.; Broccoli, A. V.; Grisham, C. M.; J. Chem. Educ. 1978, 55, 63. DOI: http://dx.doi.org/10.1021/ed055p63 PMID: 627613 42. Barreto, M. C.; J. Chem. Educ. 2005, 82, 103. DOI: http://dx.doi.org/10.1021/ed082p103 43. Perles, C. E.; Volpe, P. L. O.; J. Chem. Educ. 2008, 85, 686. DOI: http://dx.doi.org/10.1021/ed085p686 44. Taber, D. F.; Li, R.; Anson, C. M.; J. Chem. Educ. 2011, 88, 1580. DOI: http://dx.doi.org/10.1021/ed101188q 45. Valdez, H. C.; Amado, R. S.; de Souza, F. C.; D'Elia, E.; Quim. Nova 2012, 35, 601. DOI: http://dx.doi.org/10.1590/S0100-40422012000300028 |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access