Artigo
| Pyroligneous liquor produced from Acacia mearnsii de wild wood under controlled conditions as a renewable source of chemicals |
|
Carolina M. FurtadoI; Aline dos Santos StolzI; Fernanda L. PintoI; Angela B. D. MouraI; Fernando Dal Pont MorissoI; Ana Paula PitareloII; Luiz Pereira RamosII; Carin von MühlenI; Izabel C. Riegel-VidottiI,*
IInstituto de Ciências Exatas e Tecnológicas, Universidade Feevale, RS-239, 2755, 93352-000 Novo Hamburgo - RS, Brasil Recebido em 07/04/2015 *e-mail: izabel.riegel@ufpr.br Acacia mearnsii de Wild (black wattle) is one of the most important trees planted in Southern Brazil for tannin extraction and charcoal production. The pyrolysis of the black wattle wood used for obtaining charcoal is performed in brick ovens, with the gas fraction being sent directly into the environment. The present study examines the condensable compounds present in the liquor produced from black wattle wood at different thermal degradation conditions, using gas chromatography coupled with mass spectrometry (GC/MS). Branches of black wattle were thermally degraded at controlled ambient and temperature conditions. Overall, a higher variety of compounds were obtained under atmospheric air pressure than under synthetic air pressure. Most of the tentatively identified compounds, such as carboxylic acids, phenols, aldehydes, and low molecular mass lignin fragments, such as guayacol, syringol, and eugenol, were products of lignin thermoconversion. Substituted aromatic compounds, such as vanillin, ethyl vanillin, and 2-methoxy-4-propeny-phenol, were also identified. At temperatures above 200 ºC, furan, 2-acetylfuran, methyl-2-furoate, and furfural, amongst others, were identified as polysaccharide derivatives from cellulose and hemicellulose depolymerization. This study evidences the need for adequate management of the condensable by-products of charcoal production, both for economic reasons and for controlling their potential environmental impact. INTRODUCTION Wood biomass has long been used as a source of energy for heating, cooking and industry. Biomass is a general term for all living organic matter and it contributed to about 10% of the world's energy supply in 2013.1 The demand for wood biomass is constantly growing not only because it is a short-term renewable source of organic materials but also because photosynthesis is the most efficient mechanism for carbon dioxide fixation from the atmosphere. Black wattle (Acacia mearnsii de Wild) is a native species from Australia that was introduced in Brazil through seeds coming from South Africa. In Southern Brazil, its cultivation has spread throughout the Rio Grande do Sul State, such that the acacia production chain has become an important economic activity with considerable social and environmental impact in this region. As a family production activity, thousands of charcoal furnaces are distributed throughout more than 10,000 small properties in Rio Grande do Sul. The main economic interest in black wattle plantations is associated with extracting tannins from the barks and trunks. As a consequence, this large scale production generates large secondary biomass coproducts. The acacia wood has been used as a direct source of energy production or as charcoal, and is also useful for other industrial activities for the production of pulp and paper, rayon, shavings, plates, fibers, parquet, and wood chips.2 Despite the numerous advantages of using wood as fuel, the charcoal industry is responsible for discharging materials that pollute the surrounding atmosphere, water beds, soil and vegetation and may have impacts far away from the furnaces' location. Additionally, the wood charcoal production is very rudimentary, leading to low conversions and yields, generating high amounts of disposable residues. Studies have been carried out to improve the efficiency of pyrolytic processes3 as well as to identify other value-added applications for the pyrolysis residues.4 Especially for those family-based activities, it is extremely important to improve the population's standard of living as well as to reduce the environmental impact of their production activities. In Brazil, the gas phase of the pyrolytic process of acacia wood charcoal production is currently sent out into the atmosphere. However, it is known that, under controlled conditions, the semi-volatile fraction can be condensed to create a pyroligneous liquor that can be used as raw material for the pharmaceutical, cosmetic and oil industries, as well as food processing and others applications, as already reported for condensed liquors derived from other wood conversion processes. In addition, it is important to evaluate process variables such as pressure, moisture content and gas flow for an efficient energy generation during biomass conversion.5,6 Bio-oils have been characterized using modern analytical techniques such as pyrolysis- gas chromatography coupled with mass spectrometry4 and comprehensive two-dimensional gas chromatography coupled with mass spectrometry due to the high complexity of the extracts.7,8 To the best of our knowledge, there are no reports in the literature regarding the characterization of the extract resulting from controlled thermal degradation of A. mearnsii de Wild. For this reason, this study provides a detailed characterization of various pyroligneous extracts from this wood material using gas chromatography coupled with mass spectrometry (GC/MS). Prior to the GC/MS analysis, an extraction method was also developed to improve the efficiency of the analytical procedure which proved to be very successful for the characterization of very acidic samples.
