Artigo
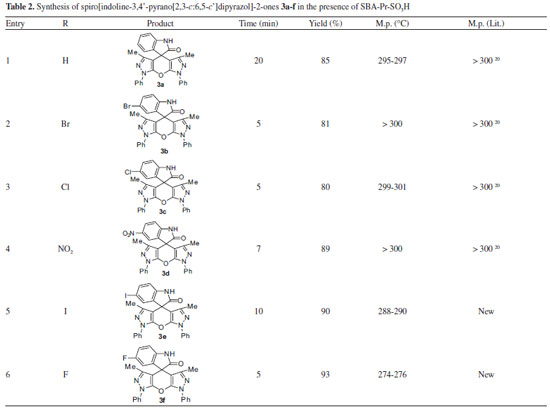
| Synthesis and biological evaluation of spiro[indoline-3,4'-pyrano[2,3-c:6,5-c']dipyrazol]- 2-ones in the presence of SBA-Pr-SO3H as a nanocatalyst |
|
Ghodsi Mohammadi ZiaraniI,*,#; Razieh MoradiI; Negar LashgariII; Alireza BadieiII; Ali Abolhasani SoorkiIII
IDepartment of Chemistry, Alzahra University, Vanak Square, Tehran, 1993893973, Iran Recebido em 23/03/2015 *e-mail: gmziarani@hotmail.com Sulfonic acid functionalized SBA-15 nanoporous material (SBA-Pr-SO3H) with a large pore size of 6 nm, a high surface area, high selectivity, and excellent chemical and thermal stability was applied as an efficient heterogeneous nanoporous acid catalyst in the reaction of isatin with pyrazolones under mild reaction conditions. A novel class of symmetrical spiro[indoline-3,4'-pyrano[2,3-c:6,5-c']dipyrazol]-2-one derivatives was successfully obtained in high yields. Comparison of these results with those reported in the literature shows that the current method is efficient, and results in better reaction times and yields of the desired products. Other advantages of this new method are its operational simplicity, easy work-up procedure, and the use of SBA-Pr-SO3H as a reusable and environmentally benign nanoreactor, such that the reaction proceeds easily in its nanopores. We also tested the antimicrobial activity of the prepared compounds using the disc diffusion method, and some of the synthesized compounds exhibited the best results against B. subtilis and S. aureus. INTRODUCTION Multi-component reactions (MCRs) are defined as processes in which three or more different starting materials react together to yield a final product in a one-pot procedure. Such reactions have emerged as a powerful and highly valuable strategy in modern organic synthesis to generate diverse heterocyclic scaffolds.1,2 These methodologies have great utility, particularly for the generation of privileged medicinal heterocyclic compounds.3-6 Spiro compounds have drawn tremendous interest of researchers in the area of synthetic organic chemistry and medicinal chemistry due to their extensive applications in biology and pharmacology.7 Indole and indoline fragments are important group of compounds widely exist in a variety of natural biologically active compounds.8 Some indolines that are spiro-annulated with heterocycles at the 3 position, have displayed remarkable biological activities.9-12 The spirooxindole ring system is the core structure of many pharmacological agents and natural alkaloids.13,14 Therefore, a number of methods have been reported for the preparation of spirooxindoles.15-18 The spiro[indoline-3,4'-pyrano[2,3-c:6,5-c'] dipyrazol]-2-ones in first, were synthesized by the reaction of isatin and two moles of pyrazolones under reflux conditions19 and later, in the presence of p-TsOH as catalyst.20 Recently, there is a rapid growth in research and development of mesoporous materials. SBA-15 is a nanoporous silica with hexagonal structure, high surface area, large pore size, high selectivity, excellent (chemical and thermal) stability, and easy isolation from products.21,22 Potential application of SBA-15 in different fields ranging from biosensors23 and drug delivery24 to separation25 and catalysis26 has attracted significant interest. In continuation of our studies toward using nanoporous solid catalysts in organic reactions,27-30 herein, we study the effect of SBA-Pr-SO3H as a heterogeneous solid acid catalyst in the synthesis of spiro[indoline-3,4'-pyrano[2,3-c:6,5-c'] dipyrazol]-2-one derivatives.
