Revisão
| Clinical aspects of the poisoning by the pesticide endosulfan |
|
Jiří PatočkaI,II; Qinghua WuIII,IV; Tanos C. C. FrançaIII,V,VI; Teodorico C. RamalhoVII; Rene PitaVIII; Kamil KučaII,III,V,*
IDepartment of Radiology and Toxicology, Faculty of Health and Social Studies, University of South Bohemia Ceske Budejovice, Czech Republic Recebido em 24/11/2015 *e-mail: kamil.kuca@fnhk.cz Endosulfan is an organochlorine insecticide, widely used in insect control. Unfortunately, it is also an acute neurotoxic compound to both insects and mammals, including humans, and has been responsible for many severe poisonings and several fatal cases. Endosulfan also imitates or enhances the effect of the female hormone estrogen, having the capability of causing reproductive and developmental damage in both, animals and humans, and its exposure has been linked to liver tissue injury. This persistent lipophilic compound is one of the most abundant organochlorine pesticides in the environment, capable of undergoing long range transport to remote locations such as the Arctic. It is practically water-insoluble, but readily adheres to clay particles and persists in soil and water for several years. Its indiscriminate and injudicious use in the control of insects on a wide range of agricultural products and in the extermination of house-hold pests, has considerably increased the hazard risk for human health. Also, this compound has a high fatality rate in humans when ingested accidentally, from food or water contaminated, or in suicidal cases. The aim of this article is to review and summarize chemical, biochemical, environmental, and toxicological data of endosulfan and draw attention to its toxicological potential risk to human health. INTRODUCTION Endosulfan is an organochlorine manufactured insecticide which was first introduced in the 1950s, and is commonly known by its trade-name Thiodan®. It is widely used to control a number of insects on a wide range of agricultural products, such as grains, tea, fruits, vegetables, tobacco and cotton, among others. It is also used as a wood preservative.1 The toxicity of endosulfan is well known on non-target organisms and has been responsible for many severe poisonings, including several fatal cases.2 Endosulfan is a highly toxic insecticide that produces tonic-clonic convulsions, headache, dizziness and ataxia. It can also cause life threatening metabolic disturbances.3 Treatment is only limited to symptomatic and supportive measures. Endosulfan is considered a persistent organic pollutant (POP) and belongs to the organochlorine insecticides group that includes molecules notorious for being POPs. High environmental persistence coupled with their potential for bioconcentration and biomagnification may lead to toxic levels in plants and animals. Nowadays, there is also a call for a worldwide ban on POPs because of their possible links to cancer and effects on hormones, the immune system, and reproduction. However, when compared with other POPs which disperse across the world, endosulfan tends to remain close to its region of use, although it has been found in high concentrations in many areas around the world because it is widely used.4 Endosulfan is associated with a high fatality rate in humans when ingested accidentally, from food or water contaminated, or in suicide cases.5-13 It is also a substance that imitates or enhances the effect of the female hormone estrogen, which means it can cause reproductive and developmental damage in both animals and humans. Researchers studying children from an isolated village in Kerala, India, have linked endosulfan exposure to delays in sexual maturity among boys.14,15 As a result of its poisonous and persistent nature, endosulfan has been banned from many developed countries, and is gradually being phased out in others although extensive use continues in less-developed parts of the world. It is already banned in Australia, New Zealand, Singapore, Bangladesh, Brazil, Indonesia, Korea, Thailand, but is still in use in India, Taiwan and the US, as well as in some EU countries such as Denmark and Great Britain.
CHEMISTRY Endosulfan (6,7,8,9,10,10-hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benzodioxathiepin 3-oxide, CAS Registry Number 6994-04-3) is a polychlorinated compound (Figure 1). It is a cream to brown colored solid that may appear crystalline or in flakes. It has a distinct odor similar to turpentine and does not burn. Its physical properties are summarized in Table 1.
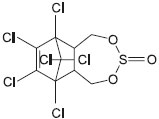 Figure 1. Chemical structure of endosulfan; 6,7,8,9,10,10-hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benzodioxathiepin 3-oxide; CAS Registry Number 6994-04-3
Endosulfan is produced by the Diels-Alder reaction of hexachlorocyclopentadiene with cis-butene-1,4-diol. The product is then reacted with thionyl chloride, liberating HCl.16 This process produces a preparation containing approximately 30% of β-endosulfan and 70% of α-endosulfan (Figure 2).