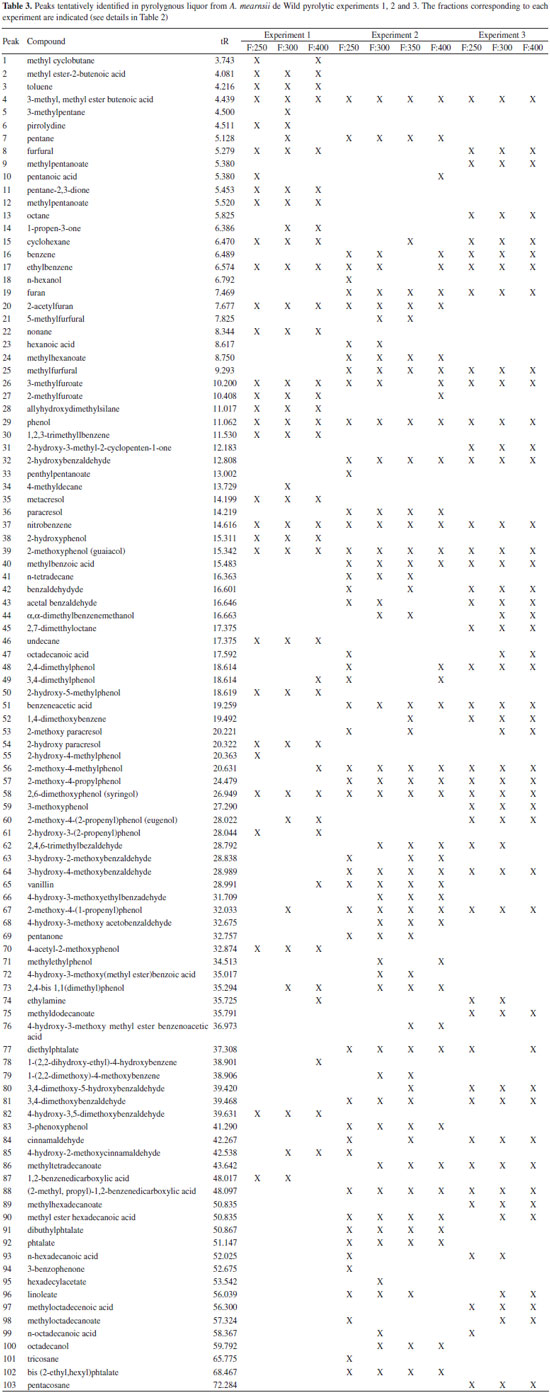
EXPERIMENTAL Sampling The botanically herborized material of Acacia mearnsii de Wild is located in the Herbarium Anchieta (PACA, São Leopoldo, RS) under the Tipping Number PACA 107,063. Branches of four-year-old trees were collected in a rural property of the Vale do Sinos region, which is located near São Leopoldo between the latitudes 29º and 30º South with altitudes ranging from 60 to 600 meters. The temperature is above 22 ºC in the warmest month, the average annual rainfall is 1,649 mm and the average annual temperature is 19.5 ºC according to the Campo Bom weather station (29º 41' S and 51º 03' W, 25.8 m alt.). The relative humidity has little variation over the year, and its average ranges from 72% to 86%. In the laboratory, the branches were peeled and triturated to produce a sawdust with an average particle size of 0.24 mm. The resulting sawdust was dried in an oven at 100 ºC for 24 hours and stored for its further processing. Sawdust chemical composition and extract content Approximately 3 g of A. mearnsii sawdust were oven dried for 2 h at 105 ºC. Ash content was determined by placing the sample into an ash cup that was heated for 2 h at 525 ºC, in accordance to the TAPPI T 211 om-23 standard method.9 The residue was cooled to room temperature and weighed. Ash content was expressed as a percentage of the initial sample mass. The total extractives content was obtained by summation of Soxhlet extractable materials that were removed from a known amount of A. mearnsii sawdust by a sequence of solvents with increasing polarity: dichloromethane, ethanol:toluene (1:2, vol/vol) and ethanol 95%, following the TAPPI T 204 om-88 standard method.9 At the end, the remaining residue was submitted to a hot water extraction according to the TAPPI T 264 om-88 standard method. After each extraction step, the solvent was evaporated under reduced pressure at 50 ºC and the extracted material was weighed. Finally, the mass recovery of each of these fractions was expressed in relation to the dry mass of the original material. The acid-insoluble lignin (Klason lignin) was determined in extractives-free materials on the basis of the TAPPI Standard Method9 T 222 os-74 with a modification in the second hydrolysis step. Approximately 300 mg of the sample was treated with 72% sulfuric acid at 20 ºC for 1 hour, with constant stirring with a glass rod. After that, the dispersion was diluted to 3% and autoclaved (118 ºC) for 1 hour in order to promote the