Artigo
| Chemical composition and anti-inflammatory activity of Roldana platanifolia |
|
Amira ArciniegasI; Ana L. Pérez-CastorenaI,*; Antonio Nieto-CamachoI; Jhon Ironzi MaldonadoI; José Luis VillaseñorII; Alfonso Romo de VivarI
IInstituto de Química, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, Coyoacán 04510, D.F., México Recebido em 21/04/2015 *e-mail: alperezc@unam.mx The chemical study of Roldana platanifolia led to the isolation of β-caryophyllene, five eremophilanolides, chlorogenic acid, and a mixture of β-sitosterol-stigmasterol, β-sitosteryl glucopyranoside, and sucrose. The anti-inflammatory activities of the extracts and isolated products were tested using the 12-O-tetradecanoylphorbol-13-acetate (TPA) model of induced acute inflammation. The acetone and methanol extracts showed dose dependent activities (ID50 0.21 and 0.32 mg/ear, respectively), while none of the isolated compounds exhibited relevant edema inhibition. The active extracts were also evaluated with the myeloperoxidase assay technique (MPO) to determine their ability to prevent neutrophil infiltration. Results showed that the anti-inflammatory activity was related to the compound's ability to inhibit pro-inflammatory mediators such as neutrophils. INTRODUCTION The species of the genus Roldana (Asteraceae, Senecioneae, Tussilagininae) are perennial herbs which may grow to shrubs, in the temperate forests between 1000 to 4000 m elevation. They are distributed from southern Arizona and New Mexico to Panama.1,2 Previous chemical studies on 12 of the 48 species grouped into the genus Roldana show that they are source mainly of sesquiterpenes of eremophilane type,3,4 although some of them contain also oplopanes,5 plastoquinones,6 and flavonoids.7 Pyrrolizidine alkaloids have not been isolated so far in spite of their close taxonomic relation with the genus Senecio. Some species of Roldana are used in Mexican folk medicine, thus, R. ehrenbergiana is employed against leprosy8 and R. sessilifolia is included in the "cachani complex", which is a mixture of plants traditionally used to treat female sterility, hemorroids, and rheumathic pains.9 In addition, bioactive metabolites with anti-oxidant, anti-inflammatory,6 insecticidal,10 and antifungal properties7 have been reported in Roldana species. As part of an ongoing project on species of Senecioneae, this paper describes the first chemical study of Roldana platanifolia, as well as, the anti-inflammatory activity of extracts and isolated compounds tested on the 12-O-tetradecanoylphorbol-13-acetate (TPA) model of induced acute inflammation. The active acetonic and methanolic extracts were also tested on the myeloperoxidase (MPO) assay to determine their effect on neutrophil recruitment.
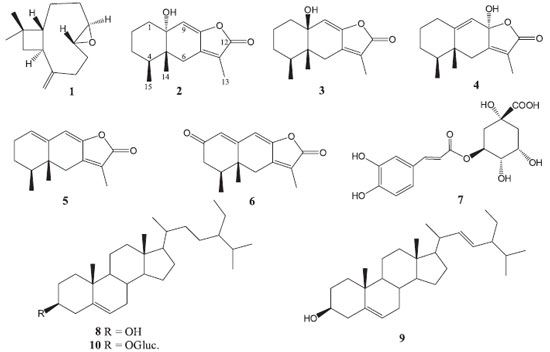
RESULTS AND DISCUSSION The chemical study of the aerial parts of Roldana platanifolia afforded β-caryophyllene 8R,9R-oxide (1), tsoongianolides A, B, and D (2-4), ligularenolide (5), 2-oxoerenophil-1(10),7(11),8(9)-trien-12,8-olide (6), chlorogenic acid (7), mixture of β-sitosterol (8) -stigmasterol (9), β-sitosteryl glucopyranoside (10), and sucrose (Figure 1). Structures of the isolated products were determined by spectroscopic analyses and comparison with reported data.