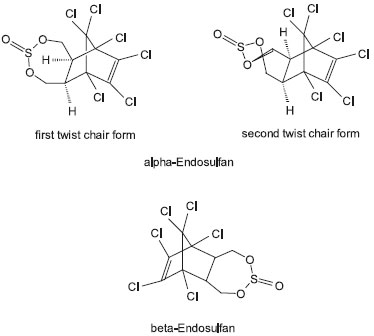 Figure 2. Endosulfan is a mixture of stereoisomers, designated α and β. β-endosulfan is a symmetrical compound, whereas α-endosulfan is asymmetric and exists as two twist chair forms. Technical product contains two stereoisomeric endosulfans, α- and β-endosulfan, in the proportion variously reported as from 4:1 to 7:3. The technical material is a 90-95 per cent pure mixture of the two isomers
STEREOCHEMISTRY Endosulfan is actually a mixture of stereoisomers, designated α and β. α-Endosulfan is more thermodynamically stable, while β-endosulfan slowly converts irreversibly to the α-form at room temperature.18 Commercial endosulfan has traditionally been described as a mixture of two diastereoisomers in a ratio of 70% of α-endosulfan and 30% of β-endosulfan. Early reports on the conformation of the endosulfan isomers were based on incorrect NMR assignments. More recently, Schmidt et al.19 used NMR spectroscopy and X-ray crystallography to determine the structure of the isomers. They reported that β-endosulfan is a symmetrical compound, whereas α-endosulfan exists as two asymmetric isomers (Figure 2). In mammals, the α-isomer is significantly more toxic than the β-isomer, with a LD50 of the α-isomer in rats of 76 mg/kg in comparison to 340 mg/kg for the β-isomer.20
ENVIRONMENTAL TOXICOLOGY Endosulfan is an important pollutant of high priority for international environmental agencies.21 It enters air, water and soil when manufactured or used as a pesticide, commonly applied to crops by using sprayers. Some endosulfan in the air may travel long distances before it lands on crops, soil, or water. Endosulfan on crops usually breaks down within a few weeks; however, it may stay in soil for several years before it all breaks down. Endosulfan does not dissolve easily in water; therefore, in surface water it is usually attached to floating soil particles or can be found attached to soil at the bottom. The small amounts of endosulfan that dissolve in water breaks down over time. Animals that live in endosulfan-contaminated soil and waters (invertebrates, insects, fish, shellfish, etc.) can build up endosulfan into their bodies, and achieve internal amounts of this pesticide that may be several times greater than in the surrounding water. Endosulfan is extremely toxic to fish and aquatic invertebrates,22,23 however, the atlantic salmon (Salmo salar) tolerates dietary technical endosulfan levels up to 500 µg/kg.24 The photochemical degradation route of endosulfan in water involves the formation of the endosulfan diol, its transformation to endosulfan ether and finally the ether's complete degradation.25 This chemical also has been gradually implicated in mammalian gonadal toxicity,26-29 genotoxicity,30 and neurotoxicity.31 In the environment, endosulfan can be converted by attack at the sulfite group via either oxidation or hydrolysis, to form the toxic endosulfate (endosulfan sulfate) and the nontoxic endodiol (endosulfan diol), respectively (Figure 3).32,33 The degradation of endosulfan is stereo-selective,34 as β-endosulfan is hydrolyzed faster than the α-isomer to endosulfan diol, which is then rapidly degraded to endosulfan ether, endosulfan α-hydroxyether (major product), and endosulfan lactone.35 The formation of endosulfate is thought to occur only through biological transformation, whereas hydrolysis to the diol occurs readily at alkaline pH.36 It was observed that isolated strains of Aspergillus niger can degrade endosulfan to endodiol.37 As endosulfan degrades to several different compounds their half-life in nature are unknown and highly dependent on a number of factors.