total hydrolysis of oligo- and polysaccharides. Next, the suspension was filtered in a medium porosity Gooch crucible and the residue was washed with hot water and oven dried to a constant mass. The residue corresponds to the acid-insoluble lignin (Klason lignin). Acid-soluble lignin (ASL) was analyzed in the sulfuric acid hydrolysates by UV spectroscopy at 210 and 280 nm, according to the TAPPI Useful Method 250,9 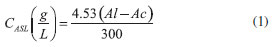 where Al and Ac correspond to the absorbance at 210 and 280 nm, respectively. Carbohydrates, organic acids and dehydration by-products were determined in acid hydrolysates of a typical Klason lignin determination by high performance liquid chromatography (HPLC). Aliquots from acid hydrolysates were centrifuged (10,000 G), filtered through 0.45 µm nylon membranes and analyzed in a Shimadzu HPLC model LC10AD. Analyses were performed in an Aminex HPX-87H (Bio-Rad) column at 65 ºC, which was preceded by a Cation-H guard column. Elution was carried out with 8 mol L-1 H2SO4 at a flow rate of 0.6 mL min-1. Quantification of the following analytes was done by external calibration: cellobiose, glucose, xylose, arabinose, formic acid, acetic acid, hydroxymethylfurfural, and furfural, taking into account their corresponding acid hydrolysis factors. These factors were 0.95 for cellobiose, 0.90 for glucose, 0.88 for xylose and arabinose, and 0.72 for acetic acid. Carbohydrates and organic acids were detected in the column eluate by differential refractometry while dehydration by-products were measured by UV spectrophotometry at 280 nm, using a diode array detector. Thermal degradation of Acacia mearnsii de Wild under controlled conditions The pyroligneous liquor from A. mearnsii de Wild wood samples was obtained under controlled conditions in a bench-scale apparatus (Figure 1) that was built based on the studies with eucalyptus wood performed by Martins.10 This system consisted of a borosilicate pyrolytic reactor placed inside a laboratory furnace that was equipped with a Novus 1100 temperature controller (type K thermocouple). The pyrolytic reactor contained a glass inlet that was used to introduce either atmospheric or synthetic air inside the reaction chamber. The products passed through a 60 cm long condenser directly connected to a cold finger that was kept at 1 ºC by a circulating water bath. The condensed compounds were collected in round-bottom flasks. Another trap was also added after the cold finger to prevent contamination.