EXPERIMENTAL Materials The chemical materials employed in this work were obtained from Merck Company and were used with no purification. Infrared (IR) spectra were recorded from KBr disks using a Fourier-transform (FT)-IR Bruker Tensor 27 instrument. Melting points were measured by using the capillary tube method with an Electrothermal 9200 apparatus. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were run on a Bruker DPX using tetramethylsilane (TMS) as internal standard in DMSO-d6 solution. Mass spectrometry (MS) analysis was performed on a model 5973 mass-selective detector (Agilent). Scanning electron microscopy (SEM) analysis was performed on a Philips XL-30 field-emission scanning electron microscope operated at 16 kV, while transmission electron microscopy (TEM) was carried out on a Tecnai G2 F30 at 300 kV. Synthesis and functionalization of SBA-15 The nanoporous compound SBA-15 was synthesized and functionalized according to our previous report31 and the modified SBA-15-Pr-SO3H was used as a nanoporous solid acid catalyst in the following reaction. Typical procedure for the preparation of spiro[indoline-3,4'-pyrano[2,3-c:6,5-c']dipyrazol]-2-ones (3a-f) A mixture of isatin (0.147 g, 1 mmol), pyrazolone (0.348 g, 2 mmol) and SBA-Pr-SO3H (0.02 g) in 10 mL EtOH (absolute) were heated and stirred under reflux condition. When the reaction was completed, as monitored by TLC, the resulting reaction mixture was cooled to room temperature and was filtered from solvent. Then the precipitate was dissolved in hot DMF and the unsolvable catalyst was removed by filtration. The filtrate was cooled to afford the pure product. The catalyst could be washed subsequently with diluted acid solution, distilled water and then acetone. After drying under vacuum, it can be reused several times without noticeable loss of reactivity. Reusability of the catalyst was investigated under optimized conditions for the synthesis of the model compound 3a. As shown in Figure 1, the process of recycling was completed four times and no significant decrease in activity was observed. The yields for the four runs were found to be 85%, 80%, 78% and 72%, respectively.
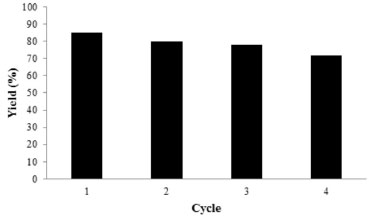 Figure 1. Reusability of SBA-Pr-SO3H in the synthesis of compound 3a
General procedure for in vitro antibacterial evaluation of compounds 3a-f The antimicrobial activities of the synthesized compounds were determined in vitro using the disc diffusion method. The microorganisms used in this study were Gram negative bacteria Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 85327), Gram positive bacteria Bacillus subtilis (ATCC 465) and Staphylococcus aureus (ATCC 25923), and fungus Candida albicans (ATCC 10231). All synthesized compounds were dissolved in DMSO (100 µg/mL) and 25 µl was loaded onto 6-mm paper discs. One hundred microliters of 109 cell/mL suspension of the microorganisms was spread on sterile Mueller-Hinton agar plates, and the discs were placed on the surface of culture plates. The minimum inhibitory concentration (MIC) of the synthesized compounds which showed antibiotic activity in disc diffusion tests was also determined by microdilution method and compared with three commercial antibiotics: chloramphenicol, gentamicin, and nystatin as references. 