Revisão
| Amazon rainforest cosmetics: chemical approach for quality control |
|
Mariko FunasakiI,II,*; Hileia dos Santos BarrosoIII; Valdelira Lia Araújo FernandesII; Ingrid Sabino MenezesIV
IMinistério da Ciência, Tecnologia e Inovação, 70067-900 Brasília - DF, Brasil Recebido em 09/06/2015 *e-mail: marikofunasaki@gmail.com The market for natural cosmetics featuring ingredients derived from Amazon natural resources is growing worldwide. However, there is neither enough scientific basis nor quality control of these ingredients. This paper is an account of the chemical constituents and their biological activities of fourteen Amazonian species used in cosmetic industry, including açaí (Euterpe oleracea), andiroba (Carapa guianensis), bacuri (Platonia insignis), Brazil nut (Bertholletia excelsa), buriti (Mauritia vinifera or M. flexuosa), cumaru (Dipteryx odorata), cupuaçu (Theobroma grandiflorum), guarana (Paullinia cupana), mulateiro (Calycophyllum spruceanum), murumuru (Astrocaryum murumuru), patawa (Oenocarpus bataua or Jessenia bataua), pracaxi (Pentaclethra macroloba), rosewood (Aniba rosaeodora), and ucuuba (Virola sebifera). Based on the reviewed articles, we selected chemical markers for the quality control purpose and evaluated analytical methods. Even though chromatographic and spectroscopic methods are major analytical techniques in the studies of these species, molecular approaches will also be important as used in food and medicine traceability. Only a little phytochemical study is available about most of the Amazonian species and some species such as açaí and andiroba have many reports on chemical constituents, but studies on biological activities of isolated compounds and sampling with geographical variation are limited. INTRODUCTION The market for natural cosmetics featuring ingredients derived from Amazon rainforest is growing worldwide. We have been attracted to exotic fragrance perfume, high potency moisturizing skin cream, etc. In Brazil, most of the cosmetics company have a series of products focused on Amazon ingredients, such as "andiroba", "copaíba", "murumuru", etc.1 Traditionally, the extracts of these plants have been used by indigenous people as sun protection, dry hair treatment, ointment for wound healing, etc. They are based on the beliefs, experiences, and the knowledge handed down from generation to generation. On the other hand, when we purchase natural cosmetics, there is no doubt that we would like to know if the cosmetics really have efficacy or if they contain genuine ingredients. Among more than 40 thousands species of Amazonian plants,2 only a few tens of species are used in cosmetic industries and, about most of them, the efficacy and its responsible chemical constituents have not been proven. To guarantee product quality, a lot of aspects can be considered: minimum threshold for natural ingredients; geographical indication, manufacturing process including extraction, preservation and transportation; extent of contamination; safety under the conditions of use; efficacy with clinical data; safety issues associated with genetic modification.3 In the herbal medicines, chemical markers are generally employed for quality control purposes.4 They are used for both identification and quantification and when they have therapeutic activity, they can serve for control of efficacy. In the same way, chemical markers can be applied to the control of cosmetic products. In this work, we reviewed chemical studies on fourteen species from Amazon rainforest used in the industry of personal hygiene, perfumery, and cosmetics, with the brief descriptions about traditional and cosmetic use of these species. Our object is to provide the information useful for quality control, principally, to pick out chemical markers which stand out chemically and may have biological activities.
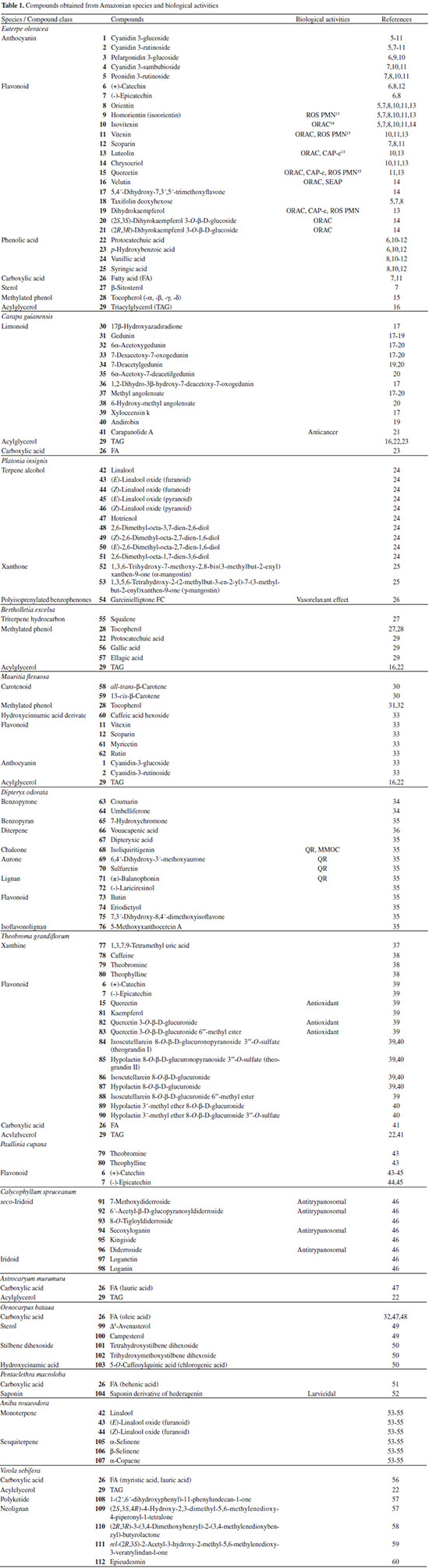
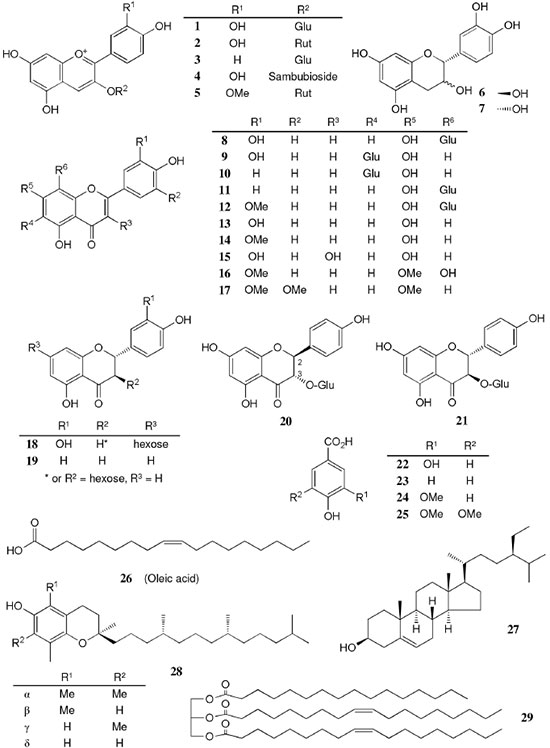
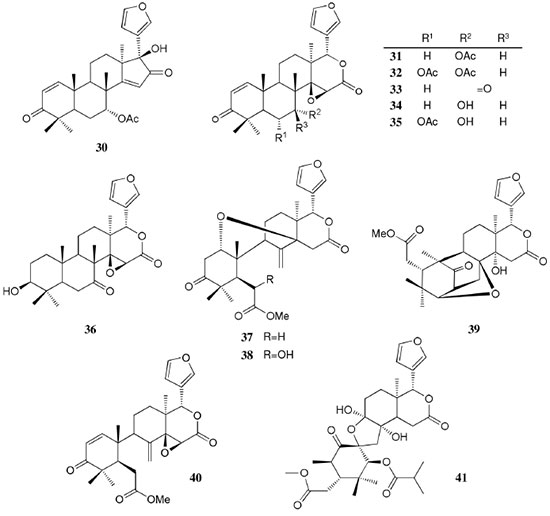
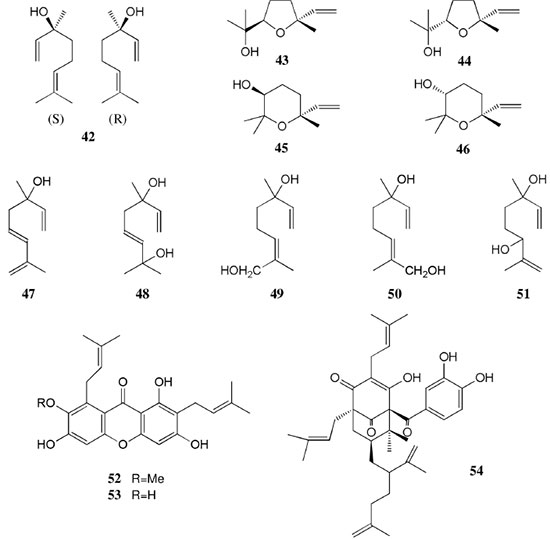
AMAZONIAN SPECIES The Table 1 summarizes the plant source, compound class, compound's name, biological activity and reference. The chemical structures of the compounds mentioned throughout this review are shown in Figures 1 to 12 with numbering 1-112.
