Assuntos Gerais
| Treatment of waste from atomic emission spectrometric techniques and reuse in undergraduate lab classes for qualitative analysis |
|
Francisco L. F. da SilvaI; Thalita O. A. DuarteI; Allan N. S. DantasII; Gisele S. LopesI; Sandro T. GouveiaI; Wladiana O. MatosI,*
IDepartamento de Química Analítica e Físico-Química, Universidade Federal do Ceará, Campus do Pici, 60451-970 Fortaleza - CE, Brasil Recebido em 06/03/2015 *e-mail: wladianamatos@ufc.br Flame atomic absorption spectrometry (FAAS) and inductively coupled plasma optical emission spectrometry (ICP OES) are widely used in academic institutions and laboratories for quality control to analyze inorganic elements in samples. However, these techniques have been observed to underperform in sample nebulization processes. Most of the samples processed through nebulization system are discarded, producing large volumes of waste. This study reports the treatment and reuse of the waste produced from ICP OES technique in a laboratory of analytical research at the Universidade Federal do Ceará, Brazil. The treatment of the waste was performed by the precipitation of elements using (NH4)2CO3. Subsequently, the supernatant solution can be discarded in accordance with CONAMA 430/2011. The precipitate produced from the treatment of residues can be reused as a potential sample in undergraduate qualitative analytical chemistry lab classes, providing students the opportunity to test a real sample. INTRODUCTION The management of chemical residues in the teaching and research laboratories of academic institutions has been extensively discussed in Brazil, both in the literature and at national/international scientific meetings.1-6 Only a few Brazilian universities have a waste management program; therefore, most residues produced in academic laboratories are inappropriately discarded or stored for years. Although the quantity of residues produced by academic research laboratories is negligible when compared with those produced by industrial activities, universities cannot ignore their responsibility for the residues generated because the environmental issue is a global concern. Moreover, this behavior toward the environmental issue appears to be contradictory to the development of environmental researches at universities. Research laboratories at universities produce several types of residues with specific characteristics depending on the studies developed by each laboratory. Thus, it is difficult to establish a standard treatment or disposal protocol for these wastes. It is very important that each laboratory assume responsibility and propose strategies for the treatment of its own waste. Some studies7-9 in the literature report the reuse of practical lessons wastes, but there is still necessary more procedures to treat wastes from labs, especially research labs. Flame atomic absorption spectrometry (FAAS) and inductively coupled plasma optical emission spectrometry (ICP OES) are spectrometric techniques typically employed in academic and industrial chemical laboratories for trace element analysis. FAAS is a monoelemental, easy-to-implement and low-cost technique. The principle of FAAS is based on the measurement of electromagnetic radiation absorption from a radiation source by gaseous atoms in a fundamental state produced by a flame at temperatures of up to 3000 K.10 ICP OES is a multielemental technique through which it is possible to identify more than 70 elements in less than 2 min. The operating principle of ICP OES is based on the excitation of elements in a plasma of argon gas. The plasma is a partially ionized gas composed of electrons, ions and neutral particles that reaches temperatures between 5000 and 8000 K, sustained by energy from a radio-frequency source operating at 27 or 40 MHz. Atoms and molecules are promoted to excited states by absorbing energy from the electrons and atoms of the excited argon gas. The excited species return to their fundamental states by emitting photons, which are measured by a detection system.11 Generally, in FAAS and ICP OES, samples are introduced in the form of solutions, which are transformed into aerosols through a nebulization process before they reach the atomizer of the spectrometer. The efficiency of nebulization of these devices is low: approximately 5-10% of a given sample reaches the atomizer. More than 90% of the sample is left to drain.12 Therefore, FAAS and ICP OES generate large quantities of waste that contains different inorganic elements, especially ICP OES, that which involves the use of a rapid and multielemental analyzer. The species found in this waste depend on the elements and samples routinely analyzed in the laboratory. Because carbon can interfere in the trace element analysis conducted using spectrometric techniques, the organic matrix of samples must usually be decomposed by using acid mixtures (HNO3, HCl and HF) before analysis; therefore, the wastes generated by FAAS and ICP OES normally have an acidic pH. This work reports the treatment of the waste generated by ICP OES analysis in a research laboratory of the Universidade Federal do Ceará, Brazil, and the possibility of applying the solid residue obtained from the waste treated in qualitative analytical chemistry undergraduate classes. The same idea applied to ICP OES wastes would be employed to FAAS wastes, since both produce wastes with the same characteristics.