3',5'-Dimethyl-1',7'-diphenyl-1',7'-dihydrospiro[indoline-3,4'-pyrano[2,3-c:6,5-c']dipyrazol]-2-one (3a):20 Cream solid, Yield (85%); M.p: 295-297 ºC. IR (KBr): υ = 3447, 3150, 1706, 1616, 1497, 1305 cm-1. 1H NMR (400 MHz, DMSO-d6) δ = 1.98 (s, 6H, 2CH3), 6.83-7.92 (m, 14H, ArH), 10.96 (s, 1H, NH) ppm. 5-Bromo-3',5'-dimethyl-1',7'-diphenyl-1',7'-dihydrospiro [indoline-3,4'-pyrano[2,3-c:6,5-c']dipyrazol]-2-one (3b):20 Cream solid, Yield (81%); M.p: > 300 ºC. IR (KBr): υ = 3447, 3142, 1704, 1618, 1577, 1497, 1309, 757, 690 cm-1. 1H NMR (400 MHz, DMSO-d6) δ = 2.01 (bs, 6H, 2CH3), 7.31-8.80 (m, 13H, ArH), 10.86 (s, 1H, NH) ppm. 5-Chloro-3',5'-dimethyl-1',7'-diphenyl-1',7'-dihydrospiro [indoline-3,4'-pyrano[2,3-c:6,5-c']dipyrazol]-2-one (3c):20 Cream solid, Yield (80%); M.p: 299-301 ºC. IR (KBr): υ = 3445, 3141, 1705, 1619, 1575, 1498, 1472, 1308, 750, 682 cm-1. 1H NMR (400 MHz, DMSO-d6) δ = 2.04 (bs, 6H, 2CH3), 7.53-8.12 (m, 13H, ArH), 10.81 (s, 1H, NH) ppm. 5-Nitro-3',5'-dimethyl-1',7'-diphenyl-1',7'-dihydrospiro [indoline- 3,4'-pyrano[2,3-c:6,5-c']dipyrazol]-2-one (3d):20 Cream solid, Yield (89%); M.p: > 300 ºC. IR (KBr): υ = 3447, 3157, 1712, 1626, 1497, 1459, 1402, 1338, 693 cm-1. 1H NMR (400 MHz, DMSO-d6) δ = 2.07 (bs, 6H, 2CH3), 7.35-8.32 (m, 13H, ArH), 11.35 (s, 1H, NH) ppm. 5-Iodo-3',5'-dimethyl-1',7'-diphenyl-1',7'-dihydrospiro [indoline-3,4'-pyrano[2,3-c:6,5-c']dipyrazol]-2-one (3e): Cream solid, Yield (90%); M.p: 288-290 ºC. IR (KBr): υ = 3139, 3086, 2973, 2876, 1702, 1619, 1577, 1464, 1305, 659 cm-1. 1H NMR (400 MHz, DMSO-d6) δ = 1.97 (s, 6H, 2CH3), 6.87-7.69 (m, 13H, ArH), 10.70 (s, 1H, NH) ppm. 13C NMR (100 MHz, DMSO-d6) δ = 13.4 (Me), 53.8 (CH, spiro), 91.2 (C-I), 118.3, 121.1, 124.3, 125.6, 128.1, 128.6, 136.7, 137.1, 137.3, 140.0, 147.3, 153.4, 176.5 (C=O) ppm. MS (m/e): 585 (M+), 429, 400, 302, 273, 258, 174, 140, 114, 91, 77, 51. 5-Fluoro-3',5'-dimethyl-1',7'-diphenyl-1',7'-dihydrospiro [indoline-3,4'-pyrano[2,3-c:6,5-c']dipyrazol]-2-one (3f): Cream solid, Yield (93%); M.p: 274-276 ºC. IR (KBr): υ = 3128, 3067, 2917, 2865, 1706, 1624, 1578, 1488, 1308, 748, 686 cm-1. 1H NMR (400 MHz, DMSO-d6) δ = 1.95 (s, 6H, 2CH3), 6.72-7.68 (m, 13H, ArH), 10.87 (s, 1H, NH) ppm. 13C NMR (100 MHz, DMSO-d6) δ = 12.1 (Me), 58.9 (CH, spiro), 109.3, 110.1, 116.4, 122.6, 126.4, 128.0, 132.8, 138.3, 143.5, 147.2, 156.7, 158.9, 177.2 (C=O) ppm. MS (m/e) : 477 (M+), 429, 400, 321, 292, 274, 223, 188, 174, 133, 105, 91, 77, 51.
