Artigo
| Kinetic study of selective gas-phase oxidation of isopropanol to acetone using monoclinic ZrO2 as a catalyst |
|
Mohammad SadiqI,*,#; Muhammad AliI; Razia AmanI; Haroon Ur RashidII; Muhammad Naveed UmarI
IDepartment of Chemistry, University of Malakand, Khyber Pakhtunkhwa, Pakistan Recebido em 19/11/2014 *e-mail: mohammad_sadiq26@yahoo.com Zirconia was prepared by a precipitation method and calcined at 723 K, 1023 K, and 1253 K in order to obtain monoclinic zirconia. The prepared zirconia was characterized by XRD, SEM, EDX, surface area and pore size analyzer, and particle size analyzer. Monoclinic ZrO2 as a catalyst was used for the gas-phase oxidation of isopropanol to acetone in a Pyrex-glass-flow-type reactor with a temperature range of 443 K - 473 K. It was found that monoclinic ZrO2 shows remarkable catalytic activity (68%) and selectivity (100%) for the oxidation of isopropanol to acetone. This kinetic study reveals that the oxidation of isopropanol to acetone follows the L-H mechanism. INTRODUCTION The industrial significance of the oxy-functionalization of alcohols results from the fact that the products of the process are valuable precursors in the manufacture of many drugs, vitamins and fragrances, etc.1 For example, cyclohexanone and adipic acid, which are the oxidation products of cyclohexanol, have been widely used in the manufacture of nylon-6, nylon-6, 6, plasticizers, food additives, insecticides and herbicides, etc.2 Keeping in view the immense significance of oxidation of alcohols to aldehydes and ketones, researchers are endeavoring to develop simple and eco-friendly methods for the chemical transformation under question. Generally, homogeneous catalysts like soluble salts and transition metal complexes in combination with oxidants like O2, H2O2 or RO2H have been used for the liquid phase oxidation of alcohols. But several problems have been encountered in these homogeneous catalytic oxidation reactions, such as, the separation of the catalyst, its recovery and recycling from the reaction mixture.3 Therefore, it is a worthwhile effort to replace homogeneous catalysts with heterogeneous counterparts. The development of heterogeneous catalysts for the oxidation of alcohols is a field of intensive research. Several different heterogeneous catalysts for the oxy-functionalization of alcohols have been reported. For example, Ilyas et al. have demonstrated the efficiency of Y2O3/ZrO2 in the dehydrogenation of cyclohexanol to cyclohexanone.4 Sugunan et al. have studied the catalytic activity of ZrO2-Y2O3 in the oxidation of cyclohexanol.5 Venkathatathri and coworkers have employed Mn-MCM-41 molecular sieves in the selective gas phase oxidation of cyclohexanol.6 Muhler and coworkers have reported the oxidation of isopropanol with oxygen using Au/TiO2 as a catalyst.7 Hutchings reported that TiO2 supported Au-Pd alloy nanocrystals give significantly enhanced activity for the oxidation of alcohol using oxygen as oxidant.8 Hutchings and coworkers have also explored the catalytic efficiency of zeolite supported Au and Au-Pd catalysts for the oxidation of benzyl alcohol with molecular oxygen.9 Stucky and coworkers have utilized gold nanoparticles in the solvent free selective aerobic oxidation of alcohols.10 Many other similar examples of attempts for carrying out the oxidation of alcohols with heterogeneous catalysts can be found in the literature.5-10 ZrO2 is of considerable interest for researchers working to develop heterogeneous catalytic systems for various applications. A lot of research work has been devoted to the application of ZrO2 as a catalyst support in various oxidative reactions such as oxidative carbonylation of methanol to dimethyl carbonate,11 combustion of trace amounts of methane under exhaust gas condition,12 oxidative dehydrogenation of propane,13,14 and oxidation of CO,15 etc. We have also reported the efficiency of ZrO2 as a support for Pt catalyst in the aerobic oxidation of toluene.16 A study of literature reveals that though ZrO2 has been an efficient support for a number of oxidation reactions but there is a lack of sufficient literature on the use of ZrO2 as a catalyst in these reactions. We were thus motivated to study the activity of ZrO2 in the oxidation of alcohols. The oxidation of isopropanol was chosen as a model reaction as it is widely used as a probe reaction to explore the activity and selectivity of various catalysts in oxidative dehydrogenation and dehydration reactions. To the best of our knowledge, ZrO2 has not been thoroughly studied in the aspect of its catalytic activity in the gas phase oxidation of isopropanol. In this paper, we report the catalytic activity of ZrO2 in the gas phase oxidation of isopropanol to acetone, using oxygen as the oxidant.