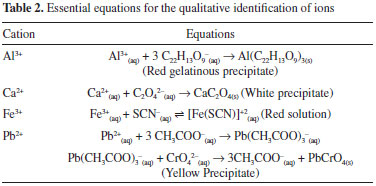
EXPERIMENTAL Samples and reagents The sample used in this work was waste collected from ICP OES analysis conducted in a research laboratory (Laboratório de Estudos em Química Aplicada - LEQA) at a university (Universidade Federal do Ceará, Brazil) between 2011 and 2013. Over this period, the laboratory handled several types of samples (water, food, plant, soil, paint, wax, etc.) and analyzed different inorganic trace elements. All solutions were prepared using ultrapure water (resistivity of 18.2 MΩ cm) obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA). All glassware and polypropylene flasks were immersed in 10% v v-1 nitric acid (Merck, Darmstadt, Germany) for 24 h and rinsed with ultrapure water prior to use. The waste sample was neutralized by using NaOH (Merck, Darmstadt, Germany), and for the precipitation of metals, (NH4)2CO3 (Merck, Darmstadt, Germany) was used. In order to extract analytes from precipitates, 37% w w-1 HCl (Sigma-Aldrich) and 65% w w-1 HNO3 (VETEC) were used. In qualitative studies, the reagents used were those available in the laboratory of Qualitative Analytical Chemistry for university graduates: 6.0 mol L-1 CH3COOH; 6.0 mol L-1 HNO3; 0.1% w v-1 C22H23N3O9 (aluminon); 0.2 mol L-1 (NH4)2C2O4; 1.0 mol L-1 (NH4)2SO4; 0.5 mol L-1 K2CrO4; 1.0 mol L-1 KSCN; 0.2 mol L-1 (NH4) C2H3O2. Reference solutions were prepared after successive dilutions from 1000 mg L-1 Ag, Al, As, B, Ba, Ca, Cd, Cr, Cu, Fe, Mg, Mn, Ni, Se, Sn, Pb and Zn stock solutions (Acros Organics, Belgium) for ICP OES waste analysis before and after treatment. For Se analysis by hydride generation-atomic absorption spectrometry (HG-AAS), the pre-reduction was performed by HCl 37% w w-1 (Merck, Darmstadt, Germany). The reducing solution of 0.6% w v-1 sodium tetrahydroborate (III) was prepared by dissolving NaBH4 (99%, Merck, Darmstadt, Germany) in a 0.5% w v-1 NaOH solution (Merck). Instrumentation A Micronal B474 pH meter and a combined glass electrode were used to measure the pH of the solutions. A heating plate (QUIMIS) was employed to heat solutions used for waste treatment and qualitative analysis. The precipitate was dried in an incubator (QUIMIS). A dual-view Optima 4300 DV (Perkin Elmer) ICP OES instrument was used for Ag, Al, As, B, Ba, Ca, Cd, Cr, Cu, Fe, Mg, Mn, Ni, Sn, Pb and Zn analysis. The sample was introduced into the ICP device using a cross-flow nebulizer with a double-pass spray chamber. The ICP operating parameters were as follows: 40 MHz generator frequency; 1.1 kW radio-frequency power; 15 L min-1 argon plasma flow rate; 0.5 L min-1 auxiliary argon flow rate; 0.8 L min-1 nebulizer argon flow rate; and 1.4 L min-1 sample flow rate. A central tube torch with an internal diameter of 2.4 mm was used. The wavelengths of the elements and the viewing position of the torch in the ICP OES measurements are presented in Table 1.