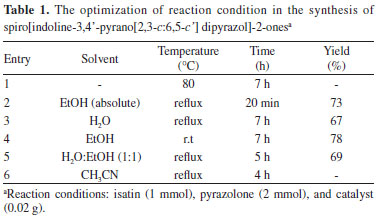
RESULTS AND DISCUSSION In this paper, we report a simple and highly efficient strategy for the synthesis of spiro[indoline-3,4'-pyrano[2,3-c:6,5-c'] dipyrazol]-2-ones by the condensation of isatin and two moles of pyrazolones in the presence of SBA-Pr-SO3H (Scheme 1). To optimize the reaction conditions, isatin 1 and two moles of pyrazolone 2 were subjected to various conditions using a catalytic amount of SBA-15-Pr-SO3H as catalyst. The reaction times and yields of products under various conditions (reflux, 80 ºC, and room temperature) and in the presence of different solvents such as H2O, EtOH, H2O:EtOH (1:1), EtOH (absolute), CH3CN and under solvent-free system were compared (Table 1). As shown in Table 1, the best result was obtained after 20 min at reflux temperature in EtOH (absolute) solvent by taking a 1:2 mol ratio mixture of isatin and pyrazolone. The reaction was also tested without any catalyst under reflux conditions in EtOH, and after 6 h related product was obtained in 80% yield.19
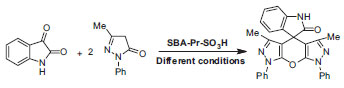 Scheme 1. Synthesis of spiro[indoline-3,4'-pyrano[2,3-c:6,5-c'] dipyrazol]-2-one under various conditions
Then, to evaluate the generality and versatility of this methodology, six types of substituted isatins 1 and pyrazolone 2 in a molar ratio of 1:2 were used in the optimum quantity of SBA-Pr-SO3H (0.02 g) (Scheme 2). Different spiro[indoline-3,4'-pyrano[2,3-c:6,5-c']dipyrazol]-2-one derivatives 3 (a-f) were prepared successfully under reflux conditions. The results were good in terms of reaction times and yields, and the procedure was simple and easy to operate (Table 2).
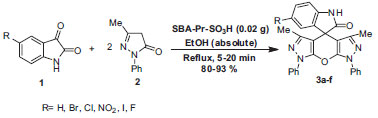 Scheme 2. Synthesis of spiro[indoline-3,4'-pyrano[2,3-c:6,5-c']dipyrazol]-2-ones in the presence of SBA-Pr-SO3H
The suggested mechanism for the reaction of pyrazolones and isatin derivatives is patterned in Scheme 3. The first step involves the protonation of carbonyl group of pyrazolone 4 by the solid acid catalyst and formation of intermediate 7 from condensation reaction of pyrazolone 5 with protonated form of isatin 1. Then, Michael addition of the second molecule of pyrazolone 2 with intermediate 7 gives the intermediate 8, which followed by cycloaddition and dehydration provides the desired product 3a-f (Scheme 3).
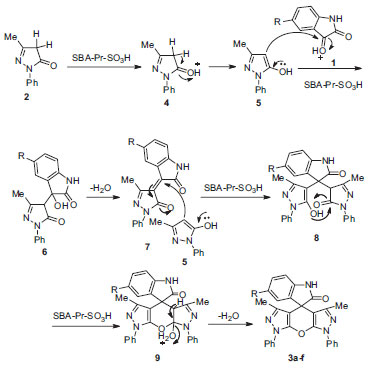 Scheme 3. Proposed mechanism for the synthesis of spiro[indoline-3,4'-pyrano[2,3-c:6,5-c']dipyrazol]-2-ones
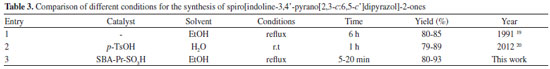
In order to show the merit of our method, we have compared our results with those reported in the literature in Table 3. It is clear from Table 3 that the current method is efficient and gives better times and yields of the desired products, when compared with other existing methods.
