Artigo
| Staphylococcus aureus biofilm formation on polypyrrole: an electrical overview |
|
Erlon R. CordeiroI,II; Antonio W. C. FernandesIII; Alessandra F. C. PereiraI; Mateus M. da CostaIII; Marcio L. F. NascimentoII; Helinando P. de OliveiraI,*
IInstituto de Pesquisa em Ciência dos Materiais, Universidade Federal do Vale do São Francisco, 48902-300 Juazeiro - BA, Brasil Recebido em 13/02/2015 *e-mail: helinando.oliveira@univasf.edu.br The development of organic devices based on conducting polymers for biofilm detection requires the combination of superior electrical response and high surface area for biofilm incorporation. Polypyrrole is a potential candidate for application in biofilm detection and control due to its characteristic superior electrical response and strong interaction with bacteria, which enables the use of the bioelectric effect in resulting devices. In this study, chemically synthesized polypyrrole was applied as a support for biofilm growth of S. aureus. Modifications in the electrical response of the polymeric template were explored to identify general mechanisms established during the deposition of the biofilm. INTRODUCTION Bacteria are available in nature as planktonic structures and as biofilms. Biofilms are composed by viscous matrix of polysaccharides with a porous structure which enables the nutrients exchange and waste elimination. Typical sequence of biofilm formation has been considered as follows: primary attachment, cell adhesion, proliferation of biofilm, maturation and planktonic cell detachment from biofilm.1 The study of biofilm has been progressively reported in the literature due to the resistance of enclosed microorganisms against conventional treatment (based on antibiotics) in comparison with planktonic form.2,3 It is reported a 50-100 times higher antibiotic resistance for these structures.3 Different physicochemical mechanisms at interface (bacteria/ electrode and bacteria/ culture media) contribute with complexity of adhesion of bacterial biofilm to specific surface.4,5 Staphylococcus aureus (S. aureus) is one of the most common nosocomial microorganisms (gram-positive cocci bacterium) with strong adaptation to the human host. Its presence is typically established in the human respiratory tract, skin and on interior surface of medical devices such as catheters and prostheses.6 S. aureus is associated in medical clinics with persistent skin infection (furuncles) and food poisoning.4,5,7 Serious diseases are associated with S. aureus due to their ability to adhere to surfaces with subsequent deposition of multilayered communities viz. biofilms, source of persistent infection after surgery.8 Particularly, biofilm formation represents a main source of risk for implant associated infections.9 Mature biofilms improve the antibiotic resistance and their action results in typical procedure of surgical replacement of infected implants. The use of alternative therapeutic agents represents important procedure in order to circumvent progressive resistance of biofilm to antibiotics (in particular to oxacilin).10 Application of essential oils,2 association of electrical current with bactericidal agent11 and electrochemical cathodic polarization for elimination of biofilm12,13 have been considered as alternative strategies for biofilm control. The emergence of antibiotic-resistant forms of pathogenic S. aureus (e.g. Methicillin-resistant S. aureus- MRSA) characterizes a worldwide problem in clinical medicine. MRSA causes diverse infections such as osteomyelitis and septic arthritis.14 Polypyrrole has been considered a potential candidate for applications involving biofilm detection and control. Zheng et al.3 reported the use of polypyrrole as in situ sensor for biofilm detection. Ungureanu et al.15 reported the use of polypyrrole as a polymeric support in association with polyethylene glycol for antibacterial activity. Zhang et al.9 reported the preparation of films of chitosan/ polypyrrole for applications using electrically driven bactericidal action. This process, termed bioelectric effect, takes place with excitation of surface of polymeric template/ biofilm with DC current and contributes with improvement in the efficacy of conventional antibiotics such as tobramycin and ciprofloxacin9 applied against biofilm-related infections in implants. The advantage of polypyrrole is related with its non-cytotoxicity and biodegradability associated with high level of conductivity. Based on these important applications, the study of biofilm formation on polymeric surface can be considered an important topic to be explored if considered the control in the resulting biofilm infections. The detection of biofilm depends on diverse processes such as non-uniform bacteria accumulation during growth and external parameters viz. nutrients, light and temperature16 and degree of immobilization on the surface of electrodes. Polymeric templates provide additional advantages relative to the available surface area and conductivity of support. Ungureanu et al. reported that roughness and wettability of polymeric surface represent important factors for bacterial adhesion and biofilm deposition.15 In this work, the process of biofilm incorporation on the surface of polypyrrole (PPy) electrodes has been characterized from the use of electrical impedance spectroscopy (EIS),17 SEM images and BET data. Interesting advantages have been observed due to novelty associated with simple and fast procedure for detection of biofilm formation using a 2-electrode configuration. With this aim, free growth of bacterial colonia of S. aureus on pellets of polypyrrole was established. Comparison of absorbance and impedance of culture media during bacterial growth was explored in order to identify bacterial and biofilm formation degree.