An atomic absorption spectrometer (Varian Model SpectrAA 430 Fast Sequential) was used to carry out selenium determination by hydride generation. The hydride was continuously produced with a Varian vapour generation accessory (Model VGA-77), equipped with gas-liquid separator adapted to a FIA system. The flows of the HCl and NaBH4 solutions in Tygon tubes were 3 mL min-1 and 2 mL min-1, respectively, and the sample flow rate was 8.0 mL min-1. A high intensity selenium hollow cathode lamp (Varian UltrAA) operating at a lamp current of 15 mA was used as an excitation source. All measurements were carried out at 196.2 nm. A spectral slit-width of 0.5 nm and a D2 background correction was used. Treatment of the waste sample To reduce the volume of the waste, 500 mL of the sample was heated under agitation using a magnetic stir bar at 80 ºC on a hot plate. The final volume of the sample after heating was 100 mL. This solution was neutralized with 3.0 mol L-1 NaOH and 3.0 mol L-1 (NH4)2CO3 was added to precipitate the metals. The mixture was left to stand overnight and filtered. The precipitate obtained was dried at 70 ºC for 24 h and used to produce an actual and unknown sample for use in qualitative analytical chemistry lab classes for the identification of cations. The supernatant resulting from filtration was analyzed by titration (S2-)13 potentiometry with ion-selective electrode (F-),14 HG-AAS (Se) and ICP OES (Ag, Al, As, B, Ba, Ca, Cd, Cr, Cu, Fe, Mg, Mn, Ni, Sn, Pb and Zn). Identification of cations in the solid residue obtained from the ICP OES waste treatment Qualitative analytical chemistry is a mandatory discipline for all courses in related science areas at university in Brazil. In addition to the area of chemistry, pharmacy, engineering, oceanography, zootechnology, geology, etc., all have an experimental qualitative analytical chemistry component. In general, at the end of the experimental courses, students receive a sample and they may spend two lab class periods identifying the cation and the anion present in the sample, which is, in fact, an unknown salt but not an actual sample. It would be interesting if students could have the opportunity to work with actual samples to approximate their experience to that of professionals. To this end, the possibility of using the solid residue produced from ICP OES waste treatment as a sample in qualitative analytical classes was explored. The same steps applied in the procedure guide of a qualitative analytical chemistry course, which follows the cations identification proposed by King, 195915 adapted, were used to investigate the cations present in the solid residue. The dried precipitate obtained from the ICP OES waste treatment was used as a sample for the qualitative identification of Al, Ca, Fe and Pb. For the identification of aluminum, the cation was extracted from 1.0 g of the precipitate using 2 mL of 37% w w-1 HCl in a heated bath for 2 min and centrifuged. To the supernatant, 0.25 ml (5 drops) of 0.2 mol L-1 (NH4)C2H3O2 and 0.15 mL (3 drops) of 15 mol L-1 NH3 were added to maintain the pH levels at approximately 5.0 and 7.2, respectively; furthermore, 0.2 mL (4 drops) of aluminon (C22H14O9) were added to the solution for precipitate formation. The appearance of a red gelatinous precipitate is considered a positive test result for the presence of aluminum. For the identification of calcium, the analyte was extracted from 1.0 g of the precipitate in 2 mL of 6 mol L-1 CH3COOH, and 0.05 mL (1 drop) of 15 mol L-1 NH3 was added to maintain the medium alkaline. The solution was centrifuged, and 0.25 mL (5 drops) of trietilanamine and 0.5 mL (10 drops) of 1 mol L-1 (NH4)2SO4 were added to the supernatant, the solution was heated and centrifuged. To the supernatant it was added 0.25 mL (5 drops) of 0.2 mol L-1 (NH4)2C2O4, than the mixture was heated in a water bath at 100 ºC for 2 min and centrifuged. The appearance of a white precipitate is considered a positive test result for the presence of calcium. For extraction of iron from 1.0 g of the precipitate, 2 mL of water was applied and the mixture was centrifuged. To the supernatant, it was added 0.25 mL (5 drops) of 37% w w-1 HCl in heated bath for 2 min in order to promote the oxidation of Fe2+ to Fe3+. Another centrifugation was performed, and 0.25 mL (5 drops) of 0.1 mol L-1 (NH4)SCN were added to the solution. The appearance of a blood-red solution is considered a positive test result for the presence iron. Fe3+ reacts with SCN- to form a soluble, colored complex. To test for the presence of lead in the sample, the analyte was extracted from 1.0 g of the precipitate in 2 mL of 68% w w-1 HNO3. The mixture was heated and centrifuged for 2 min in a water bath (100 ºC). 0.05 mL (one drop) of 6 mol L-1 CH3COOH was added to the supernatant for the elimination of possible interferences due to Cu2+ and Bi3+. 0.25 mL (five drops) of 2 mol L-1 K2CrO4 were added, and the mixture was then heated in a water bath for 2 min to observe the formation of a yellow precipitate. The sample was centrifuged, and the supernatant was discarded. The appearance of a yellow precipitate is considered a positive test result for the presence of lead. All equations for the qualitative identification of ions are presented in Table 2.