EXPERIMENTAL General All chemicals used in the study were of high purity grade and were used without further purification. .Gases like nitrogen, oxygen and hydrogen were supplied by BOC Pakistan and further purified by specific filters. Preparation of zirconia Zirconia was prepared from its precursor compound (Octahydrated zirconium oxychloride) by ammonolysis, i.e. aqueous ammonia was added drop wise to 0.5 mol L-1 solution of zirconyl chloride over a time span of approximately 4 h. White precipitate of zirconium hydroxide was obtained at pH 9. The precipitate was washed and dried overnight in oven at 383 K. The dried sample was pulverized, meshed (100-150 mesh) and calcined at a desired temperature in a temperature controlled furnace at 723 K, 1023 K and 1253 K. Characterization of catalyst The powder XRD was carried out using X-ray diffractometer (Rigaku D/Max-II, Japan) using Cuα radiation. Scherrer equation was used to calculate the crystallite sizes of the samples. The morphology and elemental composition were studied by means of SEM and EDX (JSM 5910, JEOL, Japan). The surface area and pore size were investigated using surface area analyzer Quantachrome, Nova 2200, and particle size analyzer (analysette 22), respectively. Catalytic test The catalytic activity of the zirconia was tested in a fixed-bed flow type Pyrex glass double walled reactor connected to circulating heating bath (Cole-Parmer, 12101-15) for achieving the desired temperature. A catalyst dose of 100 mg was used for the oxidation of isopropanol each time. Isopropanol vapors were fed from saturator, using nitrogen as a carrier gas with a specified flow rate. The temperature was varied in the range of 443-473 K. Reaction mixtures of 0.5 mL were injected to GC (Perkin Elmer Clarus 580) with column (rtx@-Wax 30 m, 0.5 mm ID, 0.5 nm) and FID detector at specified time intervals with six-port gas sampling valve.
RESULTS AND DISCUSSION Characterization of the catalyst The XRD patterns obtained for the zirconia sample as given in Figure 1 show that at lower temperature, zirconia was present in tetragonal phase while at higher temperature, it exhibited a purely monoclinic phase. The zirconia calcined at 1023 K is however a blend of both phases.17
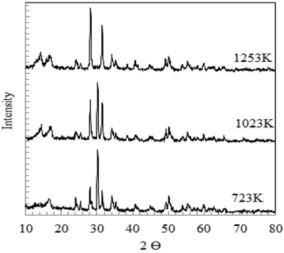 Figure 1. XRD of zirconia calcined at different temperatures
We focused only on the monoclinic phase of ZrO2 as it has been observed to show remarkable catalytic activities in the alcohol oxidation reactions.18 Particle size of the zirconia was calculated from XRD and SEM by Scherrer equation and intercept method respectively. Scherrer equation gave a particle size of 0.1-1.5 µm. Particle size analyzer gave a wide range of particle size distribution with the major fraction measuring 1.2 µm, as shown in Figure 2. Thus there is a substantial agreement between the particle size calculated from XRD and that obtained with particle size analyzer.
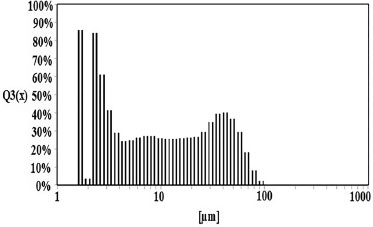 Figure 2. Particle size distribution of monoclinic zirconia
SEM images as given in Figure 3 reveal that the particles are almost spherical with a smooth morphology. Moreover, no voids and cracks were observed in the particles calcined at high temperature.19
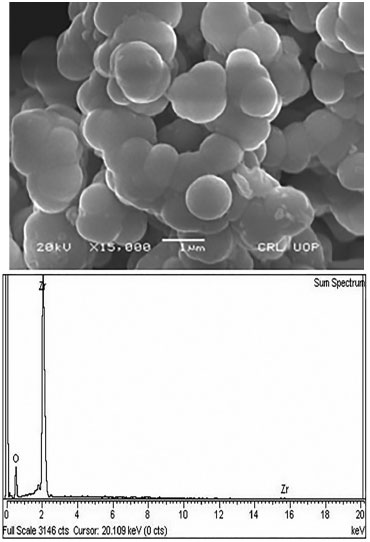 Figure 3. SEM /EDX of monoclinic zirconia