Nanoporous SBA-15 can be prepared by using triblock copolymer Pluronic P126 as a structure-directing agent.32,33 Functionalization of SBA-15 with sulfonic acid groups was usually performed though two major methods: direct synthesis or post-grafting.34,35 The SBA-15 silica was functionalized with (3-mercaptopropyl)trimethoxysilane (MPTS), and then the thiol groups were oxidized to sulfonic acid by hydrogen peroxide. Analysis of the catalyst surface was performed by various methods including thermogravimetric analysis (TGA), Brunauer-Emmett-Teller (BET) analysis, and elemental analysis (CHN), which demonstrated that the propyl sulfonic acids were successfully immobilized into the pores.31 Figure 2 illustrates the SEM and TEM images of SBA-Pr-SO3H. The SEM image (Figure 2, a) shows uniform particles about 1 µm which the same morphology was observed for SBA-15. It can be concluded that no changes occurred during surface modifications and morphology of the solid was saved. Furthermore, the TEM image (Figure 2, b) reveals parallel channels, that resemble the pore configurations of SBA-15. This indicates that the pore of SBA-15-Pr-SO3H was not collapsed during the two-step reactions.
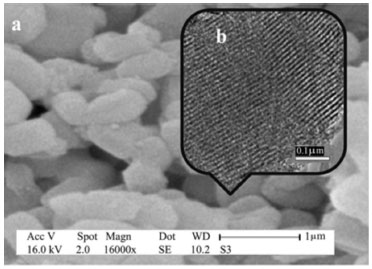 Figure 2. SEM image (a) and TEM image (b) of SBA-15-Pr-SO3H
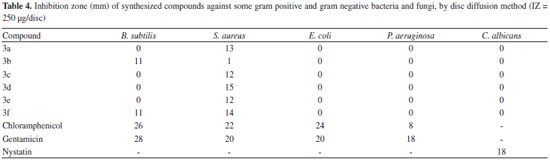
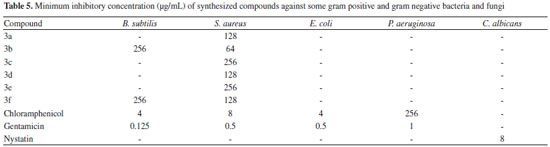
Antimicrobial activity All products were screened for antimicrobial activity using the disc diffusion method. The antimicrobial activities data are given in Tables 4 and 5. Table 4 presents the inhibition zones of compounds around the discs. The results indicated that compounds 3b and 3f (compounds containing R = Br and R = F substituted groups) were able to inhibit the growth of B. subtilis in inhibition zone values of 11 mm. Meanwhile, almost all compounds showed high antibacterial activities against S. aureus which among them, compound 3d with a value of 15 mm inhibition zone, showed the highest antibacterial activity. None of the compounds showed antibiotic activity against E. coli, P. aeruginosa or C. albicans. The minimum inhibitory concentration (MIC) of the synthesized compounds was also determined (Table 5). It was found that compounds 3a-f have higher MIC values in comparison with chloramphenicol and gentamicin as standards. The MIC results indicated that compound 3b exhibited the highest antimicrobial activity against S. aureus.
CONCLUSIONS In conclusion, we have successfully developed a simple method for the synthesis of symmetrical spirooxindoles through the reaction of isatin with two moles of pyrazolones in the presence of a catalytic amount of SBA-Pr-SO3H. Among the advantages of this new method are short reaction times, operational simplicity, high yields, easy work-up procedure, and using SBA-Pr-SO3H as a reusable and environmentally benign nano-reactor that the reaction proceeds easily in its nano-pores. The prepared compounds were also screened for their antimicrobial activities. It was found that compounds 3b and 3f were able to inhibit the growth of B. subtilis in inhibition zone values of 11 mm. Meanwhile, the MIC results indicated that compound 3b exhibited the highest antimicrobial activity against S. aureus.
