Artigo
| A theoretical evaluation of electroconductive properties for [1,2,4]-triazole 4N-substituted polymers |
|
Adolfo E. EnsunchoI; Cesar OrtegaII; Luis C. SánchezII,*
IDepartamento de Química, Universidad de Córdoba, Montería, Colombia Recebido em 23/09/2014 *e-mail: luiscarlos@correo.unicordoba.edu.co In order to evaluate a set of electrical and optical properties by using time-dependent density functional theory (TD-DFT), at level of theory B3LYP/6-31G(d), the 4N-substituted [1,2,4]-Triazole (TAZ) oligomers and substituted derivatives were studied using cyano, amino, methyl and fluoro functional groups. Additionally, specific properties were theoretically evaluated, namely electronic and geometrical properties, excitation energies, λmax, and the HOMO-LUMO orbital of the different oligomers TAZ with repeated units of 4 to 20 rings. These properties were extrapolated to polymer by using a second order polynomial fit. The oligomers studied exhibited a high molecular planarity, favoring electronic delocalization. Thus, an increase of the monomeric units in the different TAZ oligomers and their corresponding substituents led to an improvement in these properties, showing the best results for the cyano group. The findings contribute to the design of charge carrier polymeric materials and photoluminescent devices (OLEDs). INTRODUCTION Computer-aided molecular modeling based on quantum chemistry methods (also referred to as computational chemistry) constitutes a powerful alternative that effectively contributes to the development of new materials.1,2 In recent years there has been increased interest in studying heterocyclic conducting polymers that can be synthesized both chemically and electrochemically.3,4 Furthermore, these polymers can be added to various functional groups to allow easy regulation of their electrical, optical and magnetic properties.1 Therefore, studies of this nature are regularly used to interpret, predict or even replace experimental measures; thus saving time, materials and specialized laboratories, while considerably simplifying the work of highly qualified personnel.1 Consequently, a large number of π-conjugated polymers with diverse chemical structures have been studied, both theoretically and experimentally. The most representative polymers of this kind are those based on pyrrole and thiophene heterocyclic units due to their high conductivity after being subjected to a structural modification process called doping.1-2,4-6 Hence, the synthesis of new π-conjugated polymers is essential to improve the optical and electrical properties of these materials,6-9 as well as their environmental stability.1,10,11 This has led to the rational design of new polymers that involves an oriented development to obtain appropriate electronic properties, as the efficient transport of charges.7,8 Also, recent studies on the synthesis and characterization of new polymers with conductive properties containing triazoles have been developed.12-14 The studies address the use of this structural unit for designing copolymers with electroconductive properties, corresponding to heterocyclic compound 4N-[1,2,4]-triazole, also referred to as TAZ, which offers the possibility of constructing an Organic Light-Emitting Diode-OLED.15 Investigations in this field show that, by adding TAZ units to the synthesis of copolymers (based on 10-hexylphenothiazine), it is possible to favor electro-conductive properties of the polymer chain.15,16 This fact strongly suggests that TAZ compounds are key in the functional operation of polymeric structures.14 This additional benefit is associated to the generation of an agglutinator polymer in the propellant formulation that is much more energetic and environmentally friendly.8,12,15 It is worth noting that this last feature is one of the expected goals (yet to be attained) in the development of ϖ-conjugated polymers12,16,17 since conductor devices based on inorganic metals are toxic and affect some biological cycles necessary to preserve life.9,10 Due to the importance of the triazoles, as building blocks in polymer architectures, and also to the molecular modeling capabilities available to predict certain properties,17,18 it is interesting to propose structures based on monomers of the compound of 4N-substituted [1,2,4]-triazole. In this sense, we investigate, from a computational standpoint, the oligomers of 4 to 20 rings formed by the unit of these compounds, since they have not yet been synthesized and tested. The main purpose is to assess their potential for use as an electroconductive material when having a substituent on Nitrogen marked with the number 4 (Figure 1). These groups, which extract or accept electrons to the triazole ring, showed favorable features for this type of application.19,20
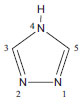 Figure 1. Structural unit 4H- [1,2,4]-Triazole (TAZ)
Recent theoretical studies have been made to estimate the properties of the excited states of heterocyclic polymers using the TD-DFT methodology,21,22 which is intended to determine the excitation energies, the involved orbitals and the UV-VIS spectra. Additionally, Ullah et al studied ammonia sensors using polypyrrole polymer chains.22 We have carried out DFT and TD-DFT calculations at B3LYP/6-31G(d) level of theory of the structural and electronic properties of oligomers 4N-[1,2,4] triazole and of the substituted derivatives.