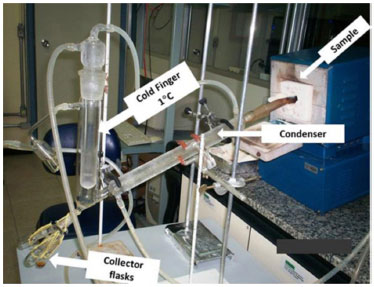 Figure 1. Laboratory apparatus used to collect the condensed fractions from the Acacia mearnsii de Wild wood pyrolysis
The methodology consisted in first pre-drying 10.0 g of A. mearnsii sawdust at 100 ºC for 10 minutes to remove any residual moisture. Then, the sample was placed into the pyrolytic reactor. Three experimental protocols were conducted. Experiment 1 was performed under ambient atmosphere pressure at temperatures ranging from 100 ºC to 400 ºC, with 10-minute isothermal steps at each 50 ºC interval. The whole extraction cycle took 160 minutes with heating rates of 5 ºC min-1. This procedure resembles the common practice in real acacia charcoal production ovens. Experiment 2 was performed under synthetic air atmosphere at a flow rate of 0.2 L min-1, using the same temperature program of Experiment 1. Experiment 3 was performed under synthetic air atmosphere as in Experiment 2, however, no isothermal steps were performed. Samples were directly heated from room temperature to the desired final temperature, at a heating rate of 50 ºC min-1, and left standing at the final temperature for 3 hours. The temperature programs of Experiments 1, 2 and 3 are given in Figure 2. For Experiments 1 and 2, fractions were collected during the 10-minutes isothermal step and subsequent heating run and during the 3 hour isothermal step for Experiment 3. All experiments were performed in triplicate.
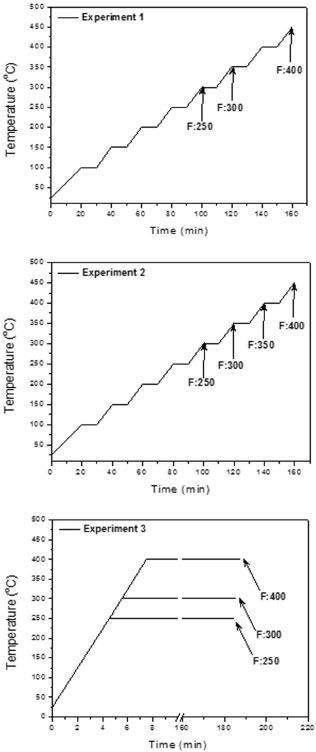 Figure 2. Temperature programs of Experiments 1, 2 and 3. The time points at which the fractions were collected are indicated. See details in Table 2
Pyroligneous liquor analysis For the GC/MS characterization of the pyroligneous extracts, a derivatization step was necessary prior to the chromatographic analysis due to the presence of a significant amount of acetic acid and other organic acids, which could potentially damage the chromatographic column. Derivatization procedure The derivatization reaction was performed with BF3 in 10 wt% methanol P.A. grade (Merck, Germany). Other reagents used in this procedure were NaCl P.A. grade (Nuclear, Brazil) and Na2SO4 anhydrous P.A. grade (Nuclear, Brazil). The derivatization method consisted of weighing 200 mg of pyroligneous extracts in an erlenmeyer and adding 3 mL of BF3 in methanol at 10 wt%. The mixture was heated in a water bath for 10 minutes at 60 ºC. The solution was cooled to room temperature and transferred to a separation funnel. 20 mL of hexane and 2 mL of saturated NaCl solution was added to perform a liquid-liquid extraction (LLE). The organic phase was removed, and the LLE was repeated twice with the aqueous phase. The three organic fractions were mixed and passed through an anhydrous Na2SO4 column to remove the excess of water. Then, the derivatized extract was transferred to a 50 mL amber flask and the total hexane volume was reduced to 2 mL by nitrogen purging. This extract was analyzed by GC/MS as described below. Gas chromatography/mass spectrometry analyses - GC/MS To identify the chemical constituents of the pyroligneous liquor, a Shimadzu GC 17A gas chromatograph coupled with a QP 5050A quadruple mass spectrometer was used. Analyses were carried out in a Restek RTX-5 capillary column (30 m × 0.25 mm × 0.25 µm) containing 5%-phenyl-95%-methylsilicone as the stationary phase. Helium was used as the mobile phase at a flow rate of 1 mL min-1, the split ratio was 1:20, and the injection temperature was 300 ºC. The oven started at 60 ºC and was temperature-programmed at 3 ºC min-1 to a final temperature of 280 ºC, where it stayed for 8 min. The MS operated with electron impact ionization of 70 eV, with interface and ion source temperatures of 300 ºC, and a mass/charge (m/z) range of 35-350 u. Retention indexes and peak identification In addition to the Mass Spectra Library search, Linear Temperature Programmed Retention Indexes11 (LTPRI) were used for qualitative analysis. To obtain the retention indexes experimentally, a mixture of n-alkanes standards ranging from C8 to C24 (Sigma-Aldrich) was injected in the same analytical conditions used for the samples. Tentative identification of compounds was performed using LTPRI and mass spectra comparison with ADAMS and NISTMS libraries using MS Search 2.0 and AMDIS 32.