EXPERIMENTAL Materials and methods Pyrrole (Aldrich) was distilled before the use while ammonium persulfate (Vetec) was used as received. The structure of resulting material was characterized by FTIR technique (KBr method) using an IR Prestige-21 Fourier Transform Infrared Spectrometer Shimadzu. The morphology of polypyrrole was analyzed from images of a SEM Vega 3XMU microscope with an accelerating voltage of 5 kV and in situ detection of biofilm formation was performed by electrical impedance spectroscopy (EIS) using a Potentiostat / Galvanostat Metrohm Autolab AUT302N and absorbance in the visible region (546 nm) using a Hach UV-vis model DR5000. Monitoring of biofilm formation by EIS was established at regular intervals of time of 20 min in which the impedance (in the range of 1 Hz to 1 MHz) of culture media with bacteria and polypyrrole was analyzed. Brunauer-Emmett-Teller (BET) surface area measurements of resulting powder (polypyrrole+biofilm) were performed on a Micromeritics ASAP 2420 surface area analyzer. Chemical synthesis of polypyrrole 200 µL of pyrrole was dispersed in 100 mL of milli-Q water and maintained under intense stirring during 5 min at 0 ºC. 0.6846 g of ammonium persulfate was dissolved in a second beaker with 50 mL of milli-Q water under intense stirring during 5 min at 0 ºC. Aqueous solution of ammonium persulfate was slowly dropwised in the first beaker (pyrrole+water). The resulting solution was kept at 0 ºC under intense stirring during 2 h. After polymerization, the solution was filtered under vacuum and washed with water in abundance. The elimination of residual water was obtained in an oven (60 ºC) during 2 h. Pellets of 50 mg of polypyrrole and diameter of 0.77 mm were pressed at 20 kN during 3 min. Biofilm formation on surface of pellets of polypyrrole Different stock cultures were maintained in Muller-Hinton Agar during 24 h at 37 ºC in order to achieve a suspension of S. aureus with turbidity (τ=0.5) according to McFarland scale.18 Five pellets of polypyrrole were dispersed into 50 mL of culture media (tryptic soy broth - TSB) containing bacterial suspension during initial minutes of bacterial growth (planktonic and biofilm forms). The comparison with polypyrrole- free culture media was established in order to identify the influence of PPy on kinetics of bacterial growth. Preparation of samples for characterization For SEM analysis, pellets of PPy were kept in S. aureus culture media during a fixed interval of time (6h to 120 h) after that resulting material was dispersed in a Petri dish, subsequently exposed to UV radiation during 1 h and dried at 60 ºC. For electrical measurements, two metallic wires of 10 cm of length were introduced into the culture media with bacteria and pellets of PPy. An AC external excitation of 100 mV was established between electrodes. As described in the literature,14,19 bacterial growth can be conveniently addressed by single point measurement of absorbance at characteristic peak of planktonic and biofilm forms of bacteria. Increasing turbidity (in the visible region) is established with progressive growth of bacteria and therefore, the absorbance level can be used as an important parameter for determination of kinetics of bacterial growth. Measurement of absorbance of resulting solution was determined from aliquots of culture media removed at fixed interval of time of 20 min.
