Artigo
| A novel polyacrylamide-based hydrogel crosslinked with cellulose acetate and prepared by precipitation polymerization |
|
Rubén O. Muñoz-GarcíaI; María E. HernándezII,*; Genaro G. OrtizIII; Víctor V. A. FernándezIV; Martín R. ArellanoII; Juan C. Sánchez-DíazIIrnández; Genaro G. Ortiz; Víctor V. A. Fernández; Martín R. Arellano; Juan C. Sánchez-Díaz
IDepartamentos de Química, Universidad de Guadalajara. Boul. M. García Barragán # 1451, Guadalajara, Jal. 44430, México Recebido em 16/02/2015 *e-mail: elena.hernandez@ymail.com The synthesis of polyacrylamide-cellulose acetate hydrogels by precipitation polymerization in acetone solution is reported herein. These hydrogels exhibit smaller swelling ratios and larger compression moduli than homo polyacrylamide hydrogels. For cellulose acetate concentrations above 20 wt.%, hydrogels with N,N'-methylenebisacrylamide as a crosslinker exhibit swelling ratios and compression moduli similar to those of the hydrogels without the crosslinker. A possible explanation for this behavior is that cellulose acetate crosslinks polyacrylamide via free-radical reaction. The hydrogels obtained without the N,N'-methylenebisacrylamide crosslinker exhibit compression moduli up to 1.7 MPa, making them suitable for tissue engineering applications such as cartilage replacement. INTRODUCTION Hydrogels are crosslinked networks that absorb large amounts of water while maintaining their tridimensional structural stability.1 The swelling capacity of the hydrogels depends on the hydrophilic groups present in the network.2 Among two-polymeric component networks, there are the so-called interpenetrating polymer networks (IPNs) and the semi-interpenetrated networks (semi-IPN). An IPN is the combination of two crosslinked polymers; meanwhile in a semi-IPN, one of the polymeric components is linear.3,4 Polyacrylamide (PAAm) hydrogels are usually prepared by free-radical crosslinking copolymerization of acrylamide (AAm) in the presence of a crosslinking agent such as N,N'-methylenebisacrylamide (NMBA). Crosslinked PAAm can form semi-IPNs5,6 as well as grafted IPNs.7 Cellulose acetate (CA) is a polymer composed of cellulose di- and triacetate commonly processed into films.8 In CA hydrogels crosslinked with molecules containing polar functional groups such as carboxylic acid, the swelling capacity can reach up to 1000%.9 CA has been used in controlled release and water retention fertilizers,10 semi-IPN with acrylic acid and copolymerized with PAAm to obtain membranes via photopolymerization.11,12 As biomedical applications, hydroxypropyl cellulose and AAm-based hydrogels have been used in tissue engineering applications13 as well as in controlled drug release.14 The compression and Young modulus of AAm-based hydrogels are important properties for some tissue engineering applications. However, values of the modulus differ up to one order of magnitude, depending upon the starting materials and the network conformation. For instance, double network hydrogels (DNH) derived from PAAm and poly(2-acrylamido-2-methy-1-propanesulphonic acid) crosslinked with NMBA exhibit compression modulus of c.a. 0.3 MPa; for DNH of bacterial cellulose and poly(dimethyl acrylamide) crosslinked with NMBA, the compression modulus is 1.7 MPa.15 Potential application for such hydrogels as artificial cartilage has been reported.15-17 Furthermore, a semi-IPN obtained with PAAm-methyl cellulose exhibit the compression modulus up to 30 kPa meanwhile for the hydrogels derived from methacrylated cashew gum and PAAm the compression modulus is 1-3 kPa.18,19 For hydrogels of hydroxypropyl cellulose crosslinked with divinyl sulphone the Young modulus reaches up to 700 kPa.20 For the case of nanostructured hydrogels of poly(N-isopropylacrylamide) crosslinked with NMBA, the Young modulus is c.a. 5 kPa;21 similar Young modulus (3 kPa) is reported for semi-IPN obtained from chitosan and a acrylamide-itaconic acid copolymer.14 Conversely, hydrogels of poly(hydroxyethylmethacrylate) crosslinked with NMBA exhibit the Young modulus up to 500 kPa.22 Nevertheless, there are reports of hydrogels derived from AAm-modified cellulosic materials, it is important to point out that the use of NMBA as crosslinker was necessary to obtain a network that is insoluble in water. However, in this work we report the preparation of hydrogels obtained from acrylamide and CA that are insoluble in water without the use of NMBA as crosslinker, for a CA composition range of 10-25 wt.%. The compression modulus exhibited by these hydrogels is high enough to consider them as suitable materials in artificial cartilage applications.