BET surface areas of the ZrO2 calcined at 723 K, 1023 K and 1253 K were 87.4 m2 g-1, 71.3 m2 g-1 and 59 m2 g-1, respectively. The BET surface area of zirconia decreased with an increase in the calcination temperature. Two processes have been found to account for the decrease in the surface area of zirconia at high calcination temperature; the growth of the crystallite accompanied by phase transformation, and inter-crystallite sintering.20,21 EDX shows that the sample is free of chloride ions and only zirconium and oxygen are present in the sample such that the %age composition of the sample is 77.43 wt% zirconium and 22.57 wt% oxygen22 as shown in Figure 3. Catalytic test GC analysis of the reaction mixtures showed that acetone was the only product of the reaction. Monoclinic zirconia thus catalyzes the oxidation of isopropanol to acetone. The activity and selectivity of the catalyst for the oxidation of isopropanol to acetone were found to be 68% and 100%, respectively. In contrast to monoclinic zirconia, tetragonal zirconia is active for the dehydration of isopropanol to propene and has a negligible activity for the oxidation of isopropanol to acetone. This is due to the difference in the geometry of zirconia and the most probable explanation for isopropanol oxidation to acetone or dehydration to propene is surface sites. Redox surface sites on monoclinic zirconia are responsible for oxidation while acidic surface sites on surface of tetragonal zirconia responsible for the conversion of isopropanol to propene.23 The reactions parameters were optimized by varying only one parameter at a time. The effect of the amount of catalyst was studied by taking various amounts of the catalyst in the range of 50-300 mg of the catalyst while keeping other reaction conditions constant such as, temperature; 473 K, vapor pressure of alcohol; 20 Torr and partial pressure of oxygen; 380 Torr. It was observed that there was a rapid increase in the yield of acetone by increasing the amount of catalyst to a double (100 mg) of the initial amount (50 mg). No further increase in the acetone yield was observed with an increase in the amount of the catalyst beyond 100 mg. With other parameters held constant, vapor pressure of isopropanol was varied in the range of 20-50 Torr and it was found that the rate of formation of acetone increased with an increase in the vapor pressure of isopropanol as shown in Figure 4.
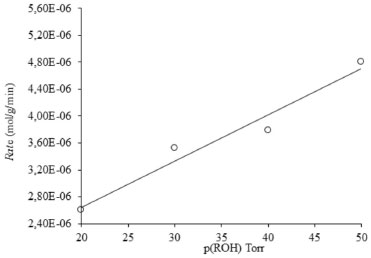 Figure 4. Effect of vapor pressure of alcohol on the rate of 2-propanol conversion to acetone on 100 mg of ZrO2(m) at 473 K
The effect of the catalyst's amount and vapor pressure of isopropanol on the reaction rate can be explained by the surface sites as well as by the ability of the catalyst to adsorb isopropanol. The more suitable the catalyst to reactant ratio, the best is the catalytic activity. The effect of partial pressure of oxygen was studied in the range of zero to 475 Torr as shown in Figure 5.
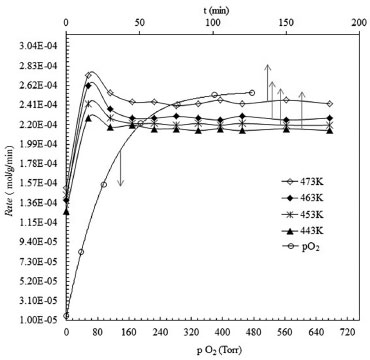 Figure 5. Variation of the rate of conversion of 2-propanol to acetone with the partial pressure of oxygen and temperature
It was observed that the reaction rate increased rapidly with an increase in the partial pressure of oxygen upto 380 Torr. The reaction rate did not respond to any further increase in the partial pressure of oxygen. The effect of reaction temperature and reaction duration was studied in the temperature range of 443-473 K, and the results thus obtained are given in Figure 5. No side products were detected in the products, thus confirming 100% selectivity of the catalyst for the oxidation of the isopropanol to acetone. Reaction kinetics The calculated activation energy according to equation 1 reveals that the reaction is in kinetic control regime as presented in Figure 6.