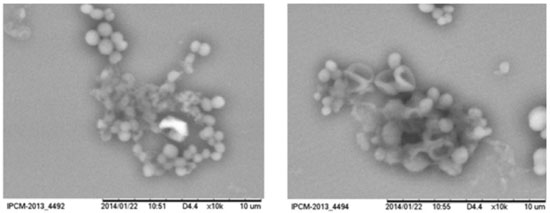
RESULTS AND DISCUSSION Biofilm formation is characterized by progressive ejection of extracellular polymeric substances rich in polysaccharides and proteins from bacteria, allowing the adhesion of bacteria on solid surface. Images in the Figure 1 reveals the adhesion of S. aureus as grape-like clusters on glass substrate (12 h of bacterial growth). Depressed surface of bacteria are progressively observed during biofilm formation and viscous solution contributes with disposition of bacterial clusters.
The use of polypyrrole as a polymeric matrix for biofilm deposition introduces interesting advantage for biofilm detection, due to the available surface area for bacterial deposition. Figure 2a shows that as-synthesized polymer is characterized by dispersion of polymeric grains and high level of rugosity.
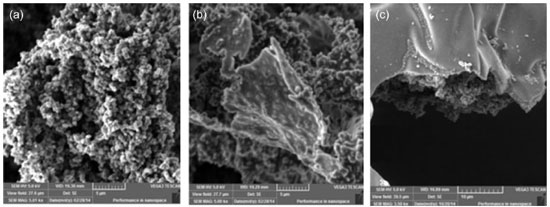 Figure 2. (a) SEM micrograph of as-synthesized polypyrrole, (b) after 6 h and (c) after 120 h of biofilm formation
Image of Figure 2b shows the progressive deposition of biofilm on the surface of polypyrrole (6 h of growth in culture media). Improved smoothness on surface of polymeric grains is a result of biofilm deposition on polypyrrole. If considered the large-scale growth process (in order of 120 h), it is possible to observe a thick layer of biofilm on the surface of polypyrrole (as shown in the Figure 2c), since in this condition the maturation of biofilm is followed by detachment of planktonic form of bacteria from biofilm (spherical particles disposed on cavities of flat surface). These results are in agreement with data obtained from BET surface area measurement: neat polypyrrole returns the higher value of available surface area (76.72 m2 g-1). The progressive incorporation of biofilm reduces the surface area of polypyrrole (as expected, due to the smoothness of biofilm) and after 24 h the BET surface area of resulting powder is in order of 34.38 m2 g-1. Progressive detachment of biofilm form surface of polypyrrole takes place with progressive biofilm formation, and a BET surface area of 54.39 m2 g-1 is obtained after 112 h of biofilm formation. FTIR spectrum of PPy and PPy + S. aureus biofilms is shown in the Figure 3. Peaks at 1548, 1468, 1045, 964 and 784 cm-1 are assigned to C=C and C-C stretching vibrations, C-N stretching vibration, C-H in-plane vibration, C-H out-of-plane vibration and pyrrole ring vibrations, respectively.20 Characteristic peaks of polypyrrole are preserved in sample (PPy+biofilm). The presence of residual water bounded to sample PPy+biofilm is verified from a broad band around 3500 cm-1.21 Mohr et al.22 reported that peak and a tiny shoulder at 862 cm-1 to 854.7 cm-1 act as indications of presence of bacteria in the resulting material (shown in the inset of Figure 3).