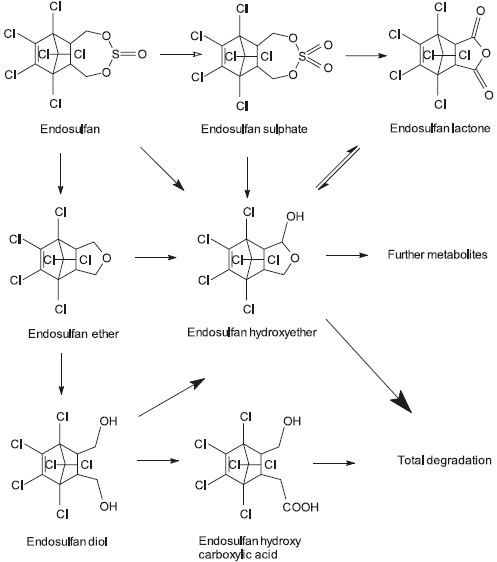 Figure 3. The proposed metabolic pathway for endosulfan in human, as published by Lee et al.33
Martens38 studied the degradation of endosulfan by different bacterial and fungal cultures, and found that endodiol and endosulfate, respectively, were the major metabolites accumulated. Besides, small amounts of endohydroxy ether and endolactone were also formed.39 He has proposed a pathway, wherein, the endosulfan is converted to endosulfate followed by endodiol, endohydroxy ether and endolactone. Formation of these metabolites has also been confirmed by other investigators.40-42 Unlike the isomers of endosulfan, the lipophilic metabolite endosulfate can accumulate in animal fat.1,43,44 As a result, pasture contamination can result in unacceptably high endosulfate residues in locally grown production animals. Endosulfan present at trace levels in air and total atmospheric precipitations of Paris was found by Trajkovska et al.45 About its distribution predicate fact, endosulfan was also found in the eggs of broad-snouted caimans (Caiman latirostris), thus caiman eggs could be useful to biomonitor local contamination by endosulfan. The origin of endosulfan in caiman eggs is not known but appears to come from food contaminated by air during spraying.46 Trace amounts of endosulfan (from non-detectable to 27 pg m-3) were recorded also in Australia's atmosphere in 2012, together with many other persistent organic pollutants.47 Endosulfan sulfate was one of the most frequently pesticide detected in the Arctic, with peak deposition fluxes of 1.0 and 0.4 pg.cm-2 per year. While endosulfan sulfate was more abundant than its parent compounds in most years, endosulfan (sum of α and β isomers) was predominant in 2003 and 2006 which, together with air mass backward trajectories, suggests a possible origin from ongoing use in Eurasia.48 Potter et al.49 measured endosulfan wet deposition in precipitation over a 4-year period within an area of high agricultural use in Southern Florida (USA) and in nearby Biscayne and Everglades National Parks. Endosulfan's two isomers and endosulfan sulfate were detected at high frequency with the order of detection and concentration being: β-endosulfan > α-endosulfan > endosulfan sulfate. Within the agricultural area, detection frequency (55 to 98%), mean concentrations (5 to 87 ng L-1) and total daily deposition (200 ng m-2 day-1) exceeded values at other sites by 5 to 30-fold. Endosulfan in soil can be degraded by some soil bacteria, as demonstrated by the findings of recently discovered endosulfan-degrading bacterial strain Alcaligenes faecalis JBW4 which was isolated from activated sludge. This strain is able to use endosulfan as a carbon and energy source.50 The estrogenic activity of environmentally relevant doses of endosulfan was investigated by injecting ovariectomized adult rats once a day for 3 days with sesame oil (control), 0.02 mg kg-1 day-1 with 17 β-estradiol (an uterotrophic dose; UD), 0.0002 mg kg-1 day-1 with 17 β-estradiol (a non-uterotrophic dose; NUD), and 0.006, 0.06, 0.6 or 6 mg kg-1 day-1 of endosulfan. After 24 hours of treatment, the uteri were weighed (uterotrophic assay) and the luminal epithelial cell height (LECH), progesterone receptor (PR) and estrogen receptor alpha (ERalpha) protein levels were measured. PR, ERalpha, and complement factor-3 (C3) mRNAs were evaluated using real-time PCR. Uterine weight and LECH were only increased in UD-treated rats. PR, ERalpha and C3 expression levels were modified in most of the endosulfan-treated groups, showing an identical pattern of expression to the NUD-group.51 These results show that endosulfan mimics non-uterotrophic actions, strengthening the hypothesis that endosulfan is a widespread xenoestrogen.