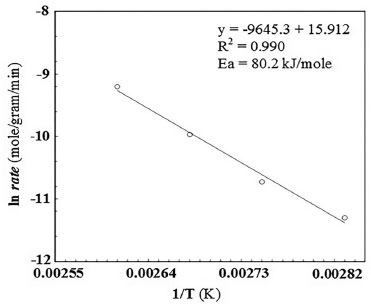 Figure 6. Arrhenius plot for activation energy
For mechanistic interpretation of the reaction kinetics, experimental data was fitted to Mars van Krevelen, Eley- Rideal and Langmuir- Henshelwood kinetic models.  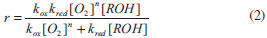 By keeping Oxygen constant and putting the constants as koxkred[O2]n = a, kox[O2]n = b and kred = c equation (2) can be modified to  According to MK model, the oxygen of the lattice is the one responsible for the oxidation of isopropanol; the lattice oxygen is then replenished by the gas phase oxygen. 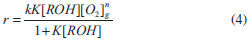 By putting k[O2]gn = a and K = b equation (4) can be written as:  ER model assumes that the gas phase isopropanol shall be oxidized by the oxygen adsorbed onto the catalyst. 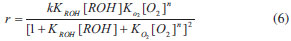 By putting kKROHKO2[O2]n = a, 1 + KO2[O2]n = b and KROH = c the equation (6) can written as; 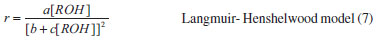 LH mechanism considers that both isopropanol and oxygen shall be adsorbed onto the catalyst, followed by an irreversible surface reaction to yield the product. Figure 7 shows non-linear least square fit to LH, ER and MK models at different reaction temperatures.
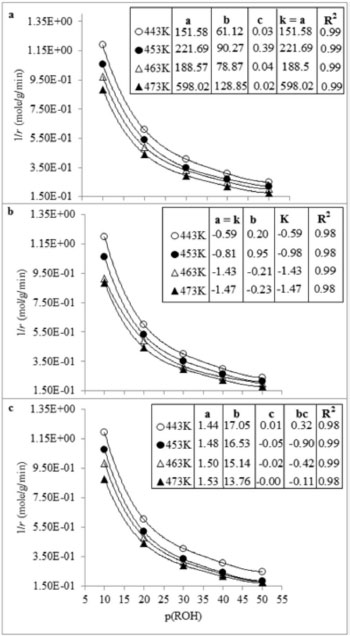 Figure 7. Plots for the comparison of various kinetic models a. L-H, b. ER and c. MK at different temperatures
From the figure, it can be seen that LH model has a better regression than the other two models at all temperatures. Arbitrary units of micro-mole g-1 s-1 were used for the rate constant as the actual units in this case are very complex. In equation 3 (Mars van Krevelen model), "a" can be taken as reaction rate constant, which requires that a = bc/0.5. However, none of the observed values satisfy this condition. Similarly the coefficient values calculated according to equation 5 (Eley-Rideal model) are negatives which shows that Eley-Rideal model does not applicable. While the coefficient values calculated according to equation 7 show that the reaction follows LH mechanism. For further confirmation of the mechanism, chemisorption of O2 was studied. Since the rate of reaction increases with an increase in partial pressure of oxygen in the feed gases, hence ER mechanism was excluded and the other two mechanisms were subjected to non-linear least square regression analysis. 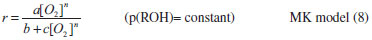 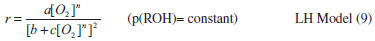 It is clear from Figure 8 that the non-dissociative adsorption of oxygen is preferred in both LH and MK models.
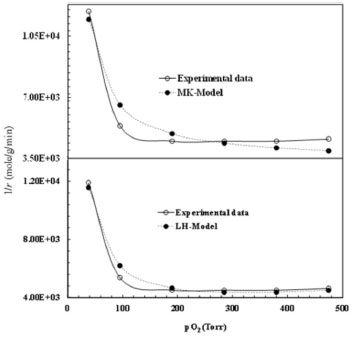 Figure 8. Comparison of Mars-van Krevelen and L-H competitive adsorption models
Furthermore, it is also evident that LH model gives a much better regression coefficient (R2=0.990) than MK model does (R2 =0.972). To sum up; gas phase oxidation of isopropanol to acetone by monoclinic zirconia is purely kinetic controlled and proceed by Langmuir-Henshelwood mechanism.
CONCLUSIONS Monoclinic zirconia is an ideal catalyst for the gas phase oxidation of isopropanol to acetone. Optimal conditions for better catalytic activity are; catalyst; 100 mg, temperature; 473 K, flow; 40 mL min-1, vapor pressure of alcohol; 20 Torr, partial pressure of oxygen; 380 Torr. The reaction in most cases is kinetically controlled and proceeds with Langmuir- Henshelwood competitive adsorption mechanism.
ACKNOWLEDGMENT The authors greatly acknowledge financial support from the Higher Education Commission of Pakistan, vide Project No. 20-1897/NRPU/R&D/HEC/116806 and Project No. 20-1604/R&D/HEC/092198, under National Research Program for Universities.