EXPERIMENTAL Materials AAm 99%, NMBA 99%, CA 97% (DS 2.5, 50,000 Da), benzoyl peroxide (BP) 99% and acetone A.C.S. grade from Aldrich Chemical were used as received. Double-distilled water was used. Preparation of hydrogels The polyacrylamide/cellulose acetate (PAAm-CA) hydrogels were synthesized by precipitation polymerization (PP) from acetone solution using several CA wt.% (0, 1, 2.5, 5, 10, 15, 20, 25). The total solids concentration in the system was fixed at 10 wt.%. AAm and CA were dissolved in acetone with BP as initiator (1.0 wt.% w.r.t. AAm) and left to polymerize at 63 ºC for 24 h. Nitrogen bubbling was applied during the entire period. Formulations with NMBA contained 1.0 wt.% w.r.t. AAm. The synthesized hydrogels, obtained in the shape of disks, were immersed in acetone and left for several days to wash out any residual CA left unincorporated to the network. Once the hydrogels were washed with acetone, the samples were immersed in water and left for several days to wash out any residual monomer and/or linear PAAm. After washing, the hydrogels were left to dry in a convection oven at 50 ºC. The global yield was calculated as 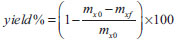 where mx0 is the xerogel initial mass before the acetone purification step and mxf is the hydrogel final mass after the water purification step. Characterization by Fourier transformed infrared spectroscopy (FTIR) Samples obtained from the acetone purification step as well as from the final xerogels were analyzed by FTIR spectroscopy looking for unreacted AAm monomer and free CA. Few drops of the acetone from the first purification step were placed on a NaCl crystal and were left to evaporate to form a thin film. The xerogel samples were prepared in 0.50 mm-thick KBr pellets, obtained by compression molding (0.6 - 0.8 mg of xerogel with 80 mg of dried KBr). The FTIR spectra between 4,000 and 400 cm-1 were recorded using a Spectrum One FTIR spectrophotometer (Perkin Elmer, Waltham, MA, USA). All spectra were obtained at ambient temperature with a resolution of 4 cm-1, and 50 scans were carried out for each sample. The final spectra of xerogels were normalized before comparison to the starting materials spectra. The spectrum of the acetone from the purification step is shown unnormalized for identification purposes only. Swelling measurements To measure the swelling capacity, disk samples (approximately 1.5 g) were immersed in an excess of water at room temperature (22 ± 1 ºC). The hydrogels were immersed in water for at least two weeks during which water was replaced every other day. The weight of the swollen samples was measured after the excess surface water was removed by gently tapping the surface with a dry piece of filter paper. The degree of swelling (S) for each disk sample at time t was calculated as S = (wt - w0) / w0 where wt and w0 are the sample's weight at any given time, and in the dry state, respectively. The swelling capacity of the hydrogels was taken as the steady state value of S. Mechanical properties Compression tests were done on the hydrogel disks once they were swollen to their swelling capacity at 20 ºC. These tests were carried out in a rheometer (AR-G2 TA Instruments). Hydrogels swollen to swelling capacity were cut into 15 mm diameter and 4 mm thickness disks. Strain-stress tests were performed with a 10 mm/s deformation rate up to 20% compression. The compression modulus was calculated at small deformations as the slope of the initial linear zone (less than 5% strain) of the stress-strain curve.