SUPPLEMENTARY MATERIAL IR, Mass, 1H NMR and 13C NMR spectra for compounds 3e and 3f are available at http://quimicanova.sbq.org.br, in pdf file with free access. ACKNOWLEDGEMENTS We gratefully acknowledge the financial support we received from the Research Council of Alzahra University and the University of Tehran.
REFERENCES 1. Kumaravel, K.; Vasuki, G.; Curr. Org. Chem. 2009, 13, 1820. DOI: http://dx.doi.org/10.2174/138527209789630514 2. Eckert, H.; Molecules 2012, 17, 1074. DOI: http://dx.doi.org/10.3390/molecules17011074 PMID: 22267194 3. Estevez, V.; Villacampa, M.; Menendez, J. C.; Chem. Soc. Rev. 2014, 43, 4633. DOI: http://dx.doi.org/10.1039/c3cs60015g PMID: 24676061 4. Cioc, R. C.; Ruijter, E.; Orru, R. V. A.; Green Chem. 2014, 16, 2958. DOI: http://dx.doi.org/10.1039/c4gc00013g 5. Rotstein, B. H.; Zaretsky, S.; Rai, V.; Yudin, A. K.; Chem. Rev. 2014, 114, 8323. DOI: http://dx.doi.org/10.1021/cr400615v PMID: 25032909 6. Rahimifard, M.; Mohammadi Ziarani, G.; Malekzadeh Lashkariani, B.; Turk. J. Chem. 2014, 38, 345. DOI: http://dx.doi.org/10.3906/kim-1307-38 7. Pradhan, R.; Patra, M.; Behera, A. K.; Mishra, B. K.; Behera, R. K.; Tetrahedron 2006, 62, 779. DOI: http://dx.doi.org/10.1016/j.tet.2005.09.039 8. Sundberg, R. J. In Indoles; Academic Press: London, 1996. 9. da Silva, J. F. M.; Garden, S. J.; Pinto, A. C. J.; J. Braz. Chem. Soc. 2001, 12, 273. DOI: http://dx.doi.org/10.1590/S0103-50532001000300002 10. Abdel-Rahman, A. H.; Keshk, E. M.; Hanna, M. A.; El-Bady, S. M.; Bioorg. Med. Chem. 2004, 12, 2483. DOI: http://dx.doi.org/10.1016/j.bmc.2003.10.063 PMID: 15080944 11. Zhu, S. L.; Ji, S. J.; Zhong, Y.; Tetrahedron 2007, 63, 9365. DOI: http://dx.doi.org/10.1016/j.tet.2007.06.113 12. Kang, T. H.; Matsumoto, K.; Tohda, M.; Murakami, Y.; Takayama, H.; Kitajima, M.; Aimi, N.; Watanabe, H.; Eur. J. Pharm. 2002, 444, 39. DOI: http://dx.doi.org/10.1016/S0014-2999(02)01608-4 13. Ma, J.; Hecht, S. M.; Chem. Commun. 2004, 1190. DOI: http://dx.doi.org/10.1039/b402925a 14. Khafagy, M. M.; Abd El-Wahab, A. H. F.; Eid, F. A.; El-Agrody, A. M.; Il Farmaco 2002, 57, 715. DOI: http://dx.doi.org/10.1016/S0014-827X(02)01263-6 15. Nandakumar, A.; Thirumurugan, P.; Perumal, P. T.; Vembu, P.; Ponnuswamy, M. N.; Ramesh, P.; Bioorg. Med. Chem. Lett. 2010, 20, 4252. DOI: http://dx.doi.org/10.1016/j.bmcl.2010.05.025 PMID: 20621732 16. Chen, H.; Shi, D.; J. Comb. Chem. 2010, 12, 571. DOI: http://dx.doi.org/10.1021/cc100056p PMID: 20515044 17. Chen, T.; Xu, X. P.; Ji, S. J.; J. Comb. Chem. 2010, 12, 659. DOI: http://dx.doi.org/10.1021/cc900127g PMID: 