Application of solid waste in experimental qualitative chemical analysis classes for undergraduates The possibility of applying the solid residue obtained from spectrometric technique waste treatment in experimental classes for qualitative chemical analysis was studied in this work. The solid waste was dried at 100 ºC for 24 h prior to qualitative analysis. A group of ten students received 10.0 g of the same solid residue sample to work following the traditional script established as described in the previous section. A questionnaire was applied in order to assess the difficulties that might be encountered in performing the analysis and the availability to identify the cation studied. The questionnaire and practical roadmap can be consulted in the supplementary material.
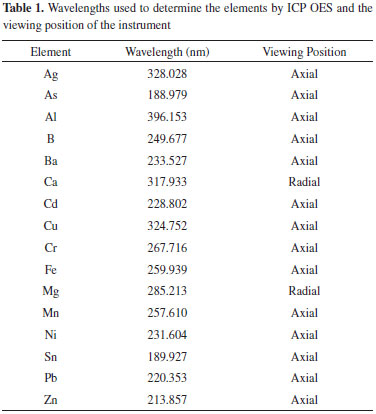
RESULTS AND DISCUSSION Treatment of waste The residue solution collected in the waste container of the ICP OES instrument was neutralized with NaOH after the volume of the sample had been reduced to approximately 100 mL by heating. During this process, the beginning of the formation of hydroxide precipitates was observed; most likely, the precipitates were composed of the main elements constituting the waste (Al, Fe and Mg), except for calcium, whose hydroxide is soluble. The precipitation process continued with the addition of (NH4)2CO3. The solid mass obtained after treatment was about 3.0 g dry mass per 500 mL of residue. This quantity is variable and depends on the routine analysis performed by the laboratory. The elements Ag, Al, As, B, Ba, Ca, Cd, Cr, Cu, Fe, Mg, Mn, Ni, Se, Sn, Pb, Zn and the anions F- and S2- present in the waste sample solution were quantified before and after the treatment. The data obtained are shown in Table 3.