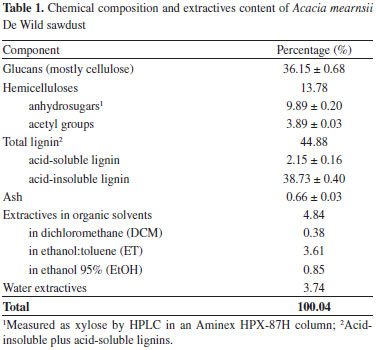
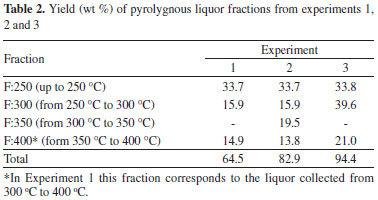
RESULTS AND DISCUSSION Acacia mearnsii De Wild chemical composition and extractives content The chemical composition of A. mearnsii De Wild sawdust is shown in Table 1. Both ash and total extractable materials were determined in the untreated sawdust but carbohydrates and total lignin contents were measured in extractive-free materials. However, all data reported in Table 1 are expressed in relation to the dry mass of the original sample. The A. mearnsii De Wild sawdust fraction was shown to contain 36.15% of glucans (mostly cellulose), 13.78% of hemicelluloses, 44.88% of total lignin, 8.58% of total extractable material and 0.66% of ash for 100.04% of total accountable matter. Hemicelluloses contained a considerable amount of acetyl groups in their chemical composition but the HPLC method used in this work was not able to resolve xylose, mannose and galactose in their acid hydrolysates. Acid-insoluble lignin was also quite high and this could not be attributed to condensation reactions involving tannins and other low molecular mass reactive components because such measurement was made in extractives-free materials. High total extractives content were also observed and these were split in two families, one mostly composed of organic solvent-extractable low molar mass carbohydrates and polyphenols and the other including water-soluble components such as tannins, pectic materials and gum exudates.12 Ash content was very small as expected for wood materials such as those used in this study. Thermal degradation experiments and pyroligneous liquor analyses For each extraction experiment, different pyroligneous fractions were collected and the mass recovery (wt %) of each fraction is presented in Table 2. For Experiments 1 and 2, that include 10-minutes isothermal steps between each desired temperature, the fractions were labeled according to the initial temperature; for example, fraction 250 ºC refers to the total amount of condensed liquids that was collected from 250 ºC up to the next temperature (isothermal step and subsequent heating). In the case of Experiment 3, the fraction corresponds to what was extracted upon the 3-hours isothermal run at the indicated temperature. In all experiments, no pyroligneous liquor was generated below 250 ºC. The total mass recovery obtained in Experiment 1 is consistent with results presented by others for other biomass sources.6 Normally, the aim of wood pyrolysis is to produce charcoal, and not to produce pyroligneous liquor. For this reason, the use of ambient atmosphere is the most common practice in commercial charcoal production, which is consistent with the results of Experiment 1. Although the percentage yield at 250 ºC was similar in all experiments, differences can be observed in all of the other fractions. For instance, the use of synthetic air pressure resulted in higher liquor yields. From a qualitative point of view, the tentative identification of compounds from each pyroligneous fraction is presented in Table 3 together with the retention index that was obtained for each peak. An illustrative chromatogram from one of the pyroligneous extracts is given in Figure 3.