BIOCHEMICAL DATA Endosulfan administration to rats significantly increased the activity of choline kinase and phosphocholine cytidylyltransferase (both cytosolic and microsomal) of lung and liver. The induction of phosphatidylcholine (PC) biosynthesis in lung and liver of rats has been shown following the intratracheal administration of endosulfan (1 mg 100 g-1 body weight) for 3 days.52 As pointed by Narayan et al.,53 biochemical effects of endosulfan are different in many cases from other organochlorine insecticide. For example, DDT and endosulfan have similar effects on microsomal lipid metabolism, but produce different biochemical manifestations on the secretion of surfactant phospholipids.54 Endosulfan significantly inhibited testicular androgen biosynthesis in adult rats (p.o. administered 7.5 and 10 mg kg-1 body weight), consecutively for 15 and 30 days. No appreciable alterations were apparent in body weights, testicular wet weights, and cytosolic and microsomal protein contents of testis in treated rats. Profound decrease in the levels of plasma gonadotrophins (FSH and LH) along with plasma testosterone and testicular testosterone were observed at both administration treatments, particularly after the longer exposure of 30 days. Activities of 3 β- and 17 β-hydroxysteroid dehydrogenases were considerably lowered on longer exposure treatments.26 Rousseau et al.55 observed that endosulfan is able to induce a substantial increase of prolactin expression while not increasing cell growth. These results suggest that endosulfan could modulate an estrogen-inducible gene such as prolactin, possibly acting via second messenger-mediated cellular mechanisms instead of solely competing with estrogens for the nuclear estrogen receptor sites. Endosulfan is reported to suppress humoral as well as cellular immune responses. It did not have any influence on nitrite production, but suppressed the LPS-induced TNF-α generation.56 Endosulfan exposure (8 and 16 mg kg-1) to rats significantly decreased the activities of superoxide dismutase and catalase, reduced glutathione levels and increased lipid peroxidation.57 Endosulfan is also able to deplete glutathione (GSH) and induce apoptosis in human peripheral blood mononuclear cells (PBMC) in vitro.58 Oral rat administration of 10 mg kg-1 body weight/day for two and four weeks showed toxic interference with liver and kidney's biochemistry and histology. Biochemical parameters, like aspartate amino transferase, alanine amino transferase, acid phosphatase, alkaline phosphatase, bilirubin, urea and creatinine, were increased, which clearly showed the hepatotoxic and nephrotoxic effects of endosulfan. Histopathologically findings included increased liver size, sinusoidal dilation, pyknotic nuclei, cytoplasmic degranulation and various nuclear aberrations. Similarly, pathological alterations were also observed in the kidney, especially chronic glomerulonephritis, glomerulosclerosis, adenoma and glomerulus deposits.59 Endosulfan is an important hepatotoxic agent that generates free oxygen radicals in the liver. Results of the enzyme and histological analyses show that exposure of rats to endosulfan cause liver tissue damage, independent of the route of exposure.60 Endosulfan can cause toxic effects on rabbit pancreas. Microscopy examination indicated degenerative changes and immunohistochemistry analyses showed marked decreases in proinsulin-, insulin-, and amylin-secreting cells and slight decreases in glucagon-secreting cells, whereas cells expressing caspase 3 increased.61 Endosulfan induced alterations in serum biochemical markers of oxidative stress and antioxidant capacity in rabbits, but vitamin C has an ameliorative effect. It was suggested that vitamin C supplementation might be helpful in preventing the detrimental effects of increased oxidative stress caused by endosulfan exposure.62 Finally, endosulfan was able to inhibit cholinesterase activity in vitro and in vivo in Wistar rats and to cross the placental barrier and/or to be eliminated through milk, affecting the enzyme activity in male rat pups.63 In vitro, the enzyme activity was found to be inhibited in a concentration dependent manner.