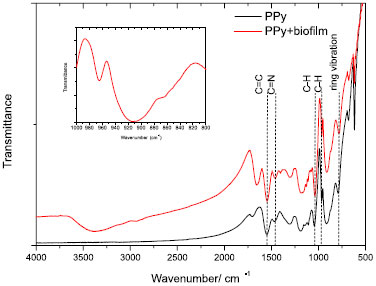 Figura 3. FTIR spectrum of polypyrrole and polypyrrole+ biofilm of S. aureus (in the inset is shown the region around 860 cm-1for sample PPy+biofilm)
For comparison with electrical response established in the presence of polypyrrole we have studied corresponding process of biofilm formation on glass surface. The biofilm deposition on surface of conducting ITO electrodes results in extremely high impedance layers (in order of 109-1010 ohms at low frequency limit - (1-100 Hz)) as shown in the Figure 4, showing that impedance increases with biofilm growth rate.
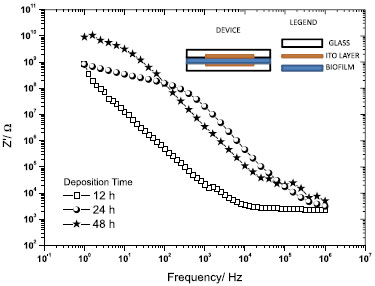 Figure 4. Real part of impedance of biofilms deposited on ITO layers/glass substrates
These data indicate that biofilms produced by S. aureus contribute with overall elevation in the level of impediment for current circulation along resulting structure. The absorbance in the visible light region indicates that kinetics of biofilm formation (polypyrrole-free culture media) follows a polynomial function as a consequence of increasing turbidity (progressive growth of bacteria) at increasing time (red curve shown in the Figure 5).
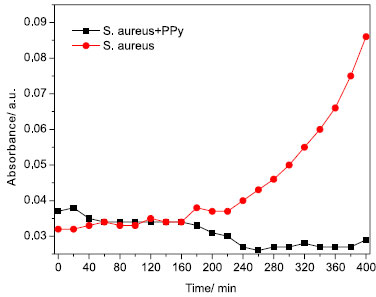 Figure 5. Comparison of kinetics of bacterial growth measured by UV-vis absorbance (polypyrrole-free sample - (S. aureus) and polypyrrole + S. aureus - (S. aureus+PPy))
If compared with mechanisms established in the presence of polypyrrole (sample S. aureus+PPy in the Figure 5), it is possible to verify that polypyrrole introduces a strong inhibitory effect on planktonic growth in culture media. The inhibition in the growth rate of bacteria is established after an initial period of induction (t < 20 min) followed by a negative slope (growth rate) after 180 min. After 240 min, the growth rate tends to zero (constant population of bacteria in culture media). As detected in the SEM images, the progressive growth of biofilm corroborates with hypothesis that competition between bacterial growth and biofilm formation takes place during initial 20 min. After induction time, a slight reduction in the bacterial population (planktonic form) is accomplished by progressive biofilm deposition. If considered the electrical signature of bacterial growth kinetics, a strong reduction in the impedance of culture media (as shown in the Figure 6) has been attributed to the elevation in the bacterial density in solution, which facilitates the current circulation between electrodes.