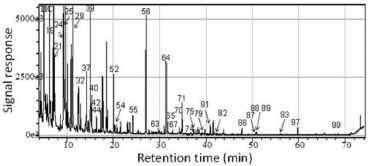 Figure 3. Total ion chromatogram from the pyroligneous liquor from A. mearnsi de Wild collected at 350 ºC at Experiment 2 (fraction F:350, according to Figure 2)
In Table 3, significant differences are observed in the composition analysis of fractions that were collected under different experimental conditions. Comparing the number of identified peaks from all extractions, a higher number of peaks was identified in Experiment 2 (66 peaks) followed by Experiment 3 (48 peaks) and Experiment 1 (43 peaks). The low mass recovery in Table 2 and the low number of identified compounds in Table 3 confirmed the loss of pyroligneous liquor in Experiment 1, which has been attributed to the air inlet of the experimental apparatus. A relatively low number of detected compounds was also observed in Experiment 3 and this was probably due to side reactions that took place during the long isothermal step, mainly at the highest conversion temperature. The GC/MS analysis of the pyroligneous extracts revealed the presence of complex chemicals that were clearly derived from the thermal degradation of black wattle lignocellulosic constituents. In fact, the phenolic nature of the pyroligneous liquor has already been observed by many in other wood species,3,13 and this is primarily related to the occurrence of low molecular mass compounds (monomers) derived from lignin. Guayacol (peak 39), syringol (peak 58), eugenol (peak 60) and substituted methoxyphenols such as 2-methoxy-4-methylphenol and 2-methoxy-4-propylphenol (peaks 56 and 57, respectively) are monomers derived from primary reactions of lignin, likewise substituted aromatic compounds such as vanillin (peak 65). A strong antioxidant activity has been attributed to the high concentration of phenolic compounds in the pyroligneous liquor.14 Highly oxygenated compounds derived from secondary radical reactions,15 such as 3-hydroxy-4-methoxybenzaldehyde (peak 66), were also observed. It is interesting to note that this compound was only detected under positive air atmosphere. Free radical reactions are also responsible for the formation of compounds with a lower molecular mass, as proven by the presence of methyl cyclobutane (peak 1), 3-methylpentane (peak 5), pentane and octane (peaks 7 and 13, respectively), amongst others. A higher number of lower molecular mass compounds were detected in Experiment 1, which was carried out under atmospheric pressure. Other substances derived from the thermal degradation of polysaccharides (cellulose and hemicelluloses) above 200 ºC were detected by GC/MS, especially furfural (peak 8), furan (peak 19), 2-acetylfuran (peak 20), and 2-methylfuroate (peak 27). These compounds were also detected in the pyroligneous liquors from other wood species.16 Substances such as 2-hydroxy-3-methyl-2-cyclopenten-1-one (Peak 31) are typical secondary degradation by-products of levoglucosan, the first derivative of cellulose degradation.3 On the other hand, compounds such as phthalate, diethyl phthalate and dibutyl phthalate may have been a contamination from plastic containers in which the samples were stored and refrigerated, so they might not be actual constituents of pyroligneous liquors. Finally, the use of a derivatization procedure prior to GC/MS analysis and the application of the LTPRI approach to characterize the pyroligneous extracts were also very successful because these samples are very acidic. Such analytical procedure, which has not been yet used for this purpose, was able to identify a series of aldehyde, phenol, ketone and carboxylic acid derivatives in pyroligneous extracts of black wattle. Economic interest in exploring the pyroligneous liquor Regarding the compounds of interest for industrial purposes, eugenol, one of the major components in pyroligneous liquors, is a well-known natural antiseptic, antibacterial, antifungal and antiviral agent that is widely used in dentistry as well as in treating nausea, indigestion