Compounds 2-4 showed the same molecular ion at m/z 248 in the EIMS experiment. Their IR spectra were similar and showed absorption bands for hydroxyl and unsaturated γ-lactone groups. These three isomeric compounds showed fifteen signals in their 13C NMR spectra (Table 1S) and characteristic resonances of eremophilanolide: a secondary, a tertiary, and a vinylic methyl groups in the 1H NMR spectra (Table 2S). Signals at δ 5.51, 5.46, and 5.60 for 2, 3 and 4, respectively, were assigned to their respective H-9 vinylic protons. Compounds 2 and 3 showed 13C NMR resonances at δ 73.4 and 74.0, assigned to C-10 by their interactions with H-9, H3-14 and H2-1, in the respective HMBC experiments, suggesting tertiary hydroxyl groups at this position and an epimeric relation between them. The chemical shift of CH3-14 at δ 0.84 in compound 2 determined its trans decaline structure, and in compound 3 the cis annelation was deduced by the CH3-14 resonance at δ 1.06.11 In compound 4, C-10 resonated as vinylic carbon at δ 136.5 and C-8 as hemi-ketal carbon at δ 102.9. The hydroxyl group at C-8 should be α-oriented since CH3-15 resonates at lower field (δ 1.00, d, J = 7.0 Hz) than CH3-14 (δ 0.83 s).12 Based on the above, compounds 2-4 were identified as tsoongianolides A, B and D previously isolated from Senecio tsoongianus.13 On the bases of 2D MNR spectroscopy, some signals of compounds 2-4 have been reassigned. Compound 5 showed a molecular ion at m/z 230 in the EIMS. The 1H NMR spectrum of 5 exhibited (Table 2S), as for compounds 2-4, an eremophilanolide structure pattern. The resonances of two vinylic protons observed at δ 5.79 (t, J = 4.0 Hz) and 5.94 (s) were assigned to H-1 and H-9, respectively, on the basis of 2D NMR experiments. The 13C NMR spectrum (Table 1S) exhibited the signals of three methyls, three methylenes, three methynes, and six non hydrogenated carbon atoms, consistent with structure 5. The spectroscopic data and physical constants of 5 are in good agreement with those of ligularenolide previously isolated from Liguaria species.13-15 Compound 6 showed a quasi-molecular ion at m/z 245 [M+H]+ in DART-MS. The IR spectrum exhibited absorptions of conjugated γ lactone and ketone groups (1777, 1663, and 1633 cm-1). The 13C NMR spectrum showed (Table 1S), besides the characteristic signals of eremophilanolide skeleton, that of a ketone carbonyl at δ 197.8 assigned to C-2 by the cross peaks between this carbon and H-1, H-3 and H-4 in the HMBC experiment. Compound 6 spectroscopic features were in agreement with 2-oxoerenophil-1(10),7(11),8(9)-trien-12,8-olide, previously isolated from Ligularia platyglossa.15,16 β-Caryophyllene 8R,9R-oxide (1),17 chlorogenic acid (7),18 mixture β-sitosterol19(8) - stigmasterol20(9), β-sitosteryl glucopyranoside21(10), and sucrose were characterized by their spectroscopic data and additionally compared with authentic samples. Based on the utilization in traditional medicine of Roldana species to alleviate rheumatic pains,9 and since anti-inflammatory agents have been isolated from species of this genus,6 extracts and isolated compounds of R. platanifolia were evaluated on the TPA model of induced acute inflammation. In a primary screening with 1 mg / ear (Table 1) the acetone and methanol extracts had a strong anti-inflammatory activity (89.34 and 82.52%, respectively, of edema inhibition) similar to that of the reference compound indomethacin. Among the isolated compounds only tsoongianolide D showed a mild anti-inflammatory effect (43.36% of edema inhibition) and was the most active. The weak activity of chlorogenic acid (21.85%) was in agreement with reported data on TPA model,22 even though its anti-inflammatory action has been reported in the carrageenan-induced inflammation model23 and its antiartritic properties demonstrated.24
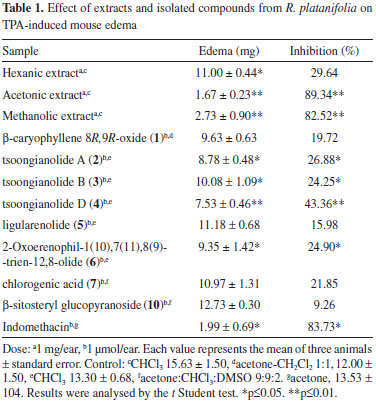
In the dose-response evaluation (Table 2) the acetonic and methanolic extracts showed dose dependent activities with ID50 0.21 and 0.32 mg / ear, respectively, and were less actives than indomethacin (0.08 mg / ear).