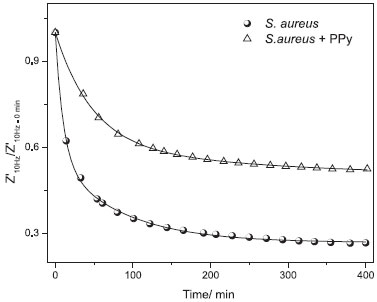 Figure 6. Kinetics of bacterial growth measured by EIS (comparison of polypyrrole-free sample (S. aureus) and polypyrrole +S. aureus (S. aureus+PPy))
Comparison of results considering polypyrrole-free culture media (S. aureus) and sample PPy+S. aureus indicates that inclusion of polypyrrole contributes with reduction in the variation of impedance, consequence of lower concentration of planktonic form in solution (in agreement with UV-vis absorbance data). Both curves are conveniently fitted by exponential decay function, according Eq. 1: 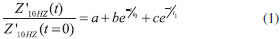 where a represents the ratio Z'10Hz(t→∞)/Z'10Hz (t=0), b and c are pre-exponential factors and t0 and t1 are characteristic time of reaction. The electrical response of culture media in the absence of bacteria is given by Z'10Hz(t)/Z'10Hz(t=0)=1 which act as a typical baseline: the variation in the impedance is attributed to external parameters than the experimental setup composed by reactor, culture media and electrodes. According Eq. 1, the higher characteristic time, lower the decay rate of impedance. Using this consideration, it is possible to correlate characteristic time with kinetics of biofilm formation. Fitting of Eq. 1 with experimental data indicates that characteristic time values for free growth of S. aureus in culture media are t0= 10.33 min and t1=84.37 min. The presence of polypyrrole in culture media during bacterial growth affects the electrical response of system and consequently the characteristic time values (t0=47.18 min and t1=265.85 min). These values correspond to plateaus identified in the UV-vis data for S. aureus+ PPy system: an initial period for inhibition of bacterial growth (t<40 min) and a negligible growth of bacteria (t>240 min) due to the inhibitory action of polypyrrole on planktonic growth. Corresponding values for characteristic times (47.18 min and 265.85 min) for samples of PPy + S. aureus are in reasonable agreement with results of absorbance. These results introduce important evidences about potential application of electrical impedance spectroscopy technique on study of biofilm formation in polymeric surfaces. The minimal interference introduced by AC excitation and low cost associated with polymer production represent important aspects associated with promising results for application of polypyrrole in the detection of S. aureus biofilm formation.
CONCLUSION The high conductivity and improved available surface area of polymeric support for biofilm deposition represent important parameters for detection of biofilm formation of S. aureus. The association of high conductivity and porosity of polypyrrole contributes with strong interaction and adsorption of biofilm on polymeric surface. This process results in different mechanisms, simultaneously detected by EIS and absorbance in the UV-vis region. An initial period in which bacterial growth is favored competes with biofilm deposition on surface of polypyrrole. This process reaches equilibrium at prolonged time of deposition (in order of 240 min), detected by constant turbidity of solution and saturation in the electrical response of media.
ACKNOWLEDGMENT The authors wish to thank CNPq, FAPESB, FACEPE and CAPES for financial support.