REFERENCES 1. Shen, J.; Shan, W.; Zhang, Y.; Du, J.; Xu, H.; Fan, K.; Shen, W.; Tang, Y.; J. Catal. 2006 237, 94. DOI: http://dx.doi.org/10.1016/j.jcat.2005.10.027 2. Chavan, S. A.;Srinivas, D.; Ratnasamy, P.; J. Catal. 2002, 212, 39. DOI: http://dx.doi.org/10.1006/jcat.2002.3756 3. Arends, W. C. E.; Sheldon, R. A.; Appl. Catal., A 2001, 212, 175. DOI: http://dx.doi.org/10.1016/S0926-860X(00)00855-3 4. Ilyas, M.; Ikramullah; Catal. Commun. 2004, 5, 1. DOI: http://dx.doi.org/10.1016/j.catcom.2003.10.010 5. Sugunan, S.; Paul, A.; React. Kinet. Catal. Lett. 1998, 65, 343. DOI: http://dx.doi.org/10.1007/BF02475274 6. Venkatathri, N.; Pillai, K. V.; Rajini, A.; Raju, M. N.; Reddy, I. A. K.; Open Catal. J. 2012, 5, 14. DOI: http://dx.doi.org/10.2174/1876214X01205010014 7. Holz, M. C.; Kahler, K.; Tolle, K.; Veen, A. C. V.; Muhler, M.; Phys. Status Solidi 2013 ,250, 1094. DOI: http://dx.doi.org/10.1002/pssb.201248504 8. Hutchings, G. J.; Enache, D. I.; Edwards, J. K.; Landon, P.; Espriu, B. S.; Carley, A. F.; Herzing, A. A.; Watanabe, M.; Kiely, C. J.; Knight, D. W.; Science 2006, 311, 362. DOI: http://dx.doi.org/10.1126/science.1120560 9. Li, G.; Enache, D. I.; Edwards, J.; Carley, A. F.; Knight, D. W.; Hutchings, G. J.; Catal. Lett. 2006, 110, 7. DOI: http://dx.doi.org/10.1007/s10562-006-0103-1 10. Stucky, G. D.; Zheng, N.; Chem. Commun. 2007, 3862. DOI: http://dx.doi.org/10.1039/b706864f 11. Keiichi, T.; Yutaka, F.; Yoshiki, I.; Mohammad, A.; Kaoru, F.; Catal. Lett. 2001, 76, 71. DOI: http://dx.doi.org/10.1023/A:1016711722721 12. Kazuhiro, N.; Kiyoshi, N.; Yasuhisa, N.; Hisao, Y.; Atsushi, S.; Tadashi, H.; Catal. Lett. 1999, 58, 127 DOI: http://dx.doi.org/10.1023/A:1019030017739 13. Andrei, K.; J, Yang.; Stephen, S.; Enrique, I.; Alexis T. B.; J. Catal. 1998, 177, 343. 14. Kaidong, C.; Shuibo, X.; Enrique, I.; Alexis T. B.; J. Catal. 2000, 189, 421. 15. Xin, Z.; Hai, W.; Bo-Qing, X.; J. Phys. Chem. B 2005, 109, 9678. DOI: http://dx.doi.org/10.1021/jp050645r 16. Sadiq, M.: Ilyas, M.; Chin. J. Chem. 2010, 28, 2216. DOI: http://dx.doi.org/10.1002/cjoc.201090366 17. Santos, V.; Zeni, M.; Bergmann, C. P.; Hohemberger, J. M.; Rev. Adv. Mater. Sci. 2008, 17, 62. 18. Ilyas, M.; Sadiq, M.; Chin. J. Chem. 2008, 26, 941. DOI: http://dx.doi.org/10.1002/cjoc.200890172 19. Xu, X.; Wang, X.; Nano Res. 2009, 2, 891. DOI: http://dx.doi.org/10.1007/s12274-009-9092-x 20. Mercera, P. D. L.; Von Ommen, J. G.; Doesburg, E. B. M.; Burggraaf, A. J.; Ross, J. R. H.; Appl. Catal. 1990, 57, 127. DOI: http://dx.doi.org/10.1016/S0166-9834(00)80728-9 21. Stichert, W.; Schuth, F.; J. Catal. 1998, 174, 2425. 22. Muhammad, S.; Hussain, S. T.; Waseem, M.; Naeem, A.; Hussain, J.; Jan, M. T.; Int. J. Sci. Technol. 2012, A4, 481. 23. Ai, M.; Suzuki, S.; J. Catal. 1973, 30, 362. DOI: http://dx.doi.org/10.1016/0021-9517(73)90151-6 |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access