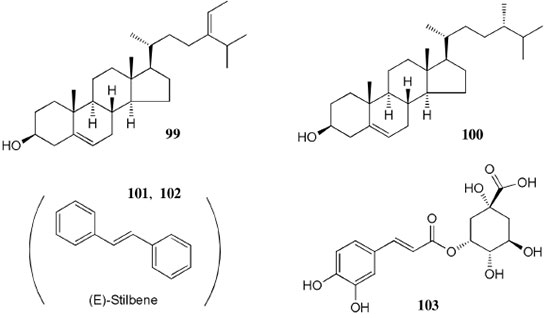 Figure 9. Structures of compounds 99-103 from Oenocarpus bataua
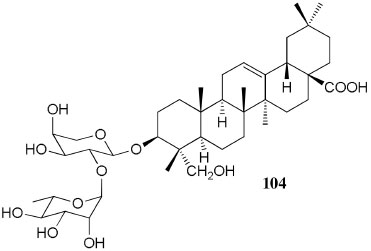 Figure 10. Structure of compound 104 from Pentaclethra macroloba
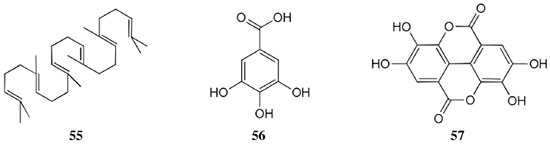
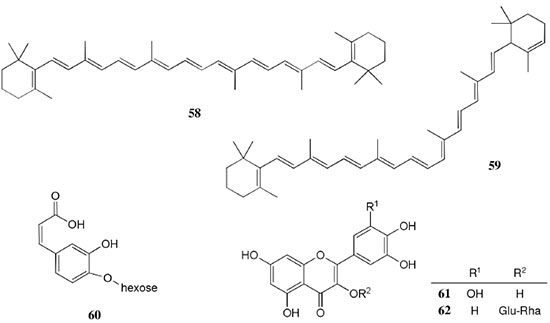
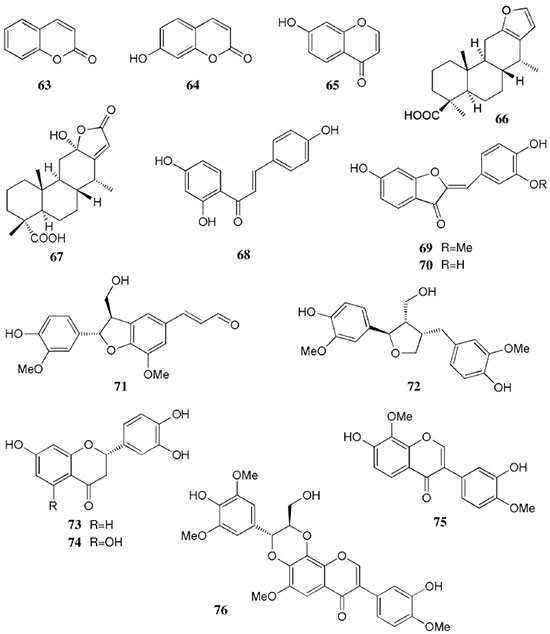
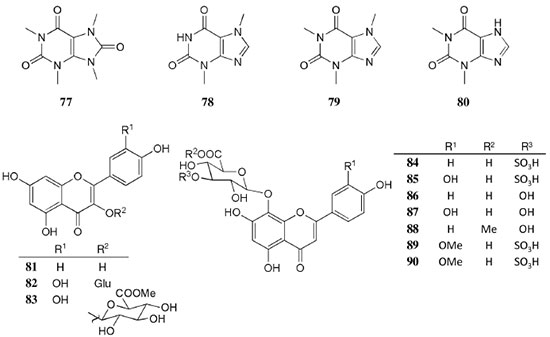
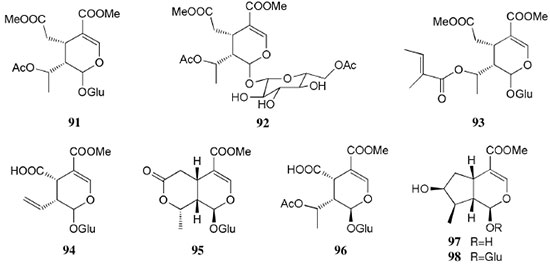
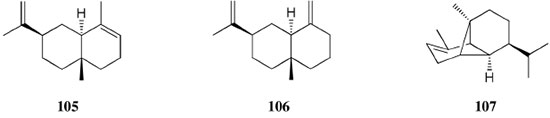
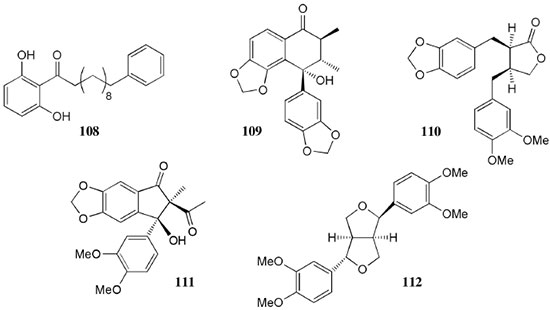
Chemical composition and biological activities of Euterpe oleracea ("açaí") Fruits of E. oleracea have been consumed as energy drink by indigenous and rural people, and are recently employed to prepare a variety of healthy foods, in both tropical and non-tropical countries.61 The oil yielded from the fruit pulp can be used in after-sun products, creams, lotions, shampoo, face masks, and other cosmetics. It is considered to have regenerative and anti-aging properties on the skin due to its constituents like essential fatty acids, phytosterol, and vitamins.61 Phenolic constituents are associated with the anti-oxidant activities in various plant-derived foods. The fruit of E. oleracea contains phenolics as major secondary metabolites remarkably anthocyanins (1-5), which is water-soluble pigments responsible for the dark purple color, and other flavonoids (6-21).5-14 Cyanidin-3-rutinoside (2) is most abundant anthocyanin followed by cyaniding-3-glucoside (1).5,7-11 Non-anthocyanin fractions include a diversity of flavonoids (6-21) and phenolic acids (22-25). Among them, flavone-C-glycosides of luteolin (orientin 8, homoorientin 9) and apigenin (isovitexin 10, vitexin 11) are dominant.5.7,8,10,11,13,14 The liquid chromatography with mass spectrometry (LC/MS) based fingerprinting analysis and mass profiling led to the identification of chemical constituents in the three different processed açaí raw materials. In the case of dichloromethane extracts containing mainly fatty acids (FA) (26), there were no significant difference in chemical profile among the three raw materials, whereas the variations and similarities were obtained in methanol extracts, which showed the presence of anthocyanin (1, 2, 4, 5), non-anthocyanin (8-12, 14, 15) polyphenols and phenolic acids (22, 24).11 Anti-oxidant capacities of flavonoids isolated from the pulp of E. oleracea were evaluated using a chemical-based assay and two cell-based assays: oxygen radical absorbance capacity (ORAC) assay, cell-based anti-oxidant protection (CAP-e) assay and reactive oxygen species formation in polymorphonuclear cells (ROS PMN) assay.13 By combining these assays, quercetin (15) and dihydrokaempferol (19) were found to be a good anti-oxidant with cell penetration ability. Anti-inflammatory assay showed that velutin (16), an uncommon flavone, inhibited secreted embryonic alkaline phosphatase (SEAP) secretion in RAW-BlueTM cells induced by LPS or oxLDL at low micromole level.14 E. oleracea fruit is rich in lipids with a high amount of unsaturated FA (26) (74%), of which oleic acid (omega-9) and linoleic acid (omega-6) are predominant with 56% and 13%, respectively.7 In addition, it has a high phytosterol level, mainly β-sitosterol (27)7 and these compounds are known to have beneficial effects on skin protection.62 Vitamin E, a general term for tocopherols and tocotrienols (α-, β-, γ-, and δ-), which are effective lipid-soluble antioxidants and α-tocopherol has been reported to play an important role in skin photoprotection.63 Darnet et al.15 reported that tocopherol (28) profiles (α, β+γ, δ) in E. oleracea fruit pulp from three different locations of Amazon estuary were found to be similar, but the mean total tocopherol value (404.5 µg g-1) was high with a concentration equivalent to many nuts.12 Chemical composition and biological activities of Carapa guianensis ("andiroba") Seed oil of C. guianensis has traditionally been used as a household liniment for treatment of sprains, rashes, sore, and inflammation, as well as insect repellent by indigenous people.64 In the cosmetic industry, the seed oil is used as soap, cream, massage oil, etc. The bitter taste, origin of name "andiroba" in the Tupi-Guarani language, is due to limonoids, highly oxygenated and modified terpenoids. Limonoids (30-41) are known to have a variety of biological activities like insecticidal, antifeedant, antibacterial, antimalarial, and antiviral.65 Recently, a number of limonoids have been isolated from the seed oil.17-21 Among them, carapanolide A (41) exhibited moderate cancer cell growth inhibition using murine L1210 leukemia cells with IC50 8.7 µM.21 High performance liquid chromatography (HPLC) method, optimized by central composite design, determined the amount of four limonoids, gedunin (31), 6α-acetoxygedunin (32), 7-desacetoxy-7-oxogedunin (33), and methyl angolensate (37), being 33 most abundant.18 Cabral et al.,23 characteryzed the triacylglycerols (TAG) (29), FA (26) and limonoid profiles of the seed oil via mass spectrometry fingerprinting using direct electrospray ionization mass spectrometry (ESI-MS). The technique, requiring no pre-separation of sample, could detect adulteration of the andiroba oil with soybean oil at levels as low as 10%. Chemical composition and biological activities of Platonia insignis ("bacuri") The sticky and white fruit of P. insignis is consumed row by local people, and is often made into various condiments and beverages due to its distinctive taste with pleasant odor and subacid flavor.66 The seeds contain high amount of oil, being used for treatment of eczemas and herpes.67 In cosmetics, the oil is used as soap, skin care product, and moisturizer,68 but the cultivation is very limited and most of the fruit production is extractive.69 Analysis of the volatile fractions of the fruits demonstrated that terpene alcohols (42-51) are the most abundant, and among them, linalool (42) and related compounds (43-51) may indicate the biosynthetic origin.24 The antioxidant and toxicity activities of the dichloromethane and ethyl acetate fractions of ethanolic extract of the seeds were evaluated.25 Both fractions demonstrated in vitro antioxidant effects, by 1,1-diphenyl-2-picryl-hydrazyl (DPPH) and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS) assays, as well as in vivo effects in antioxidant-defective Saccharomyces cerevisiae strains. These activities could be attributed to xanthones (52, 53), present in these fractions as major compounds. The xanthones isolated from other species have been reported to demonstrate anti-cancer and anti-inflammatory activities.70 Garcinielliptone FC (54), a polyisoprenylated benzophenones, present in the seed of P. insignis, promoted an endothelium-independent vasorelaxation on phenylephrine-induced vasoconstriction.26 Chemical composition and biological activities of Bertholletia excelsa (Brazil nut) B. excelsa is internationally traded seed crop and is collected exclusively from natural forests because the tree is unsuitable for cultivation. The reasons for that are the very slow growth, taking 10 to 30 years to fructify, and their unique reproductive system, which requires specific bees for pollination.71 The nut, which consists of 60-70% fat and 17% protein, has long been a valuable source of nutrient for indigenous and local people residing in the Amazon region.71 The oil extracted from the nut is high in mono- and polyunsaturated fatty acid and selenium,72 and has recently been used in cosmetic industries such as emollient or moisturizing creams.73 Squalene (55) is a triterpene hydrocarbon, a precursor in the synthesis of steroids. In human skin surface, it prevents lipid peroxidation.74 According to the studies by Maguire et al.75 and Ryan et al.,27 the Brazil nut contained the highest squalene (1377.8 µg g-1) among ten edible nuts. Tocopherols (6) are also remarkable compounds found in this nut (total tocopherol contents in oil 199.1 µg g-1)27 like as other species of nuts. The decreasing order of total tocopherol level was almond > hazelnut > walnut > pistachio > pine nut > Brazil nut > pecan > peanut > macadamia > cashew.27,75 We determined tocopherol contents of Brazil nut oils from different Amazon regions as well as some commercial oils. The rates of γ-tocopherol/α-tocopherol of authentic oils didn't show distinct variation when compared to that of