RESULTS AND DISCUSSION The AAm-based hydrogels presented in this work were synthetized by PP from an acetone solution (Figure 1). PAAm hydrogels crosslinked with NMBA obtained by PP from acetone solution are very weak, fragile and translucid (Figure 1A). However, PAAm-CA hydrogels obtained by PP are strong, rigid and opaque (Figure 1B). On the contrary, PAAm hydrogels crosslinked with NMBA obtained by aqueous solution polymerization are transparent at the same NMBA concentration (Figure 1C). Most likely, the PAAm hydrogels obtained by PP exhibit spatial inhomogeneity that render them as small blobs due to Van der Waals forces. However, in PAAm hydrogels submersed in water during 3 weeks, osmotic swelling overcame Van der Waals forces, fragmenting them in small pieces.23 The PAAm-CA hydrogels with 10 wt.% CA, either with or without NMBA, remained in one piece after being submersed in water for 3 weeks. This suggests that the intermolecular interactions in the PAAm-CA hydrogels are stronger that Van der Waals forces, as it is the case of crosslinking by covalent bonds.
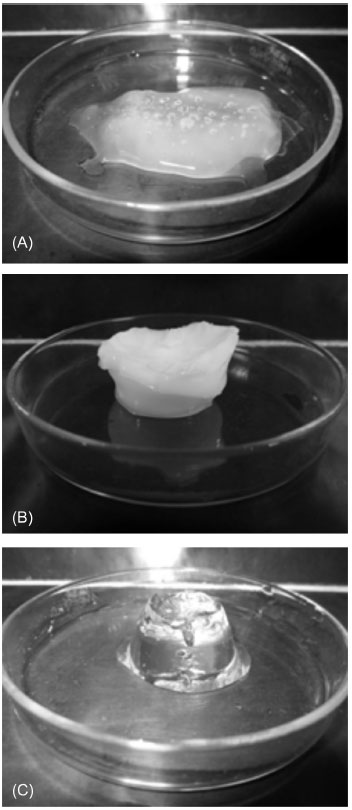 Figure 1. Polyacrylamide-cellulose acetate (PAAm-CA) hydrogels obtained by precipitation polymerization. (A) PAAm hydrogel crosslinked with N,N'-methylenebisacrylamide (NMBA), swollen in water to maximum swelling capacity. (B) PAAm-CA hydrogel, 20 wt.% of CA, without NMBA, swollen in water to maximum swelling capacity. (C) PAAm hydrogel crosslinked with N,N'-methylenebisacrylamide (NMBA), obtained by aqueous solution polymerization, swollen in water to maximum swelling capacity
FTIR Spectroscopy FTIR analysis has been utilized to prove the chemical reaction of CA with AAm for the PAAm-CA hydrogels prepared without NMBA. The FTIR spectra of PAAm, CA and the PAAm-CA xerogels for several CA concentrations up to 25 wt.% are shown in Figure 2. From the spectrum of CA several characteristic bands are evident: 3487 cm-1 (-OH), 1748 cm-1 (-CO, ether), 1236 cm-1 (-COC, ether, asymmetric stretching), 1050 cm-1 (-COC, ester, symmetric stretching), and 1369 cm-1 (-CH3 symmetric bending). The spectrum of PAAm exhibits the following characteristic AAm bands: 3363 cm-1 and 3204 cm-1 (-NH symmetric and asymmetric stretching respectively), 1680 cm-1 (-CO, amide), 1618 cm-1 (-NH stretching), and 1415 cm-1 (-CN, amide I).24 Close examination of Figure 2 shows that starting at the 10 wt.% CA concentration, the PAAm-CA hydrogels exhibit the 1748 cm-1 and 1236 cm-1 bands, characteristic of the CA ether (-CO), as well as the 1050 cm-1 band, characteristic of the CA ester (-COC). From Figure 2, it is evident that the intensity of the absorption peak at the CA characteristic bands augments as the CA wt.% increases in the PAAm-CA xerogels. Since the unbounded CA was removed from the PAAm-CA hydrogels by acetone extraction (Figure 3) and the presence of these bands gives strong evidence of crosslinking.