METHODOLOGY The oligomers of 4 to 20 rings of the compounds of the 4N-[1,2,4]-Triazol (TAZ) and their substituted derivatives (such as polymeric materials used in the fabrication of OLEDs devices) were computationally studied using TD-DFT. The geometries of oligomers TAZ and their derivatives were optimized without symmetry restrictions using the B3LYP/6-31G approximation.23 The starting geometry adopted corresponds to an anti-planar conformation, where the dihedral angles between adjacent rings are 180º. The oligomers of 4N-[1,2,4]-triazole compounds were designated as H-TAZn and the substituted as C-TAZn, A-TAZn, M-TAZn and F-TAZn (where C, A, M and F represent the functional groups Cyano, Amino, Methyl and Fluor, respectively; and n = 4, 6, 8, 10, 15 and 20 represent the number of rings TAZ in the oligomer). The properties of the excited state (such as the first excitation energies, wavelengths, simulated molecular orbitals implied on the transitions and the UV-VIS spectra) were estimated at TD-DFT-B3LYP/6-31G(d) level of theory by using the optimized geometry of the oligomers initially established.22 In this work we used the extrapolation approach to obtain electronic properties of the polymer regarding a chain of infinite length.24 Conventionally, a linear adjustment scheme is necessary for making this extrapolation. However, in this study we found that, for oligomers of the N-substituted triazole under study, a better result was obtained by using a binomial fit, which is typically defined by the following general equation: Y = a + bX + cX2, where X equals 1/n.9 The term 1/n, is the reciprocal of the number of repeating TAZ units. Y is the value of the correlative parameters to the electronic structure, such as the first excitation energy.17,22 These calculations, were developed in the gas phase by using software package Gaussian03 (rev. E.01).25
RESULTS AND DISCUSSION Optimized geometries The conduction properties of polymers are related to structural and electronic characteristics, which determine their electro-conductive capacity. This is important for understanding the mechanism of charge transport and mechanical properties of the different polymers.1,2-6 Until now the studied substituents in the polymeric chains have been substituted on the carbon atom, which has shown acceptable structural and electronic effects.7 For polymers of 4N-substituted [1,2,4]-triazole and their derivatives, the oligomers geometry (Figure 2) was studied. In this case, the oligomeric structures exhibited no distortion with respect to the initial anti-planar configuration, and this trend was maintained for the rest of substituted TAZ oligomers of higher length. These oligomers exhibit a high molecular planarity, so that the conduction is favored, since there is more overlap of the p orbitals. This fact allows considerable delocalization of the valence electrons along the system of the polymeric chain in such a way that there is emerging high energy donor bands as well as low energy acceptor bands.1
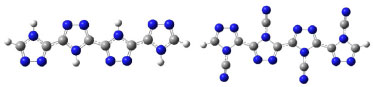 Figure 2. Optimized geometry at B3LYP/6-31G(d) level of theory of oligomers H-TAZ(4) and CN-TAZ(4), respectively. The spheres represent atoms of: Nitrogen (Blue), Carbon (grey) and Hydrogen (white)
Energetic analysis of the molecular orbitals The excitation energy values and the boundary orbitals were calculated at TD-B3LYP/6-31G(d) and B3LYP/6-31G(d) level of theory, respectively. In this work, we have first estimated the excitation energies, which provide important information about the electrical and optical properties of TAZ oligomers and their substituted derivatives. Tables 1-5 corresponds to a different TAZ oligomer each, substituted with the different functional groups.