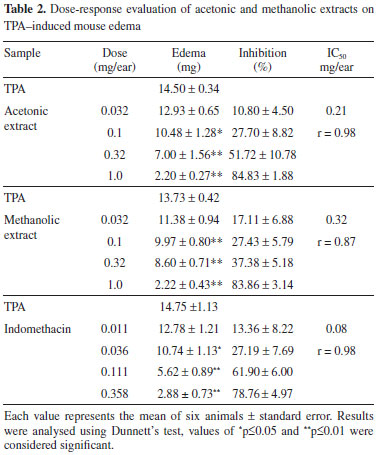
Topical application of TPA induce cell infiltration with release of mediators that increase vascular permeability and promote neutrophil influx.25 MPO activity is well correlated with the number of neutrophils in inflamed regions26,27 and is used as biological marker for tissue content of polymorphonuclear leucocytes. In order to determine the influence of neutrophil accumulation in the anti-inflammatory response of the acetonic and metanolic extracts, the MPO activity test was evaluated showing that they attenuated in a dose dependent manner the activity of MPO (Table 3), indicating that edema inhibition in the active extracts is associated with the inhibition of infiltrated neutrophils in the ear biopsy.
CONCLUSIONS The chemistry of R. platanifolia is similar to that of the species of the genus studied so far. Isolation of tsoongianolides A, B, and D (2-4), previously isolated from Senecio tsoongianus and liguranorides 4 and 5 reported in Ligularia species is not surprising since the genus Ligularia, which is distributed in the warm regions of Asia and Europe, is classified in Tussilagininae subtribe as Roldana.28 The genus Roldana has been segregated from the genus Senecio and in both the presence of sesquiterpenoids has been established, so far their main chemotaxonomic difference is the absence of pyrrolizidine alkaloids in Roldana.3 On the other hand, the anti-inflammatory activity of the acetonic and metanolic extracts is related to their capacity to inhibit neutrophil infiltration and the weak anti-inflammatory effects of the isolated compounds suggest the presence of other anti-inflammatory agents in methanol and acetone extracts, or a synergetic activity among its components.
EXPERIMENTAL General Melting points were determined using a Fisher Jones melting point apparatus and are uncorrected. IR spectra were recorded on a Nicolet Magna-IR 750 spectrometer. Optical rotations were determined on a Perkin-Elmer 343 polarimeter. EIMS data were determined on a JEOL JMS-AX505HA mass spectrometer at 70 eV, and for DART-MS a JEOL AccuTOF JMS-T100LC DART was used. 1D and 2D NMR spectra were obtained on a Bruker Avance III 400 MHz or on a Varian-Unity Inova 500 MHz spectrometer with tetramethylsilane (TMS) as internal standard. Vacuum column chromatography (VCC) was performed using Silica gel 60 G (Merck, Darmstadt, Germany). Flash column chromatography (FCC) was run using Silica gel 60 (230-400 Macherey-Nagel, Germany). Sephadex column was achieved with Sephadex LH 20 (Amersham Pharmacia Biotech AB, Sweden). Preparative TLC was carried out on Silica gel GF254 (Macherey-Nagel), layer thickness 1.0 or 2.0 mm. Plant material Roldana platanifolia (Benth.) H. Rob. & Bretell was collected at Llano de la Cantimplora, Tlalpan, D. F., México, in November 2005. A voucher specimen was deposited at the Herbarium del Instituto de Biología, UNAM, México (MEXU 1156425). Extraction and isolation Dried and ground aerial parts (569 g) of R. platanifolia were extracted successively with hexane, acetone and methanol. Solvents were removed at reduced pressure to obtain the corresponding extracts which gave negative tests with the alkaloids Dragendorff reagent. The hexane extract (11 g) was fractionated by VCC eluted with hexane-Me2CO gradient system. Fractions eluted with hexane afforded mixture A, those obtained with hexane-Me2CO 19:1 produced mixture B and the hexane-Me2CO 9:1 eluates gave mixture C. Purification of mixture A (350 mg) by FCC eluted with hexane-EtOAc 49:1 afforded after preparative TLC (benzene-EtOAc 49:1) β-caryophyllene 8R,9R-oxide (1, 8 mg).17 B gave a mixture of β-sitosterol (8)19 and stigmasterol (9)20 (123 mg) whose mother