other species. This fact indicates that the tocopherol profile can be useful to distinguish Brazil nut oil from other vegetable oil.28 John and Shahidi29 identified and quantified free- and bound phenolics in kernel, brown skin and whole nuts. Phenolic acids and flavonoids, including (+)-catechin (6), protocatechuic acid (22), vanillic acid (24), gallic acid (56), and ellagic acid (57), were found and the concentration of phenolics was the highest in the brown skin. Similarly, Trolox Equivalent Antioxidant Capacity (TEAC), DPPH, and ORAC assays showed that the brown skin had the highest antioxidant activities.29 Chemical composition and biological activities of Mauritia flexuosa ("buriti") M. flexuosa (syn. M. vinifera) is a beautiful palm tree and the common name "buriti" means "that contains water" in Tupi-Guarani language because it develops in water logged areas throughout the tropical region of South.76 The oil extracted from the fruits is traditionally used to cure respiratory problem, pneumonia, asthma, cough, influenza, fever, snake bite and heart problems as well as to treat dry hair77 and can be used as adjuvant in sun protection formulation.78 Carotenoids (58, 59) are a class of yellow to red pigment, belong to the tetraterpenoids built from four terpene units. They act as antioxidant and some of them can be converted to vitamin A in the human body. Further, when present in the skin, they have an important role in photoprotection against UV radiation.79 Among the numerous food already analyzed, the fruit of M. flexuosa has the highest β-carotene amount, as well as other provitamin A.80 Unique profile of carotenoids was obtained by HPLC- MS/MS analysis, with total carotenoid content of 513 µg g-1.30 Tocopherol (26) content of the fruit pulp of M. flexuosa is very high (1169 µg g-1 of dry matter)32 and the profile with a predominant β+γ tocopherol fraction is similar to that of other seed and nut oil, as Brazil nut, cashew nut, pecan nut, and walnut.27,75 Further, Koolen et al.33 reported that the fruits contained a considerable amount of phenolic compounds (1, 2, 11, 12, 60-62), being glycosylated flavonoids: vitexin (11), scoparin (12), and rutin (62), and anthocyanins: cyanidin-3-glucoside (1) and cyanidin-3-rutinoside (2), as the main constituents, and it may justify the observed antioxidant activities. Further studies are needed to clarify the relationship between phenolic compounds and activities as well as to obtain the quantitative and fingerprinting data. Chemical composition and biological activities of Dipteryx odorata ("cumaru") Seeds of D. odorata are also known as tonka beans and contain an important chemical component for perfumes, coumarin (63), as a major compound with yields of 1 to 3%, and other minor compounds such as diterpene, flavonoid, lignan, etc. (64-76).34,35 Jang et al.35 isolated four potential cancer chemopreventive compounds: isoliquiritigenin (68), 6,4'-dihydroxy-3'-methoxyaurone (69), sulfuretin (70), and (±)-balanophonin (71) by a bioassay-guided fractionation of an ethyl acetate-soluble extract of the seeds using the enzyme quinone reductase (QR) induction assay. With further analysis of selected compounds, 68 exhibited significant inhibition of carcinogen-induced preneoplastic lesion formation (76% at 10 µg mL-1) in the mouse mammary organ culture (MMOC) assay. Chemical composition and biological activities of Theobroma grandiflorum ("cupuaçu") Fruits of T. grandiflorum are very much appreciated for its acidic and highly-flavored pulp, that is consumed as the ingredient of juices, ice-creams, jams, and candies.69 Phytochemical analyses demonstrated in the seeds the presence of xanthine alkaloids (76-79)37,38 and flavonoids (6, 7, 15, 80-89),39,40 of which hypolaetin 8-O-β-D-glucuronide (87) and isoscutellarein 8-O-β-D-glucuronopyranoside 3"- O-sulfate (84) were dominant.40 Activity-guided fractionation using DPPH method of the seeds identified 11 flavonoid antioxidants (6, 7, 15, 81-88), of which quercetin (15) and its glycosides (82, 83) were more potent with IC50 values of 39.7-44.4.39 FA (27) and TAG (29) compositions of seed fats were compared among the eight species of the genus Theobrama.41 Significant differences in these profiles were observed between species of distinct sections (subdivision of genus according to morphological characters), whereas the profiles of species from the same section were similar. Chemical composition and biological activities of Paullinia cupana ("guarana") P. cupana is widely consumed as an effective stimulant in dietary supplements and drinks. It is due to the highest content of caffeine (78) in its seed from 3 to 6% of dry weight,42,81 but other minor compounds also contribute to pharmacological effects such as central nervous system stimulants of xanthine alkaloids (79, 80), antioxidant flavonoids (6, 7) and tannins.43,45 In the cosmetic industry, the seed extracts are used in soap, cream, and shampoo.82 Majhenič et al.83 reported that the seed extracts exhibited high antioxidant, antimicrobial, and antifungal activities, and had promising potentials as natural additives in food, cosmetic, and pharmaceutical industries. Also, because of the cellulite reduction effect of methylxanthines, such as 78-80, P. cupana is used for cosmetic formulation.84 The seed powder and commercial tablets of P. cupana were characterized by capillary electrophoresis (CE) using caffeine (78) as a marker compound and the results were compared to those obtained by HPLC.85 In this study, the CE technique showed good specificity, sensitivity and precision like HPLC and had advantages in the analysis time and solvent consumption as compared to HPLC. Chemical composition and biological activities of Calycophyllum spruceanum ("mulateiro") The wood of C. spruceanum (syn. C. multiflorum) has high economic value because of its resistance to deterioration and facility to work, being used in lumber and construction materials.86 Local people apply the bark poultice to skin, as antifungal, contraceptive, emollient, and vulnerary or take the bark decoction for diabetes and eye infection.87 The bark of C. spruceanum has recently attracted world attention as the ingredient for body care products.86 Phytochemical study showed that the ethanolic extracts of the wood bark yielded seco-iridoids and iridoids (91-98), of which 7-methoxydiderroside (91), 6'-acetyl-β-D-glucopyranosyldiderroside (92), secoxyloganin (94), and diderroside (96) exhibited weak in vitro antitrypanosomal activities.46 Screening study for antifungal activity of forty five extracts of species used in traditional medicine demonstrated that the dichloromethane extract of the bark of C. multiflorum had the highest activity,88 which may support the use for skin treatment. Chemical composition and biological activities of Astrocaryum murumuru ("murumuru") A distinguishing feature of the palm tree, A. murumuru, is innumerable thorns even on the seeds and flowers. The oil extracted from the seed is white and solid at room temperature.61 The seed butter can be added to skin care products, shampoos, and conditioners because the oil has water-retention capacity.89 The chemical studies on saponifiable compositions of the seed butter demonstrated unique TAG (29) and FA (26) profiles with lauric (43-52%) and myristic (26-37%) acid as major fatty acids.22,47 Chemical composition and biological activities of Oenocarpus bataua ("patawa") O. bataua (syn. Jessenia bataua) is one of the useful palm tree for Amazonian indigenous. The fruit is consumed as a nutritional beverage and the oil extracted from both fruit and kernel seed is traditionally used for medicinal, culinary, and cosmetic purposes, including for hair and skin care.69,90 The fruit oil has a profile of fatty acids similar to that of olive oil, with high oleic acid contents (77%), being considered to have nutritional value.32,47,48 Because in the sterol composition, the percentage of Δ5-avenasterol (99), known for effective antioxidant,91 is high and that of campesterol (100) is relatively low, these compounds could serve as markers for authentication of the oil.49 The latest report by Rezaire et al.50 dealing with the in vitro antioxidant tests showed the good activity of the fruit of O. bataua as compared to E. oleracea and other berries known as potential antioxidants. This activity