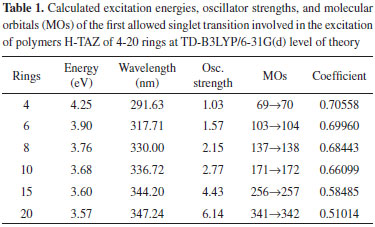
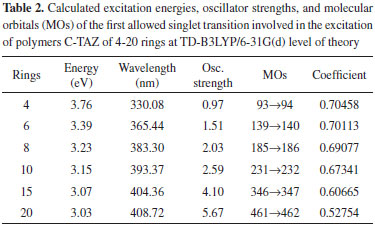
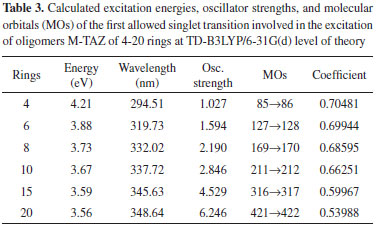
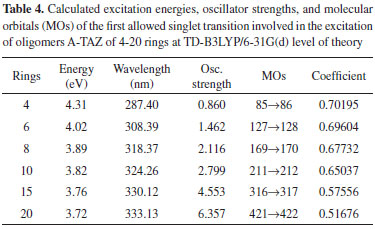
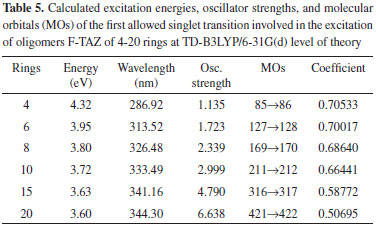
All the TAZ oligomers and their substituted derivatives show a reduction in excitation energy together with a corresponding increase of wavelengths as the number of rings in the polymeric chain increases. This can be observed by a shift toward red. Similarly, when increasing chain length from 4 to 20 rings, the excitation energies decrease due to the size of the polymeric systems.23 According to the results obtained, the functional groups that favor the electrical and optical properties of TAZ oligomers is the cyano group. This group exhibited the lowest excitation energies and highest wavelength values (see Table 2). From the point of view of the theory of bands, oxidation and reduction cause the appearance of electronic states in the band gap of the material, which greatly influence the conduction process,26 so that emerge high energy donor bands and low energy acceptor bands.1 Hence, the oligomer substituted with cyano functional group, show a significantly lower value of excitation energy; and can be explained by considering the presence of few free electrons in the oligomers, so that they can leave the valence band and reach the conduction band through an electronic transition that requires less energy than the band-gap energy. Furthermore, it can be inferred that some functional groups induce an attraction of electrons from the triazole ring as fluor and the amino group, reducing the electron density of the polymer chain, which is interesting for the design of hollow carrier materials,1,2,23 simulating an oxidation process. Therefore, these materials exhibit a high stability against oxygen, because calculations that have been performed with electron affinity of heterocyclic oligomers, where is showed the influence of heteroatoms and functional groups in decreasing the values of this property with respect to molecular oxygen (-0.59 eV), so that a greater stability of these oligomers is assumed.27 Also, the substituents that introduce electrons into the triazole ring as methyl and cyano groups induce a high electron density in the polymer chain showing a marked tendency to retain these electrons (simulating a reduction process), improving the electroconductive properties of the polymer and thus making them good electron carrier materials. In general, these polymeric systems must show good acceptor properties and electron donor. From the above observations it is clear that these polymeric materials based on TAZ units are potential candidates for the design of organic light emitting diodes (OLEDs).18 The carrier materials of hollows used for this purpose require the use of cathodes with low work functions so that the electroluminescent efficiency increases.14 However, the use of these cathodes limits the lifetime of the materials because they are susceptible to environmental molecular oxygen. Figures 3 and 4 show molecular orbitals HOMO and LUMO of H-TAZ(4) and C-TAZ(4), respectively. No significant differences were observed between the contours of the HOMO orbital of substituted and unsubstituted oligomer, although they are different functional groups. In contrast, there is a difference between the LUMO of H-TAZ and C-TAZ orbitals, namely the latter have a greater capacity to accept electrons associated with the substituent and evidenced in additional contours on the nitrogen atoms of the cyano functional group.
 Figure 3. Molecular orbitals (MOs) HOMO and LUMO of the H-AZ(4), respectively
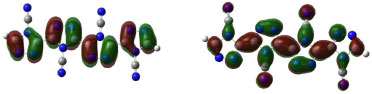 Figure 4. Molecular orbitals (MOs) HOMO and LUMO of the CN-TAZ(4), respectively
The energy of the frontier orbitals of the different TAZ oligomers were calculated at B3LYP/6-31G (d) level of the theory and are shown in Table 6. In general, a slight increase in the energy of the HOMO orbital of the TAZ oligomers is evidenced as the chain elongation increases, ie, the contribution of this orbital is very low with relation to the polymer. On the other hand, the orbital energy of LUMO decreases considerably with increasing the number of rings in the chain and this is due to charge injection from the functional groups improving the electrical and optical properties of polymers.22 With respect to the energy of the orbitals of the substituents, which contributes greater is the cyano group, since it has the lowest values for HOMO and LUMO orbital, and similarly decrease the values of band gap and likewise facilitate electronic transitions of the polymeric material.