20593846 18. Li, Y.; Chen, H.; Shi, C.; Shi, D.; Ji, S.; J. Comb. Chem. 2010, 12, 231. DOI: http://dx.doi.org/10.1021/cc9001185 PMID: 20085353 19. Joshi, K. C.; Pardasani, R. T.; Dandia, A.; Bhagat, S.; Heterocycles 1991, 32, 1491. DOI: http://dx.doi.org/10.3987/COM-90-5550 20. Rahmati, A.; Rezayan, A. H.; Alizadeh, M.; Nikbakht, A.; J. Iran. Chem. Soc. 2013, 10, 521. DOI: http://dx.doi.org/10.1007/s13738-012-0187-z 21. Bahrami, K.; Khodaei, M. M.; Fattahpour, P.; Catal. Sci. Technol. 2011, 1, 389. DOI: http://dx.doi.org/10.1039/c1cy00053e 22. Grieken, R. V.; Melero, J. A.; Morales, G.; J. Mol. Catal. A: Chem. 2006, 256, 29. DOI: http://dx.doi.org/10.1016/j.molcata.2006.04.040 23. Zhang, J.; Zhu, J.; Sci. China, Ser. B 2009, 52, 815. DOI: http://dx.doi.org/10.1007/s11426-008-0108-2 24. Song, S. W.; Hidajat, K.; Kawi, S.; Langmuir 2005, 21, 9568. DOI: http://dx.doi.org/10.1021/la051167e PMID: 16207037 25. Lee, J. W.; Cho, D. L.; Shim, W. G.; Moon, H.; Korean J. Chem. Eng. 2004, 21, 246. DOI: http://dx.doi.org/10.1007/BF02705405 26. Mohammadi Ziarani, G.; Lashgari, N.; Badiei, A.; J. Mol. Catal. A: Chem. 2015, 397, 166. DOI: http://dx.doi.org/10.1016/j.molcata.2014.10.009 27. Lashgari, N.; Mohammadi Ziarani, G.; Badiei, A.; Zarezadeh-Mehrizi, M.; J. Heterocycl. Chem. 2014, 51, 1628. DOI: http://dx.doi.org/10.1002/jhet.1746 28. Mohammadi Ziarani, G.; Badiei, A.; Mousavi, S.; Lashgari, N.; Shahbazi, A.; Chin. J. Catal. 2012, 33, 1832. DOI: http://dx.doi.org/10.1016/S1872-2067(11)60456-7 29. Gholamzadeh, P.; Mohammadi Ziarani, G.; Badiei, A.; Abolhassani Soorki, A.; Lashgari, N.; Res. Chem. Intermed. 2013, 39, 3925. DOI: http://dx.doi.org/10.1007/s11164-012-0909-y 30. Mohammadi Ziarani, G.; Faramarzi, S.; Lashgari, N.; Badiei, A.; J. Iran. Chem. Soc. 2014, 11, 701. DOI: http://dx.doi.org/10.1007/s13738-013-0342-1 31. Mohammadi Ziarani, G.; Badiei, A.; Khaniania, Y.; Haddadpour, M.; Iran. J. Chem. Chem. Eng. 2010, 29, 1. 32. Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G. H.; Chmelka, B. F.; Stucky, G. D.; Science 1998, 279, 548. DOI: http://dx.doi.org/10.1126/science.279.5350.548 PMID: 9438845 33. Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B. F.; Stucky, G. D.; J. Am. Chem. Soc. 1998, 120, 6024. DOI: http://dx.doi.org/10.1021/ja974025i 34. Lim, M. H.; Blanford, C. F.; Stein, A.; Chem. Mater. 1998, 10, 467. DOI: http://dx.doi.org/10.1021/cm970713p 35. Wight, A. P.; Davis, M. E.; Chem. Rev. 2002, 102, 3589. DOI: http://dx.doi.org/10.1021/cr010334m PMID: 12371895 |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access