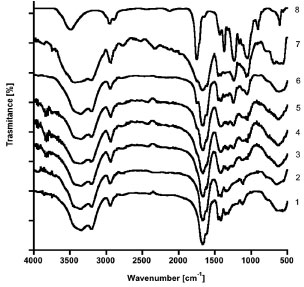 Figure 2. FTIR spectra of PAAm, CA and the PAAm-CA xerogels for several CA concentrations: (1) PAAm, (2) 1 wt.%, (3) 5 wt.%, (4) 10 wt.%, (5) 15 wt.%, (6) 20 wt.%, (7) 25 wt.%, and (8) CA
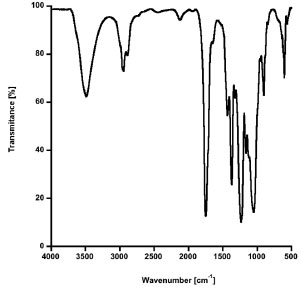 Figure 3. FTIR spectrum of an acetone residual from the acetone purification step
Global yield of the PAAm polymerization reaction with CA Table 1 shows the global yield% of the PAAm-CA hydrogels with NMBA as crosslinker for the acrylamide monomer. In this table, global yield of PAAm-CA formulations without NMBA is included for comparison purposes. The global yield was calculated for the purified xerogels as mentioned in the experimental section. Table 1 shows that for hydrogels with NMBA, the global yield increases with the CA concentration. However, it is interesting to point out that for concentrations of CA >5 wt.% the global yield% also increases for formulations without NMBA as crosslinker. Since the purification process was designed to extract soluble polymer by sequential and selective solvent extraction steps, the material recuperated is crosslinked in nature. Such crosslinked polymeric material was obtained for both formulations, with and without NMBA. This fact strongly suggests that NMBA can be used as a potential crosslinker for acrylamide monomers. In general, Table 1 shows that the global yield of formulations without NMBA is smaller than the one using NMBA as crosslinker.
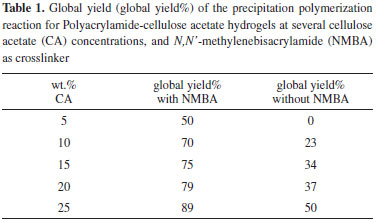
Swelling Figure 4 shows the swelling capacity of the PAAm-CA hydrogels at several CA concentrations. The hydrogels with NMBA as crosslinker exhibit a decreasing swelling capacity with CA concentration. Crosslinked hydrogels normally exhibit a decrease in swelling capacity with increasing crosslinking density. However, these PAAm-CA hydrogels are prepared with a fixed amount of NMBA, so their crosslinking density should be similar. Decreasing swelling capacity as CA concentration augments is expected for hydrogels with a hydrophobic component such as CA. Hence, as the amount of hydrophobic groups augments, the swelling capacity is reduced. Apparently, the PAAm-CA hydrogels are semi-IPNs with the cellulosic component trapped within the PAAm network crosslinked with NMBA.6 Furthermore, Figure 4 shows the swelling capacity of PAAm-CA hydrogels prepared without NMBA as crosslinker. These are the most relevant results because they indicate that the swelling capacity of NMBA-free PAAm-CA hydrogels is comparable to the swelling capacity of formulations with NMBA for CA concentrations higher than 20 wt.%. Since the swelling behavior of PAAm-CA hydrogels without NMBA exhibits evidence of PAAm being crosslinked, CA can be considered a potential crosslinker for acrylamide via free radical polymerization.
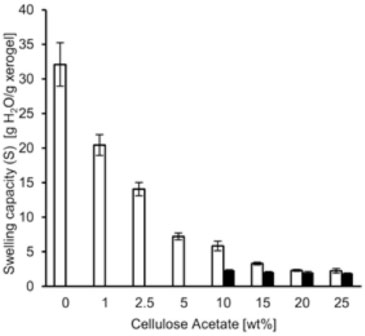 Figure 4. Swelling capacity of the Polyacrylamide-cellulose acetate (PAAm-CA) hydrogels obtained by precipitation polymerization at several CA concentrations: (white bars) crosslinked with N,N'-methylenebisacrylamide (NMBA), (black bars) without NMBA
Compression modulus Figure 5 shows the compression modulus of the PAAm-CA hydrogels at several CA concentrations. For the hydrogel formulations with NMBA as crosslinker, the compression modulus augments with the CA concentration. The PAAm-CA hydrogels obtained by precipitation solution exhibit compression moduli of 0.2 - 1.7 MPa. Typical values of compression modulus of PAAm hydrogels obtained by aqueous solution polymerization are c.a. 0.075 MPa.25 The PAAm-CA hydrogels reported hereby show an improvement in the compression modulus. This trend is exhibited by samples without NMBA too. As a matter of fact, crosslinking with NMBA shows no statistical significant effect on the compression modulus. The increment of the compression modulus with CA concentration suggests the increment in crosslinking density as well. Hence, the fact that compression moduli are similar for samples with and without NMBA also suggests the role of CA as crosslinker in the PAAm free-radical polymerization.