To improve the prediction of excitation energies, it was essential to use oligomers with 20 monomers size, which are in line with previously reports by Zade and Bendikov.26 These authors, indicated that the use of oligomers formed by ten monomeric units is not enough to predict the band gap. The calculated energies of TAZ oligomers and the substituted derivatives are observed in Tables 1 to 5, where the substituents affect the electroconductive properties of the oligomers and so they are expected to have an impact on the conductor polymers. For the excited state of the TAZ oligomer and its substituted derivatives, it can be observed that the linear prediction was not very accurate. Additionally, a good correlation was found between the excitation energy and the inverse of the number of triazole units (1/n where n=∞). For these systems, the extrapolation that best describes the relationship is a second order regression.2 In agreement with predictions based on the quadratic equation (n=20, R2=0.999), we estimated the excitation energy of all oligomers at an infinite chain, shown in the Figure 5 and listed in Table 7. These values were calculated in order to find the state with improved conductive properties and the doping type that most favors the conduction. The calculated excitation energies showed agreement with the optical band gap reported by Janietz et al.13
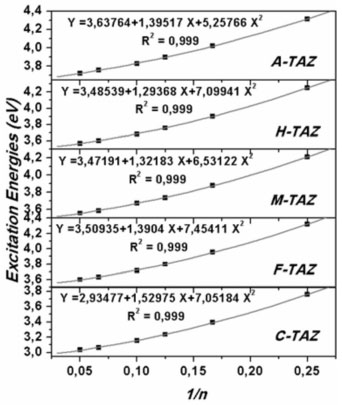 Figure 5. Excitation energies of TAZ oligomers and substituted derivatives extrapolated to an infinite chain length (polymer)
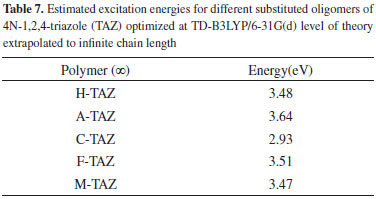
These results confirm that the cyano functional groups (2.93 eV) improve the electrical and optical properties of TAZ oligomer, exhibiting lower value of excitation energy when this is extrapolated to infinite chain length, compared with other TAZ polymers (see Table 7). These results clearly show that the delocalization in different triazole rings and the substituents provide these polymeric materials with better electrical and optical properties, due to increased accumulation of electronic density in the center of the oligomeric chain, where later the majority electronic transition occurs and it can observe this trend in the tabulated molecular orbitals for each oligomer, see Tables 1-5. However, these values of excitation ensure that these polymers behave as electron-carrier materials due to the extra loads on the chain, leading to caused defects or hollows that involve alteration of the electronic configuration and structure. The charges and unpaired electrons are not localized on the atoms, but are spread across a number of rings, which implies rearrangements of bonds in the chains, culminating in the transformation towards a quinoidea structure.23,28 Therefore, although there is no experimental information available to support this concept in this type of material, in light of the mechano-quantum calculations and the obtained results, it is important to note that these materials have excellent potential to be used as luminescent devices. Today, it is a known experimental fact that the development of hollow carrier materials has been above the development of materials for transporting electrons.18 Regarding light-emitting materials, the recombination zone is displaced towards the cathode and hence diminishes the electroluminescent efficiency due to the extinction of excitons caused by the metallic cathode.18 In terms of the excitation energy values of the studied polymers, one could establish a reasonable balance in the charge injection from two electrodes as well as an efficient transportation of holes and electrons, in case this material were to be used as luminescent layer in OLED devices. UV-Vis spectra study Figure 6 shows the UV-Vis spectra of the TAZ oligomers and the substituted derivatives of 4, 10 and 20 rings simulated at TD-B3LYP/6-31G(d) level of theory.