CONAMA Resolution 430/2011,16 National Environmental Council of Brazil, which supplements effluent discharge standards originally defined in CONAMA Resolution 357,17 establishes criteria for the conditions and standards of effluent discharges for wastewater treatment systems and industrial dischargers. Table 3 shows the maximum quantity of inorganic parameters permitted by the CONAMA Resolution 430/2011 for effluent discharges. Since the period when the ICP OES waste was collected the LEQA analysed mainly food and vegetable samples, the waste presented high concentrations of Al, Ca, Fe and Mg. Except for As, B, Se and Sn, the concentrations of all other elements in the waste before the treatment proposed in this work was above those allowed by CONAMA. The treatment applied to the waste sample substantially reduced the concentrations of the elements studied. Only aluminum did not present a concentration below the limit of detection (LOD) in the waste sample treated; nevertheless, its concentration was reduced by approximately 97%. After the treatment procedure all elements evaluated in the waste were below the values permitted by legislation. Therefore, the treatment of the waste was efficient to these elements. CONAMA Resolution 430/2011 does not include provisions for Al, Ca or Mg. In the CONAMA resolution, the allowed concentrations of chromium vary within a certain range according to the oxidation state of the element (0.1 mg L-1 for Cr(VI) and 1.0 mg L-1 for Cr(III)). Although no analysis of redox speciation in the sample was performed, after treatment, the residue showed a total chromium concentration below the limit of detection, in compliance with the maximum permissible concentration of the element in any oxidation state. Considering that the pH of the waste after treatment was 8.0; the maximum amount of mercury permitted in effluents is 0.01 mg L-1; and the solubility constant of mercury hydroxide and mercury carbonate are approximately 3.6 x 10-26 and 8.9 x 10-17, respectively; the concentration of mercury in the waste is in accordance with the legislation. The level of some anions such cyanide, fluoride, sulfide must have also to be monitored before discard effluent.10 As the samples to be analysed by ICP OES are previously decomposed using acid medium, the cyanide supposedly present in these samples would be lost by formation of volatile species during heating of the sample in the decomposition step.16 The same would occur if ammonia nitrogen was present in these samples. Therefore, it is implied that there was not cyanide or ammonia nitrogen in the waste sample. The concentration of sulfide and fluoride in the sample after treatment, determined by titration and potentiometry with ion-selective electrode, respectively, were below the limit permitted by the CONAMA. Probably the anions sulfide and fluoride precipitated in salt forms when the medium became alkaline with the presence of NaOH and (NH4)2CO3, since sulfide precipitates with diverse cations in elevated pH's and fluoride forms a insoluble salt with calcium (CaF2). According with CONAMA 430/2011, besides inorganic analysis, the concentration of some organic compounds must be also controlled; however, the effluent of this work comes from samples completely decomposed, since organic matter is an interfering to ICP OES techniques. In this way, it was unnecessary to verify the organic compounds concentration level. Taking into account the Brazilian legislation to discard effluents and the results of parameters evaluated to ICP OES waste after treatment, this effluent could be discarded in the environment. Qualitative analysis of cations in the solid residue obtained from the ICP OES waste treatment The precipitate obtained from the treatment of the ICP OES waste was used as a real sample for the identification of cations in qualitative analytical chemistry classes. To this end, it was tried to solubilize the solid precipitate to have the cations available in the solution. Following the conventional analytical guide used in qualitative analytical experimental classes, the solubilization of the precipitate was tested with water and heated water. In both tests, the complete solubilization of the precipitate was not achieved. Nitric acid with and without heating was also applied, but as the case for water, neither procedure was effective. The same occurred when hydrochloric acid with and without heating, aqua regia (3:1 mixture of HCl and HNO3) and reverse aqua regia (1:3 mixture of HCl and HNO3) were used in an attempt to solubilize the solid residue. It was supposed that the precipitate was composed of hydroxides and carbonate salts, which would readily be digested in a simple acid medium. However, no acid or acid mixture previously described was able to solubilize the solid waste. This situation most likely arose due to the presence of CaF2 in the solid waste. Before analysis by ICP OES, the samples were digested using acid mixtures. The samples that contained silicates required HF to be completely decomposed; therefore, the residue produced by ICP OES analysis may have contained excess fluoride ion that may have formed insoluble compounds such as CaF2 that even in medium-strength acids remained poorly soluble.12 The solubilization of the solid waste was effective only when HF was applied. However, hydrofluoric acid is corrosive, and its use requires special care to maintain the safety of the operator. Considering the risks posed by HF and the intended purpose of using this solid residue as an actual sample in undergraduate chemistry classes, it was decided that only the elements that were identified from the solid residue would be extracted. Thus, for each analyte, a suitable extractor was used: 37% w w-1 HCl for Al3+, 6 mol L-1 CH3COOH for Ca2+, water for Fe2+ and 68% w w-1 HNO3 for Pb2+. Only Al, Ca, Fe and Pb could be identified in the sample because a large amount of available metal is required for qualitative analysis. It was not possible to identify the other cations by semi-micro analysis. Although there may be interference in the qualitative test of the cations, it only occurs when the concentration of interfering is considerable in relation to the analyte, which was not observed in this work because, besides the medium extracting had been selective, the most part of interfering of the cations evaluated were not common in the samples worked in the LEQA. The concentration of the elements in the solid residue will depend on the routine analytical protocol employed by a given laboratory such that the elements that can be analyzed qualitatively may vary for each ICP OES waste sample. Although FAAS waste was not treated in this work, its residue has characteristics similar to those of the ICP OES residue, first primary of which is a low variability of elements because FAAS is a monoelemental technique. Thus, likely the same procedure used to treat ICP OES wastes can be applied to treat FAAS wastes. The qualitative analysis of cations in the solid residue shows that it is possible to apply this solid in qualitative analytical chemistry classes. This practice can promotes reduction of reagent volume in undergraduate laboratories. Application of solid waste in qualitative analysis to undergraduate classes The solid residue obtained from ICP OES waste treatment was applied as a real sample for identification of cations in a qualitative analytical class. After carrying out identification tests, students answered some questions about the ability to identify the cations by the proposed method. They evaluated by this method grades ranging from 1 to 5, with 1 being the ability to identify poorly and 5 excellent. The average of the students can be seen in Table 4.