 Figure 5. Compression modulus of the Polyacrylamide-cellulose acetate (PAAm-CA) hydrogels obtained by precipitation polymerization at several CA concentrations: (white bars) crosslinked with N,N'-methylenebisacrylamide (NMBA), (black bars) without NMBA
Proposed polymerization mechanism for the NMBA-free PAAm-CA hydrogels. It has been reported the chemically induced graft copolymerization of acrylamide onto cotton fibers with benzoyl peroxide used as initiator.26 Furthermore, both grafting and crosslinking have been reported for acrylic monomers onto cellulosic materials via free-radical polymerization. 27,28 The polymerization mechanism proposed for the NMBA-free PAAm-CA hydrogels is shown in the Scheme 1. This is a mechanism similar to the one reported for the grafting reaction of acrylamide into cellulose including chain transfer.28 In this case, the authors proposed a free-radical graft polymerization of acrylamide onto cellulose with crosslinking induced by chain transfer, not by the use of NMBA as crosslinker.
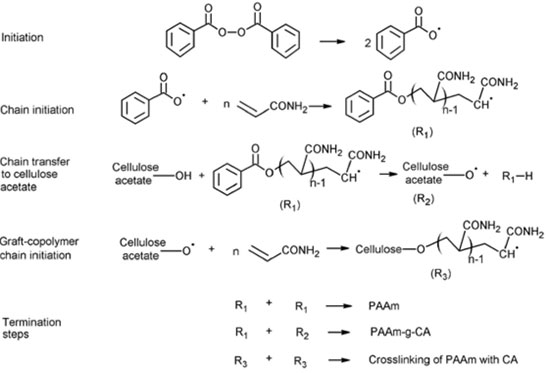 Scheme 1. Polymerization mechanism proposed for the NMBA-free PAAm-CA hydrogels
In the mechanism proposed here, during the initiation step, benzoyl peroxide forms free radicals and initiates the chain polymerization of the acrylamide monomer. Next, free radicals are formed at the OH group of CA by chain transfer from a growing PAAm chain. This reaction is statistically feasible because we used CA with a substitution degree of 2.5 providing one OH group per two anhydride glucose rings. It's in this OH group that grafting of acrylamide onto CA occurs. Furthermore, the proposed crosslinking takes place by chain transfer of two grafted PAAm growing chains. This is the most relevant step because the need for NMBA as crosslinker is eliminated. Figure 6 shows a schematic representation of the network structure obtained from the polymerization mechanism proposed hereby. PAAm chains can crosslink more than once with CA polymeric chains (Figure 6A). In the presence of NMBA, the PAAm polymer chains can crosslink with both, the NMBA and the CA as depicted in Figure 6B.
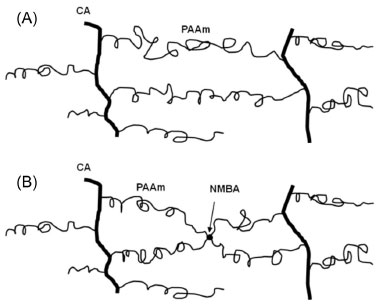 Figure 6. Schematic diagram of the network structures of the Polyacrylamidecellulose acetate hydrogels obtained: (A) without N,N'-methylenebisacrylamide (NMBA), and (B) with NMBA
It was found that for PAAm-CA hydrogels with CA concentrations of 20 and 25 wt.%, the use of NMBA in the formulations shows no significant effect on the swelling capacity (Figure 4) and the compression modulus (Figure 5). This suggests that PAAm and CA formed a crosslinked network. The PAAm-CA hydrogels without NMBA were subjected to thermal and solubility test to verify the nature of the crosslinking points. Xerogel samples of PAAm-CA hydrogels were heated-up with a Bunsen burner and no fusion point was detected; the samples were carbonized. Hence, the thermal test suggested the presence of a tridimensional chemically crosslinked network. In the solubility tests, xerogel samples of PAAm-CA hydrogels without NMBA were subjected to the action of 9 different solvents. The choice of solvents covered the polar-non-polar range from methanol to toluene. A well-known solution to dissolve cellulose, 5 wt.% of LiCl in dimethylacetamide,29 was used in the solubility test as well. None of the solvents used in the solubility tests dissolved the PAAm-CA xerogels. Solubility test results point out to the chemical nature of the crosslinks in the PAAm-CA hydrogels without NMBA as crosslinker.