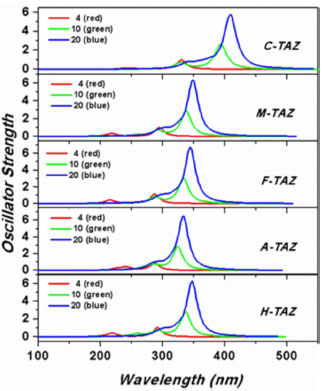 Figure 6. UV-Vis spectra of 4, 10 and 20 rings of TAZ oligomers and substituted derivatives of 4, 10 and 20 triazole rings
The spectrum of the TAZ oligomers and substituted derivatives showed a similar profile, where a main intense band occurs above 290 nm. Also, a change of this band to red is observed as increased number rings, and likewise the value of the wavelength of maximum absorption (λmax) was shifted, corresponding to dominant transitions of HOMO to LUMO orbital, mainly due to the π → π * transitions. According to the functional groups the relative trend of λmax is C-TAZ > M-TAZ > H-TAZ > F-TAZ> A-TAZ. These results indicated that cyano substituent could improve the optical properties of these polymeric materials, since it has the highest redshift (λmax = 408.72 nm) and the greatest bandwidth (see Figure 6). Moreover, these displacements describe the capacity of charge retention in the main chain of these oligomers, and are associated with the decrease in the band gap.22 The charge transference from the functional groups and chain elongation induce an electronic delocalization in the TAZ oligomers that lead to an increment in bandwidth and a displacement of λmax.29
CONCLUSIONS We have carried out DFT and TD-DFT calculations at B3LYP/6-31G (d) level of theory of the structural and electronic properties of oligomers 4N-[1,2,4] triazole (TAZ) and the substituted derivatives using cyano, methyl, amino and fluor functional groups. The studied oligomers have a high molecular planarity, favoring electronic delocalization. Also, the increase in the monomer units for the different TAZ oligomers, and the corresponding substituents, permits the modification of their electrical and optical properties, wherein the cyano group showed the best values in terms of excitation energies and wavelengths (λmax) when compared to the triazole basis (H-TAZ); associating these changes with a considerable decrease in the band gap. The LUMO orbitals provide a greater contribution than that of the HOMO orbitals when TAZ oligomers are substituted. Additionally, the bandwidth and their redshifts in the UV-Vis spectra justify the use of these polymeric materials in photoluminescent devices (OLEDs). The results presented here show that the theoretical molecular modeling techniques are suitable for evaluating these and other properties quickly and easily, also providing an understanding of the electrical and optical phenomena.
ACKNOWLEDGEMENTS The authors of this paper would like to thank Centro de Investigaciones de la Universidad de Córdoba (CIUC) for their financial support during the development of the present study, which was conducted within the framework of research project 1.2.08.109 (numeral FCB-03-09).
REFERENCES 1. Casanovas J.; Armelin E.; Iribarren J.; Alemán C.; Liesa F.; Polímeros: Ciência e Tecnologia. 2005, 15, 239. DOI: http://dx.doi.org/10.1590/S0104-14282005000400006 2. Curcó, D.; Alemán C. ; J. Comput. Chem. 2007, 28, 1743. DOI: http://dx.doi.org/10.1002/jcc.20687 PMID: 17340605 3. Roncali J. ; Chem. Rev. 1997, 97, 173; Roncali J.; Chem. Rev. 1992, 92, 711. DOI: http://dx.doi.org/10.1021/cr950257t PMID: 11848868 4. Kertesz, M.; Yang S.; Tian Y. In Handbook of Thiophene-Based Materials; Perepichka, I. F.; Perepichka, D. F., eds.; John Wiley & Sons: Hoboken, 2009. 