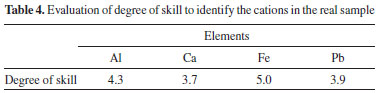
According to the results of the Table 4, it was easy for students to identify the cations in residue samples, even being necessary an extraction procedure to make the cations available in solution instead of completely dissolution of the sample. It was not observed any difficult in the iron identification for the students, they were able to see clearly red complex formed with thiocyanate as well as the aluminum precipitate after aluminon was added. The students had difficulties to identify calcium and lead in the residue sample because the few amount of precipitate formed with these cations when the precipitant was added. Typically, during the analytical chemistry course, the students used to identify the cations in a salt sample that was completely solubilized in aqueous or acid medium; therefore, this sample produced a substantial amount of precipitate when the precipitants were added to the sample. So, the observation of the precipitate in salt samples was easier than in residue samples that was not possible to be solubilized and an extraction of the cations was necessary. Even with the poor amount of calcium and lead precipitated in residue sample analysis, the results in Table 4 showed that the students overcome the problems to identify these cations. Students were also related about the difficulties faced in each step of the described analysis, and the change of pH for aluminum test was the procedure which the students had higher complicacy. The aluminum extraction is performed in acid medium and the identification reaction occurs in pH neutral to alkaline, because that a large amount of ammonia was necessary to increase the pH. In this way, the analyses of residue sample was important to the students realize the complexity of qualitative analysis in a real sample. The procedure of extraction necessary to make available the analytes from residue sample requires other chemical skills besides those used for the cation identification procedures in an unreal sample. They comprise that knowledge about extraction and sample preparation are important in real analysis situation. And, they also learn that the presence of interferences in real samples is common and it is possible to use an extractant medium as strategy to have a selective analysis. The application of the residue from ICP OES waste in the analytical lab classes provides the opportunity to discuss with the students about our responsibility related to the residues generated in chemical experiences. Considering that the cation in solid residue obtained from spectrometric techniques wastes do not have all cations studied in qualitative analytical chemistry, its application is restricted to the final test of the subject, at the end of the course, when the students need to show their ability to find the cations present in a sample. It is important to emphasize that the composition of the solid waste may change, because the variation of lab routine; therefore, a study is necessary for each solid residue produced for ICP OES waste treated before its application in qualitative analytical chemistry classes.
CONCLUSION The strategy proposed for the treatment and reuse of residues produced in the laboratory by ICP OES techniques was observed to be efficient. After the treatment procedure, the concentrations of Ag, Al, As, B, Ba, Ca, Cd, Cr, Cu, Fe, Mg, Mn, Ni, Se, Sn, Pb, Zn, S2- and F- in the solution waste were in compliance with CONAMA Resolution 430/2011. The solid residue obtained from the waste treatment can be used as a sample in experimental analytical chemistry lab classes. Students can thus have the opportunity to investigate the cations present in unknown sample. In the solid residue studied in this work, the cations Al3+, Ca2+, Fe2+ and Pb2+ could be qualitatively analysed. Solid waste have been successful to be inserted as actual sample in qualitative analysis of practical classes giving students the chance to work with real and complex samples in the graduation. The proposed procedure for the treatment of spectrometric wastes will be implemented in the LEQA protocol, and the solid residue generated from the treatment will be used to create samples for experimental qualitative analytical chemistry courses.