could be accounted for by the presence of characteristic polyphenols like stilbenes (101, 102), phenolic acids (103), and condensed tannins, identified by ultra-performance liquid chromatography/mass spectrometry (UPLC/MS). However, further experiments including structural elucidation and biological activity would be required. Chemical composition and biological activities of Pentaclethra macroloba ("pracaxi") Seed oil of P. macroloba is used in cosmetic industry and may have positive effects on wound healing.92 The oil is known to have the highest content of behenic acid, C21H43COOH, in natural products (10-25%),51 while most fruits or nuts contain less than 1%.27,48 Besides, the saponin (104) isolated from the seeds exhibited high larvicidal activity with LC50 = 18.6 µg mL-1.52 Chemical composition and biological activities of Aniba rosaeodora (rosewood) Essential oil of A. rosaeodora is worldly famous with pleasant rose-like aromas and is employed as fragrance in fine perfumery. European fragrance industry originally obtained the oil from French Guiana, but after the intensive exploitation, Brazilian rainforest is the main producer and A. rosaeodora is protected under international law.93 The oil is obtained from the wood chips and contains mainly linalool (42, 80-90%) with small amounts of (Z)- or (E)-linalool oxide (furanoid) (43, 44), α- or β-selinene (105, 106) and α-copaene (107).53-55 For the sustainable production of the oil, there are other sources including: the young leaves or branches of A. rosaeodora; synthetic linalool; another species with linalool-rich essential oils, such as Cinnamomum camphora94 and Croton cajucara.95 Yet sometimes, as obtained by low cost and considered low quality, they are used for product adulteration. Chemical study using comprehensive two-dimensional gas chromatography coupled with quadrupole mass spectrometric detection (GC×GC-qMS) identified major and minor chemical compositions of the oils extracted from the leaves collected from trees with different ages (four, ten and twenty year old) and few differences in constituents were found among all samples.96 Souza et al.97 characterized the oils from the wood and leaves by ESI-MS and the adulteration with synthetic linalool could be detected by fingerprinting. Linalool (42) has two enantiomeric forms due to a stereogenic center at C3 (Figure 3). (R)-(-)-linalool has a woody lavender scent while (S)-(+)-linalool has a sweet floral scent and both forms can be found at various proportions according to the geographic origin and part of the tree.53,55,98 Linalool has a diversity of pharmacological properties,99 but the chiral influence to the properties is not clear.55,100 Chemical composition and biological activities of Virola sebifera ("ucuuba") Yellow and aromatic fat extracted from the seeds of V. sebifera has some cosmetic properties such as emollient, moisturizer, skin conditions and antiseptic, and due to that, it is used for industrial products such as soap, cleansing, massage lotion and hair care products.69 The fat has high content of myristic (72.9%) and lauric (17.3%) acids56 and the former has high absorption into the skin.101 Study on the secondary metabolites of the seeds showed the accumulation of a variety of lignans (109-112) such as aryltetralone (109), dibenzylbutyrolactone (110), arylindan (111), and furofuran (112) type lignans.57-60
ANALYTICAL TECHNIQUES FOR QUALITY CONTROL OF COSMETIC PRODUCTS AND OTHER APPROACHES Reported chemical constituents of Amazonian species were classified from an analytical point of view: 1) TAG and FA - Major constituents of oils and fats are triacylglycerols, which are esters derived from glycerol and three fatty acids. FA analysis by gas chromatography with flame ionization detection (GC-FID) is a broadly accepted official method to characterize quality and a specific oil,102 but the analysis needs a conversion of TAG to fatty acid methyl esters by acid or base, and provides only total percentage of FA.103 The separation of TAG can be achieved by HPLC,104 but diverse factors, sample preparations, stationary and mobile phases, columns and detectors, have to be considered depend on the number of carbon atoms and their saturation.105 A refractive index (RI) was the most widely used detector for the HPLC analysis despite its low sensibility.106,107 Recently, advance of mass spectrometry has enabled the direct analysis of TAG in Amazon oils. First, Saraiva et al.22 characterized Amazon vegetable oils (andiroba, Brazil nut, buriti, passion fruit, cupuaçu, and ucuuba) via dry matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) without pre-separation or derivatization. Easy ambient sonic-spray ionization mass spectrometry (EASI-MS) has, then, realized instantaneous analysis of Amazon vegetable oils, including açaí, andiroba, Brazil nut, and buriti, by both TAG and free fatty acid profiles.16 Moreover, several samples of Brazil Nut oil (authentic oils of different geographic origin, commercial oils) were evaluated using EASI-MS TAG profiles and adulteration of Brazil nut oil with soy bean oil could be detected at a level of 5% or higher.108 2) Unsaponifiable fraction or minor components - This fraction contains minor constituents, which have also been employed for oil characterization. Tocopherols, carotenoids, and sterols are usually contained in any oils, but its amount varies depending on species and environmental conditions. Their qualitative and quantitative analyses can be performed by chromatographic methods such as HPLC coupled with photodiode array detection (DAD), fluorescence detection (FLD) or MS for tocopherol or carotenoid30,109,110 and GC-FID for sterols.49,109 Phenolic compounds have also been investigated because they are responsible for the antioxidant potential, stability and organoleptic characteristics and the use of these compounds would permit the authentication and quality assessment.11,50 3) Volatile constituents- Volatile compounds obtained from D. odorata and A. rosaeodora are ingredients of perfumes. The universally adapted analytical technique is GC including GC-FID and GC-MS. These techniques have recently been substituted by direct infusion mass spectrometry without or with simple pre-treatment because of the fast and simple measurement.97 Besides these techniques mentioned above, infrared111 and NMR112 spectroscopy have been applied to the authentication of natural products. Analyses of stable isotope ratio and trace element have also allowed us to determine the origin of food products.113 The fingerprinting techniques have become more and more popular for the purpose of quality control.16,22,23,108 They enable the handling of a large amount of data and provide quantitative and qualitative information of the compounds as well as the relationship between chemical information and efficacy with the aid of chemometric tools.114 Even though chemical composition analyses have been a great success to detect adulteration, there are difficulties in the discrimination of cultivars because of the high variability influenced by environmental conditions. In order to overcome this problem, molecular approach has been used in food115 and medicine116 traceability. In particular, for complex matrix like olive oil, molecular markers techniques such as random amplified polymorphic DNA (RAPD), amplified fragment length polymorphism (AFLP), and simple sequence repeat (SSR) are useful.117 Special attention has, however, to be paid for extraction and purification of DNA because samples (oil or extract) are usually complex and highly processed.118
CONCLUDING REMARKS Table 2 summarized possible chemical markers of reviewed Amazonian species for control purpose of cosmetic products. Chemical markers have been used in the regulatory agencies of herbal medicines or by scientists and each agency or scientist has own classification.4 With reference to them, the following criteria were used to select chemical markers: (a) the constituents have biological activities which are or are not related to efficacy as cosmetics; (b) their biological activities are not known, but they are abundant or the isolation yield was the highest; (c) they are not abundant, but characteristic (not common); (d) the fingerprintings (or profiles) of the groups of constituents are distinguishable when compared to those of other species.