liquours (222 mg) were further purified by FCC (hexane-Me2CO 4:1) followed of preparative TLC (hexane-Me2CO 9:1, x 2) to obtain tsoongianolide A (2, 8 mg)13 and tsoongianolide B (3, 12 mg).13 Purification of mixture C (681 mg) by FCC eluted with hexane-Me2CO 4:1 followed of preparative TLC (benzene-EtOAc 4:1) produced 20 mg of 3. The acetone extract (16 g) was purified by VCC using hexane-EtOAc mixtures of increasing polarity. The hexane eluates afforded mixture D; mixtures E, F, and G were obtained with hexane-EtOAc 19:1, and mixture H with hexane-EtOAc 9:1. Mixture D (408 mg) was purified using a VCC eluted with hexane to obtain a mixture of 8 - 9 (20 mg). Mixture E afforded by recrystallization (hexane-EtOAc) ligularenolide (5, 58 mg).13-15 Mother liquors of 5 (624 mg) were purified by FCC eluted with hexane-EtOAc 19:1 to obtain 85 mg of 5. Mixture F (960 mg) was subjected to FCC (benzene-EtOAc 9:1) to obtain compound 3 (17 mg) and tsoongianolide D (4, 80 mg).13 Mixture G (180 mg) was purified by preparative TLC (hexane-EtOAc 3:1) to obtain compound 4 (35 mg). Mixture H (655 mg) produced by VCC (hexane-EtOAc 9:1) compound 4 (32 mg) and 2-oxoerenophil-1(10),7(11),8(9)-trien-12,8-olide (6, 11 mg).15,16 The methanol extract (25 g) was fractionated by VCC eluting with EtOAc-MeOH mixtures of increasing polarity to produce 350 mg of sucrose, β-sitosteryl glucopyranoside (10, 180 mg)21 and mixture I (1.2 g) from the EtOAc-MeOH 3:2 eluates. Mixture I (950 mg) was purified through a sephadex LH-20 column eluted with H2O-MeOH 1:1 to obtain chlorogenic acid (7, 45 mg).18 Tsoongianolide A (2): colorless oil; [α]25 +15 (c 0.12, CHCl3); IR (CHCl3) νmax / cm-1: 3390, 1762, 1651; 1H NMR (CDCl3, 400 MHz) see Table 2S; 13C NMR (CDCl3, 100 MHz) see Table 1S; EIMS m/z 248 [M]• (45), 230 (85), 178 (100). Tsoongianolide B (3): colorless needles; mp 104-106 ºC; [α]25 +107 (c 0.13; CHCl3); IR (CHCl3) νmax / cm-1 3596, 1768, 1655; 1H NMR (CDCl3, 500 MHz) see Table 2S; 13C NMR (CDCl3, 125 MHz) see Table 1S; EIMS m/z 249 [M+H]+ (55), 230 (100). Tsoongianolide D (4): colorless needles; mp 201-203 ºC; [α]25 -250 (c 0.17, CHCl3); IR (CHCl3) νmax / cm-13315, 1742; 1H NMR (CDCl3, 500 MHz) see Table 2S; 13C NMR (CDCl3, 125 MHz) see Table 1S; EIMS m/z 244 [M-CH3COOH]+ (100), 229 (75), 215 (70); Ligularenolide (5): light yellow prisms; mp 134-136 ºC; [α]25 -331 (c 0.16, CHCl3); IR (CHCl3) nmax / cm-1 1754, 1645; 1H NMR (CDCl3, 500 MHz) see Table 2S; 13C NMR (CDCl3, 125 MHz) see Table 1S; EIMS m/z 230 [M]+• (100), 215 (90). 2-Oxoerenophil-1(10),7(11),8(9)-trien-12,8-olide (6): light yellow prisms; mp 147-149 ºC; [α]25 -541 (c 0.16, CHCl3); IR (CHCl3) νmax / cm-1 1777, 1663, 1633; 1H NMR (CDCl3, 500 MHz) see Table 2S; 13C NMR (CDCl3, 125 MHz) see Table 1S; DART-MS m/z 245 [M+H]+. Evaluation of the anti-inflammatory activity Animals Male NIH mice weighing 25-30 g were maintained under standard laboratory conditions in the animal house (temperature 27 ± 1 ºC) with a 12/12 h light-dark cycle, according with the Mexican official norm MON-062-Z00-1999. They were fed laboratory diet and water ad libitum. TPA-induced edema model The TPA-induced ear edema assay in mice was performed as previously reported29 (Tables 1 and 2). Myeloperoxidase assay Tissue MPO activity was measured using an adapted method of Bradley et al.26 and Suzuki et al.27 as previously reported30 (Table 3). Statistical analysis All data were represented as percentage mean ± standard error of mean (SEM). The statistical analysis was done by means of Student's t-test, whereas analysis of variance ANOVA followed by Dunnett test were used to compare several groups with a control. P values p<0.05 and p<0.01 were considered to be significant.