HUMAN EXPOSURE Humans are exposed to various environmental chemicals such as organochlorine pesticide residues, heavy metals, polychlorinatedbiphenyls (PCBs), among many others. The most likely way for human endosulfan exposure is through consumption of contaminated food in countries where this pesticide was not banned yet. In fact, endosulfan has been found in food products such as oils, fats, and fruit and vegetable products.64,65 Exposure can also occur by smoking cigarettes made from tobacco that has endosulfan residues on it and by skin contact or inhalation, especially if safe handling and application procedures are not followed. Accidental spills and releases to the environment at hazardous waste disposal sites are other possible sources of endosulfan exposure. Exposure to endosulfan has been reported in people living near hazardous waste sites through contact with contaminated soils.66 Pathak et al.67 first reported endosulfan levels in North Indian population. This study was designed to analyze the levels of organochlorine pesticide residues in maternal and cord blood samples of normal healthy women with full term pregnancy to gain insight into the current status of pesticide burden in newborns. Hexachlorocyclohexane was the main organochlorine present, followed by endosulfan. Published data indicates a transfer rate of 60-70% of these pesticides from mothers to newborns, which is of great concern as it may adversely affect the growth and development of newborn. No studies of fatal cases after inhalation exposure to endosulfan have been found. Symptoms of endosulfan poisoning Occupational exposure during manufacture and application has resulted in human poisoning cases. Symptoms of endosulfan poisoning have also been seen in people who intentionally or accidentally ingested large amounts of endosulfan.5 Most of these people experienced nausea, vomiting, headache, dizziness, convulsions or other nervous system effects, begining 2.7 ± 0.5 h after ingestion.68 Endosulfan poisoning in humans causes blood pressure and eletrocardiogram alterations.8 Over half of the patients developed complications, such as rhabdomyolysis, hepatic toxicity and hypotension.69 These complications resolved without sequelae in the survival group. Refractory status epilepticus70 was the most common cause of death in this series (75%). An ingestion of more than 35 g of endosulfan is considered as an independent variable to predict patient prognosis. Patients who have ingested more than 35 g (referred to a human weighing 70 kg) must then be immediately treated. Treatment is symptomatic, and includes gastric lavage, avoiding aspiration into the lungs, followed by intragastric administration of activated charcoal and 30 g of magnesium or sodium sulfate in 30% solutions.71 Convulsions require i.v. administration of benzodiazepines. In cases of skin contamination, decontamination with soap and water should also be included in the treatment. Neurotoxicity Endosulfan is an acute neurotoxic compound in humans. The US Environmental Protection Agency classifies it as Category I (Highly Acutely Toxic) while the World Health Organization classifies it as Class II (Moderately Hazardous). Symptoms of acute poisoning include hyperactivity, tremors, convulsions, lack of coordination, staggering, difficulty breathing, nausea, vomiting, and diarrhea. Doses as low as 500 mg kg-1 have been documented to cause death in humans and, in many cases, led to permanent brain damage. Farm workers with chronic endosulfan exposure have shown rashes and skin irritation.72 Convulsions were reported in nine individuals exposed to the endosulfan-containing insecticide Thiodan® during bagging.73 Other effects noted in at least one of the subjects prior to the onset of convulsions, included malaise, nausea, vomiting, dizziness, confusion and weakness. Endosulfan can induce convulsions that could lead to brain damage at low doses.74 A case of long-term, possibly permanent brain damage in an industrial worker was attributed by Aleksandrowicz75 to endosulfan exposure. As for its neurotoxicological mechanism of action, endosulfan interferes with the function of γ-aminobutyric acid (GABA), the main inhibitory neurotransmitter.71 It inhibits the binding of [35S]-t-butylbicyclophosphorothionate (TBPS) to the picrotoxinin-binding site of the GABA receptor in rat brain synaptic membranes, interfering with chloride ion flux through the GABA-gated chloride channels (GABAA), responsible for decreasing neuronal excitability. Cardiotoxicity Cardiovascular effects were part of the clinical syndrome displayed by a man who attempted suicide by ingesting 200 mL of a 30% endosulfan formulation.76 Although the man's stomach contents were aspirated and he was given activated charcoal to limit absorption during the first 16 hours following ingestion, episodes of tachycardia and hypertension occurred, followed by cardiogenic shock. Similar observations have been described in other fatal cases of acute intoxication.67,77,78 Hepatotoxicity Elevated serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were reported in a woman 2 days after being admitted to hospital because of ingestion of endosulfan-contaminated food.68 The patient died 8 days after admission, following acute renal failure, disseminated intravascular coagulation, thrombi in the pulmonary arteries and aorta, and cardiogenic shock. Postmortem examination revealed dilation and congestion of hepatic sinusoids. Centrilobular congestion and slight prominence of the bile canaliculi were among postmortem observations in an additional fatal case of acute poisoning with endosulfan.78 The autopsy of a man who ingested approximately 260 mg kg-1 showed liver congestion 4 days after exposure.77 Nefrotoxicity Hemorrhage of the medullary layer of the kidneys was reported in three persons who died following ingestion of endosulfan.12 Acute renal failure was a major contributor