REFERENCES 1. Szczuka, E.; Urbanska, K.; Petryka, M.; Kaznowski, A.; Folia Microbiol. 2013, 58, 47. DOI: http://dx.doi.org/10.1007/s12223-012-0175-9 2. Nostro, A.; Roccaro, S.; Bisignano, G.; Marino, A.; Cannatelli, M. A.; Pizzimenti, F. C.; Cioni, P. L.; Procopio, F.; Blanco, A. R.; J. Med. Microbiol. 2007, 56, 519. DOI: http://dx.doi.org/10.1099/jmm.0.46804-0 PMID: 17374894 3. Zheng, L. Y.; Congdon, R. B.; Sadik, O. A.; Marques, C. N. H.; Davies, D. G.; Sammakia, B. G.; Lesperance, L. M.; Turner, J. M.; Sens. Actuators, B 2013, 182, 725. DOI: http://dx.doi.org/10.1016/j.snb.2013.03.097 4. Munõz-Berbel, X.; Munõz, F. J.; Vigués, N.; Mas, J.; Sens. Actuators, B 2006, 118, 129. DOI: http://dx.doi.org/10.1016/j.snb.2006.04.070 5. Giaouris, E.; Chapot-Chartier, M.-P.; Briandet, R.; Int. J. Food Microbiol. 2009, 131, 2. DOI: http://dx.doi.org/10.1016/j.ijfoodmicro.2008.09.006 PMID: 18954916 6. Singh, V.; Arora, V.; Alam, M. J.; Garey, K. W.; Antimicrob. Agents Chemother. 2012, 56, 4360. DOI: http://dx.doi.org/10.1128/AAC.00544-12 PMID: 22664967 7. Davis, C. A.; Pyrak-Nolte, L. J.; Atekawana, E. A.; Werkeman Jr., D. D.; Haugen, M. E.; J. Geophys. Res. 2010, 115, G00. 8. Lorenz, U.; Schäfer, T.; Ohlsen, K.; Tiurbe, G.C.; Bühler, C.; Germer, C.-T.; Kellersmann, R.; European Journal of Vascular and Endovascular Surgery 2011, 41, 68. DOI: http://dx.doi.org/10.1016/j.ejvs.2010.09.007 9. Zhang, J.; Neoh, K.G.; Hu, X.; Kang, E.-T.; Biomater. 2014, 35, 7690. DOI: http://dx.doi.org/10.1016/j.biomaterials.2013.09.082 10. Iorio, N.L.P.; Lopes, A. P. C. N.; Schuenck, R.P.; Barcellos, A. G.; Olendzki, A. N.; Lopes, G. L.; dos Santos, K.R. N.; Microbiol. Immunol. 2010, 55, 28. DOI: http://dx.doi.org/10.1111/j.1348-0421.2010.00288.x PMID: 21175771 11. Jass, J.; Lappin-Scott, H. M.; J. Antimicrob. Chemother. 1996, 38, 987. DOI: http://dx.doi.org/10.1093/jac/38.6.987 PMID: 9023646 12. Dargahi, M.; Hosseinidoust, Z.; Tufenkji, N.; Omanovic, S.; Colloids Surf., B 2014, 117, 152. DOI: http://dx.doi.org/10.1016/j.colsurfb.2014.02.021 13. Schaule, G. ; Rumpf, A. ; Weeidilich, C. ; Mangold, K. -M.; Flemming, H. -C.; Water Sci. Technol. 2008, 58, 2165. DOI: http://dx.doi.org/10.2166/wst.2008.529 PMID: 19092192 14. Wu, W.-S.; Chen, C. -C.; Chuang, Y.-C.; Su, B.-A.; Chiu, Y.-H.; Hsu, H.-J.; Ko, W.-C.; Tang, H.-J.; J. Microbiol., Immunol. Infect. 2013, 46, 89. DOI: http://dx.doi.org/10.1016/j.jmii.2011.12.024 15. Ungureanu, C.; Pirvu, C.; Mindroiu, M.; Demetrescu, I.; Prog. Org. Coat. 2012, 75, 349. DOI: http://dx.doi.org/10.1016/j.porgcoat.2012.07.015 16. Wang, X.; Lin, H.; Wang, J. ; Xie, B.; Huang, W. ; Mater. Lett. 2012, 78, 174. DOI: http://dx.doi.org/10.1016/j.matlet.2012.02.068 17. Barsoukov, E.; Macdonald, J. R.; Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed., Wiley: New York, 2005. 18. Mcfarland, J.; J. Am. Medical Assoc. 1907, 49, 1176. DOI: http://dx.doi.org/10.1001/jama.1907.25320140022001f 19. Nassar, H. M.; Li, M.; Gregory, R. L.; Appl. Environ. Microb. 2012, 78, 536. DOI: http://dx.doi.org/10.1128/AEM.05538-11 20. de Oliveira, H. P.; Sydlik, S. A.; Swager, T. M.; J. Phys. Chem. C 2013, 117, 10270. DOI: http://dx.doi.org/10.1021/jp400344u 21. Wan, L. S.; Huang, X. J.; Xu, Z. K.; J. Phys. Chem. B 2007, 111, 922. DOI: http://dx.doi.org/10.1021/jp065152g PMID: 17266244 22. Mohr, J. E.; Carter, P. B.; Cochran, G. W; Appl. Microbiol. 1962, 10, 176. PMID: 14475079 |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access