and other gastrointestinal tract ailments. Moreover, it is also used in perfumes, in food flavoring, in food protection due to its antioxidant activity, in addition to being toxic to phytophagous insects, amongst other uses.17,18 Guaiacol is usually used in the pharmaceutical industry and medicine as an expectorant, antiseptic, local anesthetic, and for treating asthma and bronchitis. Syringol is considered a light fuel and is also used as a fuel additive. This chemical is also an important additive for tanning processes and is useful for its surfactant and waterproofing properties for the bio accessibility of dyes to fungi.19,20 Other important compounds for food flavoring and food industries are furfural and benzaldehyde derivatives. Two of the major components of the pyroligneous extracts were 5-methylfurfural (peak 21) and 4-hydroxy-3-methoxyethylbenzaldehyde (peak 64) (Figure 1), which was detected only in experiment 2. Among other furfural derivatives (peak 8 was detected in all fractions of Experiments 1 and 3 but was not detected in Experiment 2), 5-methylfurfural is one of the most important flavors of balsamic vinegars. Besides, this chemical has been used to evaluate organoleptic properties and identify possible commercial frauds.20 4-hydroxy-3-methoxyethylbenzaldehyde (among other benzaldehydes detected as peaks 32, 43, 60, 61, 62, 64 and 66 in Figure 1 and Table 2) is a derivative of benzaldehyde (almond flavor), which has a high commercial value for its nutraceutical, pharmaceutical, cosmetics, agrochemical and dyeing properties. Due to its importance, there are recent studies on how to improve its bioproduction from plant materials.21 Specifically referring to the pyroligneous liquor, many applications and features can be assigned such its use as biofuel, insecticide, plant growth-inducing agents, emulsifiers, flavoring, among others.18 These, together with the possibility of isolating value-added compounds of industrial or direct commercial use, may justify the dissemination of pyroligneous liquor processing plants using the by-products of the charcoal industry. However, due to the complexity of the extracts, the use of analytical techniques with a higher resolution, such as GC×GC/TOFMS, are recommended in order to perform a more detailed characterization.
CONCLUSIONS The controlled thermal degradation of Acacia mearnsii De Wild released various organic compounds whose composition varied depending on the process parameters such as the applied thermal treatment and the use of atmospheric air. In ambient atmospheric air with no induced air flux, which resembles the real practice of charcoal artisanal producers, a vast amount of high value-added compounds were detected which are systematically delivered to the ambient atmosphere in the real practice, despite their high polluting potential. Considering the environmental and economic effects, the present study contributes to a better exploitation of Acacia wood during charcoal production by adapting the brick ovens in order to collect the pyroligneous liquor. In addition, the use of a derivatization procedure prior to GC/MS analysis and the application of the LTPRI approach to characterize the pyroligneous extracts were also very successful because these samples are very acidic. Such analytical procedure, which has not been often used for this purpose, was able to identify a series of aldehyde, phenol, ketone and carboxylic acid derivatives in pyroligneous extracts of black wattle. However, other derivatizing strategies are yet to be developed to account for methyl esters that are usually present in these samples.
ACKNOWLEDGMENTS The authors acknowledge the Brazilian funding agencies, including CNPq (Grants 479921/2007-5 and 577232/2008-8) and CAPES (scholarship granted to A.S.S). We also thank EMBRAPA (Brazilian Agricultural Research Corporation - Temperate Agriculture) for its efforts on supporting the Acacia mearnsii De Wild productive chain.