These chemical markers have been proposed based on the reviewed articles. It is noted that markers have different role according to the type. When the markers don't have biological activities, they can provide quantitative information. It seems that the fingerprintings of TAG would be applicable to most of the fixed oils for quantitative assessment, especially identification and detection of adulteration. Essential fatty acids such as linoleic acid may be useful as chemical markers to evaluate efficacy, but they would not be adequate for the use of authentication purpose or stability test due to the susceptibility to autooxidation.119 In view of increasing demand for Amazon cosmetics, little data are available about the chemical studies on Amazonian species. Even though chemical constituents of some species, such as E.oleracea, C. guianensis, have been studied by many researchers, their biological activities, especially cosmetic efficacies, have hardly been evaluated. Furthermore, there are few studies focused on the origin of extract and in many cases researchers have obtained or purchased previously prepared extracts because of the difficulty of access to the Amazon raw materials. This problem has to be overcome to study geographical origin, seasonality, cultivar, and extraction method that affect the quality of natural cosmetics. Thus, it is essential that quality control of natural products would be accomplished by multi/interdisciplinary approaches including chemistry, biology, and agriculture. In addition, the access to the Amazon biodiversity should be controlled properly by international regime120 for further research and development.
ACKNOWLEDGMENTS This work was supported by Brazilian Science foundation CNPq and CAPES.
REFERENCES 1. http://www1.folha.uol.com.br/folha/equilibrio/noticias/ult263u2714.shtml, accessed in May 2015. 2. Pyers, G.; Biodiversity of Rainforests, Macmillan Education: South Yarra, 2013. 3. http://www.ecocert.com/en, accessed in May 2013; http://www.wto.org/english/tratop_e/trips_e/gi_background_e.htm, accessed in August 2013; Nohynek, G. J. ; Antignac, E.; Re, T.; Toutain, H.; Toxicol. Appl. Pharm. 2010, 243, 239. 4. Li, S.; Han, Q.; Qiao, C.; Song, J.; Cheng, C. L.; Xu, H.; Chinese Medicine 2008, 3, 1; EMA-European Medicines Agency. Reflection paper on markers used for quantitative and qualitative analysis of herbal medicinal products and traditional herbal medicinal products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003211.pdf DOI: http://dx.doi.org/10.1186/1749-8546-3-7 5. Gallori, S.; Bilia, A. R.; Bergonzi, M. C.; Barbosa, W. L. R.; Vincieri, F. F.; Chromatographia 2004, 59, 739. 6. Pozo-Insfran, D. D.; Brenes, C. H.; Talcott, S. T.; J. Agric. Food Chem. 2004, 52, 1539. DOI: http://dx.doi.org/10.1021/jf035189n PMID: 15030208 7. Schauss, A. G.; Wu, X.; Prior, R. L.; Ou, B.; Patel, D.; Huang, D.; Kababick, J. P.; J. Agric. Food Chem. 2006, 54, 8598. DOI: http://dx.doi.org/10.1021/jf060976g PMID: 17061839 8. Pacheco-Palencia, L. A.; Duncan, C. E.; Talcott, S. T.; Food Chem. 2009, 115, 1199. DOI: http://dx.doi.org/10.1016/j.foodchem.2009.01.034 9. Pacheco-Palencia, L. A.; Mertens-Talcott, S. U.; Talcott, S. T.; Food Chem. 2010, 119, 1071. DOI: http://dx.doi.org/10.1016/j.foodchem.2009.08.017 10. Gordon, A.; Cruz, A. P. G.; Cabral, L. M. C.; Freitas, S. C. de; Dib Taxi, C. M. A.; Donangelo, C. M.; Mattietto, R. de A.; Friedrich, M.; Matta, V. M. da; Marx, F.; Food Chem. 2012, 133, 256. DOI: http://dx.doi.org/10.1016/j.foodchem.2011.11.150 PMID: 25683393 11. Mulabagal, V; Calderón, A. I.; Food Chem. 2012, 134, 1156. DOI: http://dx.doi.org/10.1016/j.foodchem.2012.02.123 PMID: 23107743 12. Pacheco-Palencia, L. A.; Mertens-Talcott, S.; Talcott, S. T.; J. Agric. Food Chem. 2008, 56, 4631. DOI: http://dx.doi.org/10.1021/jf800161u PMID: 18522407 13. Kang, J.; Li, Z.; Wu, T.; Jensen, G. S.; Schauss, A. G.; Wu, X.; Food Chem. 2010, 122, 610. DOI: http://dx.doi.org/10.1016/j.foodchem.2010.03.020 14. Kang, J.; Xie, C.; Li, Z.; Nagarajan, S.; Schauss, A. G.; Wu, T.; Wu, X. Food Chem. 2011, 128, 152. DOI: http://dx.doi.org/10.1016/j.foodchem.2011.03.011 PMID: 25214342 15. Darnet, S.; Serra, J. L.; Rodrigues, A. M. C.; Silva, L. H. M.; Food Res. Int. 2011, 44, 2107. DOI: http://dx.doi.org/10.1016/j.foodres.2010.12.039 16. Simas, R. C.; Catharino, R. R.; Cunha, I. B. S.; Cabral, E. C.; Barrera-Arellano, D.; Eberlin, M. N.; Alberici, R. M.; Analyst 2010, 135, 738. DOI: http://dx.doi.org/10.1039/b923272a PMID: 20349539 17. Ambrozin, A. R. P.; Leite, A. C.; Bueno, F. C.; Vieira, P. C.; Fernandes, J. B.; Bueno, O. C.; Silva, M. F. G. F.; Pagnocca, F. C.; Hebling, M. J. A.; Bacci, M.; J. Braz. Chem. Soc. 2006, 17, 542. 