SUPPLEMENTARY MATERIAL Tables 1S and 2S, 1H and 13C NMR of isolated compounds and 2D NMR spectra of compounds 2-6 are freely available at http://www.quimicanova.sbq.org.br in PDF.
ACKNOWLEDGEMENTS We are indebted to Rubén Gaviño, Ma. de los Angeles Peña, Elizabeth Huerta, Isabel Chávez, Héctor Ríos, Beatriz Quiroz, Rocío Patiño, Javier Pérez, and Luis Velasco for technical assistance.
REFERENCES 1. Robinson, H.; Brettell, R. D.; Phytologia 1974, 27, 402. 2. Funston, A. M.; Ph.D. Thesis, Kansas State University, USA, 1999. 3. Romo de Vivar, A.; Pérez-Castorena, A. L.; Arciniegas, A.; Villaseñor, J. L.; J. Mex. Chem. Soc. 2007, 51, 160. 4. Arciniegas, A.; Maldonado, J. I.; Pérez-Castorena, A. L.; González, K.; Villaseñor, J. L.; Romo de Vivar, A.; J. Mex. Chem. Soc. 2013, 57, 16. 5. Joseph-Nathan, P.; Villagómez, J. R.; Román, L. U.; Hernández, J. D.; Phytochemistry 1990, 29, 977; Joseph-Nathan, P.; Villagómez, J. R.; Rojas-Gardida, M.; Román, L. U.; Hernández, J. D.; Phytochemistry 1989, 28, 2397. DOI: http://dx.doi.org/10.1016/0031-9422(90)80060-T 6. Pérez-Castorena, A. L.; Arciniegas, A.; Ramírez, A. M. T.; Villaseñor, J. L.; Romo de Vivar, A.; Planta Med. 2002, 68, 645. DOI: http://dx.doi.org/10.1055/s-2002-32890 PMID: 12143002 7. Arciniegas, A.; Pérez-Castorena, A. L.; Maldonado, J.; Avila, G.; Villaseñor, J. L.; Romo de Vivar, A.; Fitoterapia 2008, 79, 47. DOI: http://dx.doi.org/10.1016/j.fitote.2007.07.002 PMID: 17913387 8. Martínez, M.; Las plantas medicinales de México, 4th ed., Botas: México, 1959, p. 176. 9. Linares, E.; Bye, R. A.; J. Ethnopharmacol. 1987, 19, 153. DOI: http://dx.doi.org/10.1016/0378-8741(87)90039-0 PMID: 3613608 10. Céspedes, C. L.; Torres, P.; Marín, J. C.; Arciniegas, A.; Romo de Vivar, A.; Pérez-Castorena, A. L.; Aranda, E.; Phytochemistry 2004, 65, 1963. DOI: http://dx.doi.org/10.1016/j.phytochem.2004.03.037 PMID: 15280003 11. Samek, Z.; Harmatha, J.; Novotny, J.; Šorn, F.; Collect. Czech. Chem. Commun. 1969, 34, 2792. DOI: http://dx.doi.org/10.1135/cccc19692792 12. Naya, K.; Shimizu, M.; Nishio, H.; Takeda, M.; Oka, S.; Hirota, K.; Bull. Chem. Soc. Jpn. 1991, 64, 1071. DOI: http://dx.doi.org/10.1246/bcsj.64.1071 13. Zhao, Y.; Jiang, H.; MacLoed, M.; Parsons, S.; Rankin, D. W. H.; Wang, P.; Cheng, C. H. K.; Shi, H.; Hao, X.; Gueritte, F.; Chem. Biodiversity 2004, 1, 1546. DOI: http://dx.doi.org/10.1002/cbdv.200490115 