REFERENCES 1. Vakkilainen, E.; Kuparinen, K.; Heinimö, J.; Large industrial uses of energy biomass. IEA Bioenergy Task 40: Sustainable International Bioenergy Trade. 2013, http://www.bioenergytrade.org/publications.html, accessed in March, 2015. 2. Beck-Pay, S. L.; S. Afr. J. Bot. 2012, 78, 285. DOI: http://dx.doi.org/10.1016/j.sajb.2011.08.001 3. Demirbas, A.; Energy Convers. Manage. 2000, 41, 633. DOI: http://dx.doi.org/10.1016/S0196-8904(99)00130-2 4. Alsabou, E.; Helleur, R.; J. Anal. Appl. Pyrolysis 2013, 101, 222. DOI: http://dx.doi.org/10.1016/j.jaap.2013.01.003 5. Antal Jr., M.; Gronli, M.; Ind. Eng. Chem. Res. 2000, 39, 4024. DOI: http://dx.doi.org/10.1021/ie000511u 6. Bridgwater, A. V.; Biomass Bioenergy 2012, 38, 68. DOI: http://dx.doi.org/10.1016/j.biombioe.2012.01.044 7. Cunha, M. E.; Schneider, J. K.; Brasil, M. C.; Cardoso, C. A.; Monteiro L. R.; Mendes, F. L.; Pinho, A.; Jacques, R. A.; Machado, M. E.; Freitas, L. S.; Caramão, E. B.; Microchem. J. 2013, 110, 113. DOI: http://dx.doi.org/10.1016/j.microc.2013.03.004 8. Silva, R. V. S.; Casilli, A.; Sampaio, A. L.; Ávila, B. M. F.; Veloso, M. C. C.; Azevedo, D. A.; Romeiro, G. A.; J. Anal. Appl. Pyrolysis 2014, 106, 152. DOI: http://dx.doi.org/10.1016/j.jaap.2014.01.013 9. Dence, C. W.; Lin, S. Y.; Methods in Lignin Chemistry, Springer Verlag: New York, 1992. 10. Martins, F.; Diniz, J.; Stahl, J. A.; Cardoso, A. L.; Quim. Nova 2007, 30, 873. DOI: http://dx.doi.org/10.1590/S0100-40422007000400021 11. van Den Dool, H.; Kratz, P.D. J. Chromatogr. A 1963, 11, 463. DOI: http://dx.doi.org/10.1016/S0021-9673(01)80947-X 12. Vassilev S. V.; Baxter, D.; Andersen, L. K.; Vassileva, C. G.; Morgan, T. J.; Fuel 2012, 94, 1. DOI: http://dx.doi.org/10.1016/j.fuel.2011.09.030 13. Martins, A. F.; Diniz, J.; Stahl, J. A.; Cardoso, A. L.; Bioresour. Technol. 2007, 98, 1095. DOI: http://dx.doi.org/10.1016/j.biortech.2006.04.024 PMID: 16790341 14. Ma, C.; Song, K.; Yu, J.; Yang, L.; Zhao, C.; Wang, P.; J. Anal. Appl. Pyrolysis 2013, 104, 38. DOI: http://dx.doi.org/10.1016/j.jaap.2013.09.011 15. Demirbas, A.; Energy Convers. Manage. 2002, 43, 1801. DOI: http://dx.doi.org/10.1016/S0196-8904(01)00137-6 16. Souza, J. B.; Ré-Poppi, N.; Raposo Jr., J. L.; J. Braz. Chem. Soc. 2012, 23, 610. 17. Park, I. K.; Lee, H. S.; Lee, S. G.; Park, J. D.; Ahn, I. J.; J. Agric. Food Chem 2000, 48, 2528. DOI: http://dx.doi.org/10.1021/jf9904160 PMID: 10888580 18. Alfredo, E.; Souza, E. S.; Marchesan; M. A.; Paulino, S. M.; Gariba-Silva, R.; Sousa-Neto, M. D.; Braz. Dent. J. 2006, 17, 130. DOI: http://dx.doi.org/10.1590/S0103-64402006000200009 PMID: 16924340 19. Hernandez, J. F.; Morla, J. C.; Energy Fuels 2003, 17, 302. DOI: http://dx.doi.org/10.1021/ef020030i 20. Giordano, L.; Calabrese, R.; Davoli, E.; Rotilio, D.; J. Chromatogr. A 2003, 1017, 141. DOI: http://dx.doi.org/10.1016/j.chroma.2003.08.029 PMID: 14584699 21. Craig, T.; Daugulis, A. J.; Biochem. Eng. J. 2014, 82, 97. DOI: http://dx.doi.org/10.1016/j.bej.2013.11.008 |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access