18. Tappin, M. R. R.; Nakamura, M. J.; Siani, A. C.; Lucchetti, L.; J. Pharm. Biomed. Anal. 2008, 48, 1090. DOI: http://dx.doi.org/10.1016/j.jpba.2008.08.027 PMID: 18845411 19. Silva, V. P.; Oliveira, R. R.; Figueiredo, M. R.; Phytochem. Anal. 2009, 20, 77. DOI: http://dx.doi.org/10.1002/pca.1100 PMID: 19003936 20. Silva, S. G.; Nunomura, R. C. S.; Quim. Nova 2012, 35, 1936. DOI: http://dx.doi.org/10.1590/S0100-40422012001000009 21. Inoue, T.; Nagai, Y.; Mitooka, A.; Ujike, R.; Muraoka, O.; Yamada, T.; Tanaka, R.; Tetrahedron Lett. 2012, 53, 6685. DOI: http://dx.doi.org/10.1016/j.tetlet.2012.09.108 22. Saraiva S. A.; Cabral, E. C.; Eberlin, M. N.; Catharino, R. R.; J. Agric. Food Chem. 2009, 57, 4030. DOI: http://dx.doi.org/10.1021/jf900043u 23. Cabral, E. C.; Cruz, G. F.; Simas, R. C.; Sanvido, G. B.; Gonçalves, L. V.; Leal, R. V. P.; Silva, R. C. F.; Silva, J. C. T.; Barata, L. E. S.; Cunha, V. S.; França, L. F.; Daroda, R. J.; Sá, G. F.; Eberlin, M. N.; Anal. Methods 2013, 5, 1385. DOI: http://dx.doi.org/10.1039/c3ay25743f 24. Boulanger, R.; Chassagne, D.; Crouzet, J.; Flavour Fragr. J. 1999, 14, 303. DOI: http://dx.doi.org/10.1002/(SICI)1099-1026(199909/10)14:5<303::AID-FFJ834>3.0.CO;2-C 25. Costa-Júnior J. S.; Ferraz, A. B. F.; Sousa, T. O.; Silva, R. A. C.; De Lima, S. G.; Feitosa, C. M.; Citó, A. M. G. L.; Melo Cavalcante, A. A. C.; Freitas, R. M.; Moura Sperotto, A. R. M.; Péres, V. F.; Moura, D. J.; Saffi, J.; Basic Clin. Pharmacol. Toxicol. 2013, 112, 34. DOI: http://dx.doi.org/10.1111/j.1742-7843.2012.00924.x PMID: 22788872 26. Arcanjo, D. D. R.; Costa-Júnior, J. S.; Moura, L. H. P.; Ferraz, A. B. F.; Rossatto, R. R.; David, J. M.; Quintans-Júnior, L. J.; Oliveira, R. C. M.; Cito, A. M. G. L; Oliveira, A. P.; Nat. Prod. Res. 2014, 28, 923. DOI: http://dx.doi.org/10.1080/14786419.2014.889136 PMID: 24579922 27. Ryan, E.; Galvin, K.; O'Connor, T. P.; Maguire, A. R.; O'Brien, N. M.; Int. J. Food Sci. Nutr. 2006, 57, 219. DOI: http://dx.doi.org/10.1080/09637480600768077 PMID: 17127473 28. Funasaki, M.; Menezes, I. S.; Barroso, H. S.; Zanotto, S. P.; Carioca, C. R. F.; Acta Amaz. 2013, 43, 505. DOI: http://dx.doi.org/10.1590/S0044-59672013000400012 29. John, J. A.; Shahidi, F.; J. Funct. Foods 2010, 2, 196. DOI: http://dx.doi.org/10.1016/j.jff.2010.04.008 30. Rosso, V. V.; Mercadante, A. Z; J. Agric. Food Chem. 2007, 55, 5062. DOI: http://dx.doi.org/10.1021/jf0705421 PMID: 17530774 31. Silva, S. M.; Taham, S. T.; Rocco, S. A.; Ceriani, R.; Meirelles, A. J. A.; J. Am. Oil Chem. Soc. 2009, 86, 611. DOI: http://dx.doi.org/10.1007/s11746-009-1400-9 32. Darnet, S. H.; Silva, L. H. M.; Rodrigues, A. M. C.; Lins, R. T.; Cienc. Tecnol. Aliment. 2011, 31, 488. DOI: http://dx.doi.org/10.1590/S0101-20612011000200032 33. Koolen, H. H. F.; Silva, F. M. A.; Gozzo, F. C.; Souza, A. Q. L.; Souza, A. D. L.; Food Res. Int. 2013, 51, 467. DOI: http://dx.doi.org/10.1016/j.foodres.2013.01.039 34. Sullivan, G.; J. Agric. Food Chem. 1982, 30, 610. DOI: http://dx.doi.org/10.1021/jf00111a052 35. Jang, D. S.; Park, E. J.; Hawthorne, M. E; Schunke Vigo, J.; Graham, J. G.; Cabieses, F.; Santarsiero, B. D.; Mesecar, A. D.; Fong, H. H. S.; Mehta, R. G.; Pezzuto, J. M.; Kinghorn, A. D.; J. Nat. Prod. 2003, 66, 583. DOI: http://dx.doi.org/10.1021/np020522n PMID: 12762787 36. Godoy, R. L. O.; Lima, P. D. D. B.; Pinto, A. C.; Aquino Neto, F. R.; Phytochemistry 1989, 28, 642. DOI: http://dx.doi.org/10.1016/0031-9422(89)80073-1 37. Vasconcelos, M. N. L; Silva, M. L.; Maia, J. G. S.; Gottlieb, O. R.; Acta Amaz. 1975, 5, 293. 38. Lo-Coco, F.; Lanuzza, F.; Micali, G.; Cappellano, G.; J Chromatogr. Sci. 2007, 45, 273. DOI: http://dx.doi.org/10.1093/chromsci/45.5.273 PMID: 17555636 39. Yang, H.; Protiva, P.; Cui, B.; Ma, C.; Baggett, S.; Hequet, V.; Mori, S.; Weinstein, I. B.; Kennelly, E. J.; J. Nat. Prod. 2003, 66, 1501. DOI: http://dx.doi.org/10.1021/np034002j PMID: 14640528 40. Pugliese, A. G.; Tomas-Barberan, F. A.; Truchado, P.; Genovese, M. I.; J. Agric. Food Chem., 2013, 61, 2720. DOI: http://dx.doi.org/10.1021/jf304349u PMID: 23431956 41. Gilabert-Escrivá, M. V.; Gonçalves, L. A. G.; Silva, C. R. S.; Figueira, A.; J. Sci. Food Agric. 2002, 82, 1425. DOI: http://dx.doi.org/10.1002/jsfa.1107 42. Henman, A. R.; J. Ethnopharmacol. 1982, 6, 311. DOI: http://dx.doi.org/10.1016/0378-8741(82)90054-X PMID: 7154700 43. Heard, C. M.; Johnson, S.; Moss, G.; Thomas, C. P.; Int. J. Pharm. 2006, 317, 26. DOI: http://dx.doi.org/10.1016/j.ijpharm.2006.02.042 PMID: 16600539 44. Yamaguti-Sasaki, E.; Ito, L. A.; Canteli, V. C. D.; Ushirobira, T. M. A.; Ueda-Nakamura, T.; Dias Filho, B. P., Nakamura C. V.; Mello, J. C. P.; Molecules 2007, 12, 1950. DOI: http://dx.doi.org/10.3390/12081950 PMID: 17960098 45. Ushirobira, T. M. A.; Yamaguti, E.; Uemura, L. M.; Nakamura, C. V.; Dias Filho, B. P.; Mello, J. C. P.; Lat. Am. J. Pharm. 2007, 26, 5. 46. Zuleta, L. M. C.; Cavalheiro, A. J.; Silva, D. H. S.; Furlan, M.; Young, M. C. M.; Albuquerque, S.; Castro-Gamboa, I.; Bolzani, V. S.; Phytochemistry 2003, 64, 549. DOI: http://dx.doi.org/10.1016/S0031-9422(03)00153-5 47. Mambrim, M.C.T.; Barrera-Arellano, D.; Grasas Aceites 1997, 48, 154. DOI: http://dx.doi.org/10.3989/gya.1997.v48.i3.783 48. Rodrigues, A. M. C.; Darnet, S.; Silva, L. H. M.; J. Braz. Chem. Soc. 2010, 21, 2000. DOI: http://dx.doi.org/10.1590/S0103-50532010001000028 49. Montufar, R.; Laffargue, A.; Pintaud, J.; Avallone, S. H. S.; Dussert, S.; J. Am. Oil Chem. Soc. 2010, 87, 167. DOI: http://dx.doi.org/10.1007/s11746-009-1490-4 50. Rezaire, A.; Robinson, J.-C.; Bereau, D.; Verbaere, A.; Sommerer, N.; Khan, M. K.; Durand, P.; Prost, E.; Fils-Lycaon, B.; Food Chem. 2014, 149, 62. DOI: http://dx.doi.org/10.1016/j.foodchem.2013.10.077 PMID: 24295677 51. http://www.amazonoil.com.br/en/products/oils/pracachy.htm, accessed in October, 2013. 52. Santiago, G. M. P.; Viana, F. A.; Pessoa, O. D. L.; Santos, R. P.; Pouliquen, Y. B. M.; Arriaga, A. M. C.; Andrade-Neto, M.; Braz-Filho, R.; Rev. Bras. Farmacogn. 