to the deaths of two individuals who ingested unknown amounts of endosulfan.68,78 In both cases, postmortem examination revealed extensive tubular necrosis. In contrast, no kidney lesions were found in a man who died 4 days after ingesting approximately 260 mg kg-1 of endosulfan.77 Immunotoxicity The immune system is adversely affected by endosulfan due to white blood cell count decreases.79 Endosulfan also inhibits leucocytes and macrophage migration, causing adverse effects on humoral and cell-mediated immune system.80 Genotoxicity The genotoxic effect of endosulfan is questionable. DNA damage in mononuclear leukocytes, as measured with the alkaline comet assay, was significantly increased in two of four French agricultural workers on the day following the application of pesticide mixtures (including endosulfan), when compared to levels of DNA damage prior to application.81 However, the contribution of endosulfan to the observed effect is uncertain because of co-exposure to fungicides, herbicides, and other insecticides. Evaluations for micronuclei in human peripheral blood lymphocytes provided mixed results, depending on the analytical method used.82 The results of all genotoxicity studies in humans should be treated with caution because the multiple-chemical exposures confound the interpretation, and exposure levels of endosulfan were not reported.83-85 Carcinogenicity According to Antherieu et al.,86 endosulfan has not been classified by the International Agency for Research on Cancer (IARC) as a carcinogen, and was described by the International Programme on Chemical Safety (IPCS)87 as not carcinogenic. However, despite no accurate data related to the carcinogenicity of endosulfan in humans is available, there are concerns about possible carcinogenic properties in chronic exposures.88 Some studies have also shown that it induces proliferation of human breast estrogen sensitive MCF7 cells in vitro, which may lead to greater breast cancer risk.87-90 Studies also indicate the contribution of endosulfan in the combined effect of environmental estrogens in inducing breast cancer.88-93 Teratogenicity Physical malformations observed in fetus which fathers were exposed to endosulfan 8 hours per day, in spring and winter, during 1 - 20 years of work, include cleft palates, harelips, club feet, limb malformations, eye deformities and extra fingers and toes.94 Low concentrations of endosulfan around 0.1 nM have also shown to strongly inhibit the ability of human sperm to fertilise ova in vitro.95 According to Lemaire et al.96 endosulfan disrupts the retinoid signalling pathway in cells, and this is thought to explain the teratogenic effect of long-term exposure to low levels of the chemical, as has been experienced in India,94 as retinoids play an essential role in the proliferation, development and differentiation of cells.
ANIMAL TOXICITY Similar to the data from humans, respiratory effects have been observed in animals tested almost exclusively in acute, lethal-dose exposure. Male rats given single gavage doses of 200 mg kg-1 of endosulfan exhibited dyspnea and cyanosis prior to death.12 Necropsy revealed hemorrhages in the interalveolar partitions of the lung and acute emphysema of the lungs.97 Also studies in which a limited number of dogs were given single oral doses of endosulfan as low as 10 mg kg-1 or 50 mg kg-1, demonstrated respiratory paralysis and death. Autopsy of the dogs revealed congestion of the lungs. Local inflammation of the lungs and dilated alveoli were observed in rats administered 10 mg kg-1 day-1 of endosulfan in peanut oil by gavage for 15 days.98 Acute toxicity of endosulfan for different animals and administration routes is summarized in Table 2.
Neurotoxicity Sheep was reported to become blind one week after exposure to pasture previously sprayed with endolsulfan, although recovery was observed after one month.110 Similar exposure in lambs and pigs showed ataxia and inability to stand.111 Genotoxicity Lajmanovich et al.112 used the micronucleus test in erythrocytes of Hyla pulchella tadpoles as an experimental model for detecting genotoxic effects of endosulfan. The frequency of micronuclei was examined in blood smears obtained from tadpoles exposed in vivo to three different concentrations (2.5, 5, and 10 µg L-1) and fixed at two sampling times (48 and 96 h). As a positive control larvae were exposed to 40 mg L-1 of cyclophosphamide. Results obtained in this study demonstrated the genotoxic effects of endosulfan. Carcinogenicity As evidences on the carcinogenicity of endosulfan to animals are regarded as inconclusive, it has been reported that this chemical has no carcinogenic potential for animals.113 However, increases in several kinds of tumors in rats and mices, have already been reported.95,114,115 α-Endosulfan was also reported as a tumour promoter causing a significant and dose-related increase in hepatocytes,95,116 and to rapidly inhibit gap junctional intercellular communication (GJIC) in liver cells.95,117,118 Teratogenicity A relationship has been observed between maternal exposure and fetal malformations in the skull, ribs and spine of rats.100,119 Similar teratogenic effects, as well as accumulation of cerebrospinal fluid in the brain, underdeveloped cerebrum, incomplete ossification of skull bones, and malformations of the liver, kidneys, ribs and renal pelvis were observed.120 Reproductive toxicity Detailed studies in adult rats exposed to endosulfan for 5 days per week for 10 weeks showed reduced intratesticular spermatid counts, sperm abnormalities, and changes in the marker enzymes of testicular activities, providing further evidence of effects on spermatogenesis.121 A study by Dalsenter et al.122 indicates that pre and postnatal exposure to low doses of endosulfan (0.5 and 1.5 mg kg-1) does not induce significant adverse effects in the reproductive system of male off-spring Wistar rats at adulthood.