SUPPLEMENTARY MATERIAL In the supplementary material is presented the questionnaire applied to students after qualitative analysis of solid residue from ICP OES waste treatment, as well as the practical guide with which they followed for qualitative tests. This supplementary material has free access in Química Nova website (http://quimicanova.sbq.org.br/)
ACKNOWLEDGMENTS The authors acknowledge CAPES (Coordenaçao de Apefeiçoamento de Pessoal do Nível Superior, Brazil) and the Universidade Federal do Ceará for their financial support and the Laboratório de Análises de Traços (LAT), the Laboratório de Métodos de Análises (LABMA) and the Programa de Educaçao Tutorial of the Chemistry Course for providing their facilities to complete this work.
REFERENCES 1. Jardim, W. S.; Quim. Nova 1998, 21, 671. DOI: http://dx.doi.org/10.1590/S0100-40421998000100011 2. Amaral, S. T.; Machado, P. F. L.; Peralba, M. C. R.; Camara, M. R.; Santos, T; Berleze, A. L.; Falcao, H. L.; Martinelli, M.; Gonçalves, R. S.; Oliveira, E. R.; Brasil, J. L.; Araújo, M. A.; Borges, A. C. A.; Quim. Nova 2001, 24, 419. DOI: http://dx.doi.org/10.1590/S0100-40422001000300022 3. Cunha, C. J.; Quim. Nova 2001, 24, 424. 4. Afonso, J. C.; Noronha, L. A.; Felipe, R. P.; Freidinger, N.; Quim. Nova 2003, 26, 602. DOI: http://dx.doi.org/10.1590/S0100-40422003000400027 5. Alberguini, L. B. A.; Silva, L. C.; Rezende, M. O. O.; Quim. Nova 2003, 26, 291. DOI: http://dx.doi.org/10.1590/S0100-40422003000200026 6. Gerbase, A. E.; Coelho, F. S.; Machado, P. F. L.; Ferreira, V. F.; Quim. Nova 2005, 28, 1. DOI: http://dx.doi.org/10.1590/S0100-40422005000100001 7. Gomes, M. G.; Borges, S. S. S.; Junior, C. A. E.; Silva, R. S.; Silva, F. J. S.; Oliveira, S. N.; Revista Universo e Extensao 2013, 1, 61. 8. Vaz, C. R.; Fagundes, A. B.; Oliveira, I. L.; Kovaleski, J. L.; Selig, P. M.; Revista GEPROS 2010, 3, 45. 9. Nolasco, F. R.; Tavares, G. A.; Bendassouli, J. A.; Revista Engenharia Sanitária e Ambiental 2006, 11, 118. 10. Harris, D. C.; Análise Química Quantitativa, 7ª ed., LTC: Rio de Janeiro, 2008. 11. Guiné-Rosias, M. F.; Espectrometria de emissao atômica com plasma acoplado indutivamente (ICP AES), CENA-US: Piracicaba, 1998. 12. Mora, J.; Maestre, S.; Hernandis, V.; Todolí, J. L.; Trends Anal. Chem. 2003, 22, 123. DOI: http://dx.doi.org/10.1016/S0165-9936(03)00301-7 13. Furman, N. H.; Welcher, F. J.; Standard methods of chemical analysis, 20th ed., Litton Education Publishing: Florida, 1962. 14. Harwood, J. E.; Water Res. 1969, 3, 273. DOI: http://dx.doi.org/10.1016/0043-1354(69)90024-4 15. King, J. E.; Qualitative Analysis and Electrolytic Solutions, 2nd ed., Harcourt, Brace: San Diego, 1959. 16. Conselho Nacional do Meio Ambiente (2005), Resoluçao Nº 357, Brasília, 17 de março. 17. Conselho Nacional do Meio Ambiente (2011), Resoluçao Nº 430, Brasília, 16 de maio. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access