2005, 15, 187. DOI: http://dx.doi.org/10.1590/S0102-695X2005000300003 53. Zellner, B. D.; Lo Presti, M.; Barata, L. E. S.; Dugo, P.; Dugo, G.; Mondello, L.; Anal. Chem. 2006, 78, 883. DOI: http://dx.doi.org/10.1021/ac051337s 54. Maia, J. G. S.; Andrade, E. H. A.; Couto, H. A. R.; Silva, A. C. M.; Marx, F.; Henke, C.; Quim. Nova 2007, 30, 1906. DOI: http://dx.doi.org/10.1590/S0100-40422007000800021 55. Sampaio, L. F. S.; Maia, J. G. S.; Parijós, A. M.; Souza, R. Z.; Barata, L. E. S.; Phytother. Res. 2012, 26, 73. DOI: http://dx.doi.org/10.1002/ptr.3518 56. Soares, B. M. C.; Gamarra, F. M. C.; Paviani, L. C.; Gonçalves, L. A. G.; Cabral, F. A.; J. Supercrit. Fluids 2007, 43, 25. DOI: http://dx.doi.org/10.1016/j.supflu.2007.03.013 57. Lopes, L. M. X.; Yoshida, M.; Gottlieb, O. R.; Phytochemistry 1982, 21, 751. DOI: http://dx.doi.org/10.1016/0031-9422(82)83181-6 58. Lopes, L. M. X.; Yoshida, M.; Gottlieb, O. R.; Phytochemistry 1983, 22, 1516. DOI: http://dx.doi.org/10.1016/S0031-9422(00)84055-8 59. Lopes, L. M. X.; Yoshida, M.; Gottlieb, O. R.; Phytochemistry 1984, 23, 2021. DOI: http://dx.doi.org/10.1016/S0031-9422(00)84962-6 60. Lopes, L. M. X.; Yoshida, M.; Gottlieb, O. R.; Phytochemistry 1984, 22, 2647. DOI: http://dx.doi.org/10.1016/S0031-9422(00)84118-7 61. Burlando, B.; Verotta, L. Cornara L.; Bottini-Massa, E.; Herbal Principles in Cosmetics: Properties and Mechanisms of Action, CRC Press, Taylor & Francis Group: Boca Raton, 2010. 62. Elias, P. M.; Brown, B. E.; Ziboh, V. A.; J. Invest., Dermatol. 1980, 74, 230; Rafia, B.; Int. J. Sci. Res. Rev. 2013, 2, 1. DOI: http://dx.doi.org/10.1111/1523-1747.ep12541775 63. Anstey, A. V.; Clin. Exp. Dermatol. 2002, 27, 170. DOI: http://dx.doi.org/10.1046/j.1365-2230.2002.01040.x PMID: 12072001 64. Ferraz, I. D. K.; Camargo, J. L. C.; Sampaio, P. T. B.; Acta Amaz. 2002, 32, 647; Kilham, C. Tales from the Medicine Trail: Tracking Down the Health Secrets of Shamans, Herbalists, Mystics, Yogis, and Other Healers; Rodale Press: Emmaus, 2000. 65. Roy, A.; Saraf, S.; Biol. Pharm. Bull. 2006, 29, 191. DOI: http://dx.doi.org/10.1248/bpb.29.191 PMID: 16462017 66. http://www.hort.purdue.edu/newcrop/morton/bakuri.html, accessed in June 2013. 67. Agra, M. F.; Freitas, P. F.; Barbosa-Filho, J. M.; Rev. Bras. Farmacogn. 2007, 17, 114. DOI: http://dx.doi.org/10.1590/S0102-695X2007000100021 68. Bolton, E. R.; Hewer, D. G.; Analyst 1922, 47, 282; Schiller, C.; Schiller, D.; The Aromatherapy Encyclopedia: A Concise Guide to Over 385 Plant Oils, Basic Health Publications, Inc.: Laguna Beach, 2008. DOI: http://dx.doi.org/10.1039/AN9224700282 69. Menezes, A. J. E. A; Carvalho, J. E. U.; Homma, A. K. O.; Matos, G. B. In Congresso Brasileiro de Sistemas Agroflorestais, 7, 2009, Luziánia, Sociedade Brasileira de Sistemas Agroflorestais: Brasília, DF, Emater-DF, Embrapa, 2009. 70. Gutierrez-Orozco, F.; Failla, M. L.; Nutrients 2013, 5, 3163. DOI: http://dx.doi.org/10.3390/nu5083163 PMID: 23945675 71. http://www.rain-tree.com/brazilnu.htm, accessed in June 2013. 72. Yang, J.; LWT-Food Sci. Technol. 2009, 42, 1573; Costa, P. A.; Ballus, C. A.; Teixeira-Filho, J.; Godoy, H. T.; Ciênc. Tecnol. Aliment. 2011, 31, 950 . DOI: http://dx.doi.org/10.1016/j.lwt.2008.03.016 73. Athar, M.; Nasir, S.M.; Afr. J. Biotechnol. 2005, 4, 36. 74. Kohno, Y.; Egawa, Y.; Itoh, S.; Nagaoka, S.; Takahashi, M.; Mukai, K.; Biochim Biophys Acta 1995, 1256. 52. DOI: http://dx.doi.org/10.1016/0005-2760(95)00005-W PMID: 7742356 75. Maguire, L. S.; O'Sullivan, S. M.; Galvin, K.; O'Connor, T. P.; O'Brien, N. M.; Int. J. Food Sci. Nutr. 2004, 55, 171. DOI: http://dx.doi.org/10.1080/09637480410001725175 PMID: 15223592 76. Prance, G. T.; Futures 1990, 22, 891. DOI: http://dx.doi.org/10.1016/0016-3287(90)90059-Q 77. Martins, R. C.; Filgueiras, T. S.; Albuquerque, U. P.; Econ. Bot. 2012, 66, 91. DOI: http://dx.doi.org/10.1007/s12231-011-9182-z 78. Zanatta, C. F.; Mitjans, M.; Urgatondo, V.; Rocha-Filho, P. A.; Vinardell, M. P.; Food Chem. Toxicol. 2010, 48, 70. DOI: http://dx.doi.org/10.1016/j.fct.2010.05.032 PMID: 19766688 79. Anunciato, T. P.; da Rocha Filho, P. A.; J. Cosmet. Dermatol. 2012, 11, 51. DOI: http://dx.doi.org/10.1111/j.1473-2165.2011.00600.x PMID: 22360335 80. Taipina, M. S.; International Journal of Nutrology 2013, 6, 102. 81. Mumford ,G. K.; Holtzman ,S.G.; J. Pharmacol. Exp. Ther. 1991, 258, 857. PMID: 1890622 82. Hamerski, L.; Somner, G. V.; Tamaio, N.; J. Med. Plants Res. 2013, 7, 2221. 83. Majhenič, L.; Škerget, M.; Knez, Ž.; Food Chem. 2007, 104, 1258. DOI: http://dx.doi.org/10.1016/j.foodchem.2007.01.074 84. Hexsel, D.; Orlandi, C.; Zechmeister do Prado, D.; Dermatol. Surg. 2005, 31, 866. DOI: http://dx.doi.org/10.1111/j.1524-4725.2005.31733 PMID: 16029680 85. Sombra, L. L.; Gómez, M. R.; Olsina, R.; Martínez, L. D.; Silva, M. F.; J. Pharm. Biomed. Anal. 2005, 36, 989. DOI: http://dx.doi.org/10.1016/j.jpba.2004.08.026 PMID: 15620524 86. http://www.rain-tree.com/mulaterio.htm, accessed in october 2013. 87. Duke, J. A.; Bogenschuts-Godwin, M. J.; Ottesen, A. R.; Duke's Handbook of Medicinal Plants of Latin America, CRC Press,Talor & Francis Group: Boca Raton, 2009. 88. Portillo, A.; Vila, R.; Freixa, B.; Adzet, T.; Cañigueral, S.; J. Ethnopharmacol. 2001, 76, 93. DOI: http://dx.doi.org/10.1016/S0378-8741(01)00214-8 PMID: 11378288 89. Msika, P.; Piccirelli, A.; EP1461060 A2 2004. 90. Plotkin, M. J.; Balick, M. J.; J. Ethnopharmacol. 