CONCLUSIONS It is possible to obtain hydrogels of PAAm-CA that exhibit small swelling capacity and high compression modulus. Relevant results are obtained in the absence of the crosslinking agent NMBA. The use of NMBA as crosslinking agent has no effect on either the swelling capacity for CA concentrations of 20 - 25 wt.%, or the compression modulus for CA concentrations of 10 - 25 wt.%. The CA most likely acts a crosslinking agent for PMMA via free radicals mechanism. The PAAm-CA obtained without the use of NMBA as crosslinker exhibit swelling and compression properties that make them potential candidates for cartilage replacement applications.
ACKNOWLEDGEMENTS This research was supported by CONACYT (APOY-COMPL- 2009-SNI-1 119176). One of us (ROMG) thanks CONACYT for the student grant.
REFERENCES 1. Peppas, N. A.; Bures, P.; LeoBandung, W.; Ichikawa, H.; Eur. J. Pharm. Biopharm. 2000, 50, 27. DOI: http://dx.doi.org/10.1016/S0939-6411(00)00090-4 PMID: 10840191 2. Baumgartner, S.; Kristl, J.; Peppas, N. A.; Pharmaceutical Res. 2002, 19, 1084. DOI: http://dx.doi.org/10.1023/A:1019891105250 3. Hennik, W. E.; Van Nostrum, C. F.; Adv. Drug Delivery Rev. 2012, 64, 223. DOI: http://dx.doi.org/10.1016/j.addr.2012.09.009 4. Shin, M. S.; Kim, S. I.; Kim, Y.; Kim N. G.; Song, C. G.; Kim, S. J.; Appl. Polym. Sci. 2002, 84, 2591. DOI: http://dx.doi.org/10.1002/app.10365 5. Tuncer, C.; Gamze, S.; Gökcen, B.; Macromol. Mater. Eng. 2006, 291, 1044. DOI: http://dx.doi.org/10.1002/mame.200600063 6. Chauhan, G. S.; Lal, H.; Mahajan, S.; J. Appl. Polym. Sci. 2004, 91, 479. DOI: http://dx.doi.org/10.1002/app.13135 7. Das, R.; Panda, A. B.; Sagar, P.; Cellulose 2012, 19, 933. DOI: http://dx.doi.org/10.1007/s10570-012-9692-6 8. Tamborim, S.M.; Dias, S. L. P.; Silva, S. N.; Dick, L. F. P.; Azambuja, D. S.; Corros. Sci. 2011, 53, 1571. DOI: http://dx.doi.org/10.1016/j.corsci.2011.01.034 9. Botaro, V. R.; Santos, C. G.; Oliveira, V. A.; Revista Polímeros 2009, 19, 278. DOI: http://dx.doi.org/10.1590/S0104-14282009000400006 10. Wu, L.; Liu, M.; Polym. Adv. Technol. 2008, 19, 785. DOI: http://dx.doi.org/10.1002/pat.962 11. Bajpai, S. K.; Banger, P.; Polym. Eng. Sci. 2013, 53, 2129. 