5. MacDiarmid, A. G.; Mammone, R. J.; Kaner, R. B.; Porter, S. J.; Pethig, R.; Heeger, A. J.; Rosseinsky, D. R.; Philos. Trans. R. Soc. London, Ser. A 1985, 314, 3. DOI: http://dx.doi.org/10.1098/rsta.1985.0004 6. Heeger, A. J.; Angew. Chem. Int. Ed. 2001, 40, 2591. DOI: http://dx.doi.org/10.1002/1521-3773(20010716)40:14<2591::AID-ANIE2591>3.0.CO;2-0 7. Vivas-Reyes, R.; Mercado, L. D.; Anaya-Gil, J.; Marrugo A. G.; Martínez, E.; J. Mol. Struct.: THEOCHEM 2008, 861, 137. DOI: http://dx.doi.org/10.1016/j.theochem.2008.04.019 8. Zhu, Y.; Heim, I.; Tieke, B.; Macromol. Chem. Phys. 2006, 207, 2206. DOI: http://dx.doi.org/10.1002/macp.200600363 9. Liu, Y.; Zhan, X.; Macromol. Chem. Phys. 2011, 212, 428. DOI: http://dx.doi.org/10.1002/macp.201000448 10. Haye, S.; Slaveykova, V.I.; Payet, J.; Chemosphere 2007, 68, 1489. DOI: http://dx.doi.org/10.1016/j.chemosphere.2007.03.019 PMID: 17467037 11. Nahmani, J.; Hodson, M. E.; Black, S.; Environ. Pollut. 2007, 149, 44. DOI: http://dx.doi.org/10.1016/j.envpol.2006.12.018 PMID: 17316938 12. Shin, J. A.; Lim, Y. G; Lee, K.; Bull. Korean Chem. Soc. 2011, 32, 547. DOI: http://dx.doi.org/10.5012/bkcs.2011.32.1.157 13. Janietz, S.; Barche, J.; Wedel, A.; Sainova, D.; Macromol. Chem. Phys. 2004, 205, 1916. DOI: http://dx.doi.org/10.1002/macp.200400095 14. Jansson, E.; Chandra, J. P.; Ågren, H.; Chem. Phys. 2006, 330, 166. DOI: http://dx.doi.org/10.1016/j.chemphys.2006.08.010 15. Kido, J.; Hongawa, K.; Okuyama, K.; Nagai, K.; Appl. Phys. Lett. 1993, 63, 2627. DOI: http://dx.doi.org/10.1063/1.110402 16. Choi, J.; Lee, B.; Kim, J. H.; Synth. Met. 2009, 159 (19-20), 1922. DOI: http://dx.doi.org/10.1016/j.synthmet.2009.03.029 17. Shin, J. A.; Lim, Y. G; Lee, K.; Bull. Korean Chem. Soc. 2011, 32, 547. DOI: http://dx.doi.org/10.5012/bkcs.2011.32.2.547 18. Nazare, A. N.; Asthana, S. N.; Singh, H.; J. Energ. Mater. 1992, 10, 43. DOI: http://dx.doi.org/10.1080/07370659208018634 19. Hosoya, H.; Aida, M.; Kumagai, R.; Watanabe, K.; J. Comput. Chem. 1987, 8, 358. DOI: http://dx.doi.org/10.1002/jcc.540080412 20. Hughes, G.; Bryce, M. R.; J. Mater. Chem. 2005, 15, 94. DOI: http://dx.doi.org/10.1039/b413249c 21. Azazi, A.; Mabrouk, A.; Alimi, K.; Comput. Theor. Chem. 2011, 978, 7. DOI: http://dx.doi.org/10.1016/j.comptc.2011.08.020 22. Ullah, H.; Ayub, K.; Ullah, Z.; Hanif, M.; Nawaz, R.; Shah, A. A.; Bilal, S.; Synth. Met. 2013, 172, 14. DOI: http://dx.doi.org/10.1016/j.synthmet.2013.03.021 23. Vivas-Reyes ; Nuñez-Zarur, R. F. ; Martínez, E.; Org. Electron. 2008, 9, 625. DOI: http://dx.doi.org/10.1016/j.orgel.2008.04.004 24. Wang, J. F.; Feng, J. K.; Ren, A. M.; Liu, X. D.; Ma, Y. G.; Lu, P.; Zhang, H. X.; Macromolecules 2004, 37, 3451. DOI: http://dx.doi.org/10.1021/ma035566x 25. Gaussian 03, Revision E.01, Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery Jr., J. A.; Vreven, T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; and Pople, J. A.; Gaussian, Inc., Wallingford CT, 2004. 26. Zade, S. S.; Bendikov, M.; Org. Lett. 2006, 8, 5243. DOI: http://dx.doi.org/10.1021/ol062030y PMID: 17078688 27. Risko, C.; Zojer, E.; Brocorens, P.; Marder, S. R.; Brédas, J.; Chem. Phys. 2005, 313 (1-3), 151. DOI: http://dx.doi.org/10.1016/j.chemphys.2004.12.020 28. Brédas, J. L.; Thémans, B.; Fripiat, J. G.; André, J. M.; Phys. Rev. B 1984, 29, 6761. DOI: http://dx.doi.org/10.1103/PhysRevB.29.6761 29. Chitpakdee, C.; Namuangruk, S.; Khongpracha, P.; Jungsuttiwong, S.; Tarsang, R.; Sudyoadsuk, T.; Promarak, V.; Spectrochim. Acta, Part A 2014, 125, 36. DOI: http://dx.doi.org/10.1016/j.saa.2013.12.111 |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access