1984, 10, 157. DOI: http://dx.doi.org/10.1016/0378-8741(84)90001-1 PMID: 6727398 91. Gordon, M. H.; Magos, P.; Food Chem. 1983, 10, 141. DOI: http://dx.doi.org/10.1016/0308-8146(83)90030-4 92. Banov, D.; Banov, F.; Bassani, A. S.; Dermatol. Ther. 2014, 4, 259. DOI: http://dx.doi.org/10.1007/s13555-014-0065-y 93. Wilson, R.; Aromatherapy: Essential Oils for Vibrant Health and Beauty, Avery: New York, 2002. 94. Roszaini, K.; Nor Azah, M. A.; Mailina, J.; Zaini, S.; Mohammad Faridz, Z.; Wood Sci. Technol. 2013, 47, 1273. DOI: http://dx.doi.org/10.1007/s00226-013-0576-1 95. Rosa, M. S. S.; Mendonça-Filho, R. R.; Bizzo, H. R.; Rodrigues, I. A.; Soares, R. M. A.; Souto-Padrón, T.; Alviano, C. S.; Lopes, A. H. C. S.; Antimicrob. Agents Chemother. 2003, 47, 1895. DOI: http://dx.doi.org/10.1128/AAC.47.6.1895-1901.2003 96. Fidelis, C. H. V.; Sampaio, P. T. B.; Krainovic, P. M.; Augusto, F.; Barata, L. E. S.; Microchem. J. 2013, 109, 73. DOI: http://dx.doi.org/10.1016/j.microc.2012.03.034 97. Souza, R. C. Z.; Eiras, M. M.; Cabral, E. C.; Barata, L. E. S.; Eberlin, M. N.; Catharino, R. R.; Anal. Lett. 2011, 44, 2417. DOI: http://dx.doi.org/10.1080/00032719.2011.551852 98. Chantraine, J. M.; Dhénin, J. M.; Moretti, C.; J. Essent. Oil Res. 2009, 21, 486. DOI: http://dx.doi.org/10.1080/10412905.2009.9700225 99. Venâncio, A. M.; Marchioro, M.; Estevam, C. S.; Melo, M. S.; Santana, M. T.; Onofre, A. S. C. Guimarães, A. G.; Oliveira, M. G. B.; Alves, P. B.; Pimentel, H. C.; Quintans-Júnior, L. J.; Braz. J. Pharmacogn. 2011, 21, 1043; Batista, P. A.; Werner, M. F. F.; Oliveira, E. C.; Burgos, L.; Pereira, P.; Brum, L. F. D.; Santos, A. R. S.; Neurosci. Lett. 2008, 440, 299. DOI: http://dx.doi.org/10.1590/S0102-695X2011005000147 100. Sousa, D. P.; Nóbrega, F. F. F.; Santos, C. C. M. P.; Almeida, R. N.; Nat. Prod. Commun. 2010, 5, 1847. PMID: 21299105 101. Becker, L. C.; Bergfeld, W. F.; Belsito, D. V.; Hill, R. A.; Klaassen, C. D.; Marks, J. G. Jr; Shank, R. C.; Slaga, T. J.; Snyder, P. W.; Alan-Andersen, F.; Int. J. Toxicol. 2010, 29, 162S. DOI: ht,,,, , tp://d, x.doi.org/10.1177/1091581810374127 PMID: 20634506 102. D'Imperio, M.; Dugo, G.; Alfa, M.; Mannina, L.; Segre, A. L.; Food Chem. 2007, 102, 956. DOI: http://dx.doi.org/10.1016/j.foodchem.2006.03.003 103. Christie, W. W. In Advances in Lipid Methodology - Two, Christie, W. W., ed.; Oily Press: Dundee, 1993. 104. Lee, D. S.; Lee, E. S.; Kim, H. J.; Kim, S. O.; Kim, K.; Anal. Chim. Acta 2001, 429, 321. DOI: http://dx.doi.org/10.1016/S0003-2670(00)01289-7 105. Aparicio, R.; Morales, M. T.; Aparicio-Ruiz, R.; Tena, N.; Garcia-Gonzalez, D. L.; Food Res. Int. 2013, 54, 2025. DOI: http://dx.doi.org/10.1016/j.foodres.2013.07.039 106. Endo, Y.; Ohta, A.; Kido, H.; Kuriyama, M.; Sakaguchi, Y.; Takebayashi, S.; Hirai, H.; Murakami, C.; Wada, S.; J. Oleo Sci. 2011, 60, 451. DOI: http://dx.doi.org/10.5650/jos.60.451 PMID: 21852743 107. Pacheco, C.; Palla, C.; Crapiste, G. H.; Carrín, M. E.; Food Anal. Methods 2014, 7, 2013. DOI: http://dx.doi.org/10.1007/s12161-014-9830-x 108. Funasaki, M; Oliveira, R. S.; Zanotto, S. P.; Carioca, C. R.; Simas, R. C.; Eberlin, M. N.; Alberici, R. M.; J. Agric. Food Chem. 2012, 60, 11263. DOI: http://dx.doi.org/10.1021/jf303877t PMID: 23113649 109. Costa, P. A.; Ballus, C. A.; Teixeira-Filho, J.; Godoy, H. T.; Food Res. Int. 2010, 43, 1603. DOI: http://dx.doi.org/10.1016/j.foodres.2010.04.025 110. Albuquerque, M. L. S.; Guedes, I.; Alcantara Jr., P.; Moreira, S. G. C.; Barbosa Neto, N. M.; Correa, D. S.; Zilio, S. C.; J. Braz. Chem. Soc. 2005, 16, 1113. 111. Wang, J; Jun, S; Bittenbender, H. C.; Gautz, L; Li, Q. X.; J. Food Sci. 2009, 74, C385; Tres, A.; van der Veer, G.; Perez-Marin, M. D.; van Ruth, S. M.; Garrido-Varo, A.; J. Agric. Food Chem. 2012, 60, 8129. DOI: http://dx.doi.org/10.1111/j.1750-3841.2008.01002.x PMID: 19646032 112. Lucas-Torres, C.; Pérez, A.; Cabañas, B.; Moreno, A.; Food Chem. 2014, 165, 21; Sciubba, F.; Capuani, G.; Cocco, M. E. D.; Avanzato, D.; Delfini, M.; Food Res. Int. 2014, 62, 66. DOI: http://dx.doi.org/10.1016/j.foodchem.2014.05.092 PMID: 25038644 113. Anderson, K. A.; Smith, B. W.; J. Agric. Food Chem. 2006, 54, 1747; Benincasa, C.; Lewis, J.; Perri, E.; Sindona, G.; Tagarelli, A.; Anal. Chim. Acta 2007, 585, 366; Bandoniene, D.; Zettl, D.; Meisel, T.; Maneiko, M.; Food Chem. 2013, 136, 1533. DOI: http://dx.doi.org/10.1021/jf052928m PMID: 16506828 114. Liang, Y.; Xie, P.; Chau, F.; J. Sep. Sci. 2010, 33, 410. DOI: http://dx.doi.org/10.1002/jssc.200900653 PMID: 20099260 115. Bazakos, C.; Dulger, A. O.; Uncu, A. T.; Spaniolas, S.; Spano, T.; Kalaitzis, P.; Food Chem. 2012, 134, 2411; Torelli, A.; Marieschi, M.; Bruni, R.; Food Control 2014, 36, 126. DOI: http://dx.doi.org/10.1016/j.foodchem.2012.04.031 PMID: 23442703 116. Seethapathy, G. S.; Balasubramani, S. P.; Venkatasubramanian, P.; Food Chem. 2014, 145, 1015. DOI: http://dx.doi.org/10.1016/j.foodchem.2013.09.027 PMID: 24128578 117. Ben-Ayed, R.; Kamoun-Grati, N.; Rebai, A.; Compr. Rev. Food Sci. Food Saf. 2013, 12, 218. DOI: http://dx.doi.org/10.1111/1541-4337.12003 118. Costa, J.; Mafra, I.; Oliveira, M. B. P. P.; Trends Food Sci. Technol. 2012, 26, 43. DOI: http://dx.doi.org/10.1016/j.tifs.2012.01.009 119. Duh, P.-D.; Yen, W. J.; Yen, G.-C.; J. Am. Oil Chem. Soc. 1999, 76, 201. DOI: http://dx.doi.org/10.1007/s11746-999-0218-9 120. http://brazil-works.com/wp-content/uploads/2012/11/Fact-sheet_india_final.pdf, accessed in May 2015. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access