12. Chatterjee, S.; Sarkar, S.; Bhattacharyya, S. N.; Barat, P.; J. Appl. Polym. Sci. 1996, 59, 1973. DOI: http://dx.doi.org/10.1002/(SICI)1097-4628(19960328)59:13<1973::AID-APP2>3.0.CO;2-F 13. Yue, Z.; Wen, F.; Gao, S.; Ang, M. Y.; Pallathadka, P. K.; Liu, L.; Yu, H.; Biomaterials 2010, 31, 8141. DOI: http://dx.doi.org/10.1016/j.biomaterials.2009.09.032 PMID: 20691470 14. Martinez-Ruvalcaba, A.; Sanchez-Diaz, J.C.; Becerra, F.; Cruz-Barba, L. E.; Gonzalez-Alvarez, A.; eXPRESS Polym. Lett. 2009, 3, 25. DOI: http://dx.doi.org/10.3144/expresspolymlett.2009.5 15. Azuma, C.; Yasuda, K.; Tanabe, Y.; Taniguro, H.; Kanaya, F.; Nakayama, A.; Chen, Y. M.; Gong, J. P.; Osada, Y.; J. Biomed. Mater. Res. 2006, 81, 373. 16. Tanabe, Y.; Yasuda, K.; Azuma, C.; Taniguro, H.; Onodera, S.; Suzuki, A.; Chen, Y.M.; Gong, J. P.; Osada, Y.; J. Mater. Sci.: Mater. Med. 2008, 9, 1379. 17. Yasuda, K.; Gong, J. P.; Katsuyama, Y.; Nakayama, A.; Tanabe, Y.; Kondo, E.; Ueno, M.; Osada, Y.; Biomaterials 2005, 26, 4468. DOI: http://dx.doi.org/10.1016/j.biomaterials.2004.11.021 PMID: 15701376 18. Aouada, F. A.; Chiou, B.; Orts, W. J.; Mattoso, L. H. C.; Polym. Eng. Sci. 2009, 49, 2467. DOI: http://dx.doi.org/10.1002/pen.21505 19. Guilherme, M. R.; Campese, G. M.; Radovanovic, E.; Rubira, A. F.; Feitosa, J. P. A.; Muniz, E. C.; Polymer 2005, 46, 7867. DOI: http://dx.doi.org/10.1016/j.polymer.2005.06.068 20. Hinkley, J. A.; Morgret, L. D.; Gehrke, S. H.; Polymer 2004, 45, 8837. DOI: http://dx.doi.org/10.1016/j.polymer.2004.09.088 21. Fernandez, V. V. A.; Tepale, N.; Sanchez-Diaz, J. C.; Mendizabal, E.; Puig, J. E.; Soltero, J. F. A.; Colloid Polym. Sci. 2006, 284, 387. DOI: http://dx.doi.org/10.1007/s00396-005-1395-1 22. Zhang, Z.; Chao, T.; Liu, L.; Cheng, G.; Ratner, B. D.; Jiang, S.; J. Biomater. Sci., Polym. Ed. 2009, 20, 1845. DOI: http://dx.doi.org/10.1163/156856208X393473 23. Lopour, P.; Seveik, S.; Labsky, J.; Dednicky, F.; Die Angewandte Makromolekulare Chemie 1996, 243, 151. DOI: http://dx.doi.org/10.1002/apmc.1996.052430113 24. Silverstein, R. M.; Webster, F. X.; Spectrometric Identification of Organic Compounds, 6th ed., Wiley: Hoboken, 1997. 25. Muniz, E. C.; Geuskens, G.; Macromolecules 2001, 34, 4480. DOI: http://dx.doi.org/10.1021/ma001192l 26. Pulat, M.; Isakoca, C.; J. Appl. Polym. Sci. 2006, 100, 2343. DOI: http://dx.doi.org/10.1002/app.23060 27. Ghosh, P.; Das, D.; Eur. Polym. J. 2011, 36, 2505. DOI: http://dx.doi.org/10.1016/S0014-3057(00)00028-8 28. Roy, D.; Semsarilar, M.; Guthrie, J.; Perrier, S.; Chem. Soc. Rev. 2009, 38, 2046. DOI: http://dx.doi.org/10.1039/b808639g PMID: 19551181 29. Chang, C.; Zhang, L.; Carbohydr. Polym. 2011, 84, 40. DOI: http://dx.doi.org/10.1016/j.carbpol.2010.12.023 |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access