14. Tanahashi, Y.; Ishizaki, Y.; Takahashi, T.; Tori, K.; Tetrahedron Lett. 1968, 3739. DOI: http://dx.doi.org/10.1016/S0040-4039(00)75530-4 15. Jenniskens, L. H. D.; Groot, A.; Tetrahedron 1998, 54, 5617. DOI: http://dx.doi.org/10.1016/S0040-4020(98)00233-6 16. Liu, J.; Zhang, C.; Zhang, M.; Wang, Z.; Qi, H.; Zhongguo Yaoke Daxue Xuebao 2005, 36, 114. 17. Heymann, H.; Tezuka, Y.; Kikuchi, T.; Supriyatna, S.; Chem. Pharm. Bull. 1994, 42, 138. DOI: http://dx.doi.org/10.1248/cpb.42.138 18. Han, T.; Li, H.; Zhang, Q.; Zheng, H.; Qin, L.; Chem. Nat. Compd. 2006, 42, 567; Kavtaradze, N. S.; Chem. Nat. Compd. 2003, 39, 314. DOI: http://dx.doi.org/10.1007/s10600-006-0215-2 19. Aldrich Library of 13C and 1H FT NMR Spectra, 1993, 1st ed., vol. 3, 569 A. 20. Aldrich Library of 13C and 1H FT NMR Spectra, 1993, 1st ed., vol. 3, 569 B. 21. Paulo, A.; Jimeno, M. L.; Gomes, E. T.; Houghton, P. J.; Phytochemistry 2000, 53, 417. DOI: http://dx.doi.org/10.1016/S0031-9422(99)00568-3 PMID: 10703068 22. Huang, M.-T.; Lysz, T.; Ferraro, T.; Abidi, T. F.; Laskin, J. D.; Conney, A. H.; Cancer Res. 1991, 51, 813. PMID: 1899046 23. Dos Santos, M. D.; Almeida, M. C.; Lopes, N. P.; Petto de Souza, G. E.; Biol. Pharm. Bull. 2006, 11, 2236. DOI: http://dx.doi.org/10.1248/bpb.29.2236 24. Chauhan, P. S.; Satti, N. K.; Sharma, P.; Shatma, V. K.; Suri, K. A.; Bani, S.; Phytother. Res. 2012, 26, 1156. DOI: http://dx.doi.org/10.1002/ptr.3684 PMID: 22180146 25. Rao, T. S.; Currie, J. L.; Shaffer, A. F.; Isakon, P. C.; Inflammation 1993, 17, 723. DOI: http://dx.doi.org/10.1007/BF00920477 PMID: 8112831 26. Bradley, P. P.; Priebat, D. A.; Christensen, R. D.; Rothstein, G.; J. Invest. Dermatol. 1982, 78, 206. DOI: http://dx.doi.org/10.1111/1523-1747.ep12506462 PMID: 6276474 27. Suzuki, K.; Ota, H.; Sasagawa, S.; Sakatani, T.; Fujikura, T.; Anal. Biochem. 1983, 132, 345. DOI: http://dx.doi.org/10.1016/0003-2697(83)90019-2 PMID: 6312841 28. Bremer, K.; Asteraceae: Cladistics & Classification, 1st ed., Timer press: Portland, 1994, p. 752. 29. Arciniegas, A.; Pérez-Castorena, A. L.; Nieto-Camacho, A.; Villaseñor, J. L.; Romo de Vivar, A.; J. Mex. Chem. Soc. 2009, 53, 229. 30. Arciniegas, A.; Pérez-Castorena, A. L.; Nieto-Camacho, A.; Villaseñor, J. L.; Romo de Vivar, A.; J. Braz. Chem. Soc. 2013, 24, 99. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access