CONCLUSION The chemical, biochemical, environmental, and toxicological data of endosulfan reviewed in this paper show its toxicological potential risk to human health. In 2010, the Persistent Organic Pollutants Review Committee (POPRC) nominated endosulfan to be added to the Stockholm Convention in April 2011, which should finally result in a global ban.123 In Brazil the comercialization and use of endosulfan is already banned since July 31st 2013, according to the resolution RDC nº 28 of August 9th 2010.
ACKNOWLEDGEMENTS This work was supported by Long term development plan of University Hospital Hradec Kralove and Excelence project University of Hradec Kralove.
REFERENCES 1. Maier-Bode, H.; Residue Rev. 1968, 22, 1. PMID: 4868139 2. Naqvi, S. M.; Vaishnavi, C.; Comp. Biochem. Physiol., C: Comp. Pharmacol. 1993, 105, 347. DOI: http://dx.doi.org/10.1016/0742-8413(93)90071-R 3. Sharma, R. K.; Kaul, A.; Gupta, A.; Bhadauria, D.; Prasad, N.; Jain, A.; Gurjar, M.; Rao, B. P.; Indian J. Pharmacol. 2011, 43, 469. DOI: http://dx.doi.org/10.4103/0253-7613.83126 PMID: 21845009 4. Becker, L.; Scheringer, M.; Schenker, U.; Hungerbühler, K.; Environ. Pollut. (Oxford, U. K.) 2011, 159, 1737. 5. Brandt, V. A.; Moon, S.; Ehlers, J.; Metehner, M. M.; Struttmann, T.; Am. J. Ind. Med. 2001, 39, 643. DOI: http://dx.doi.org/10.1002/ajim.1064 PMID: 11385649 6. Demeter, J.; Heyndrickx, A.; J. Anal. Toxicol. 1978, 2, 68. DOI: http://dx.doi.org/10.1093/jat/2.2.68 7. Kucuker, H.; Sahin, O.; Yavuz, Y.; Yürümez, Y.; Basic Clin. Pharmacol. Toxicol. 2009, 104, 49. DOI: http://dx.doi.org/10.1111/j.1742-7843.2008.00216.x PMID: 19152551 8. Moon, J. M.; Chun, B. J.; Hum. Exp. Toxicol. 2009, 28, 309. DOI: http://dx.doi.org/10.1177/0960327109106488 PMID: 19755461 9. Moses, V.; Peter, J. V.; Clin. Toxicol. (Philadelphia, Pa.) 2010, 48, 539. DOI: http://dx.doi.org/10.3109/15563650.2010.494610 10. Parbhu, B,; Rodgers, G.; Sullivan, J. E.; Clin. Toxicol. (Philadelphia, Pa.) 2009, 47, 899. DOI: http://dx.doi.org/10.3109/15563650903328879 11. Satar, S.; Sebe, A.; Alpay, N. R.; Bratisl. Lek. Listy 2009, 110, 301. PMID: 19507667 12. Terziev, G.; Dimitrova, N.; Rusev, F.; Folia Med. (Plovdiv) 1974, 16, 325. PMID: 4219948 13. Wesseling, C.; Corriols, M.; Bravo, V.; Toxicol. Appl. Pharmacol. 2005, 207(2 Suppl), 697. DOI: http://dx.doi.org/10.1016/j.taap.2005.03.033 PMID: 16153991 14. Saiyed, H.; Dewan, A.; Bhatnagar, V.; Shenoy, U.; Shenoy, R.; Rajmohan, H.; Patel, K.; Kashyap, R.; Kulkarni, P.; Rajan, B.; Lakkad, B.; Environ. Health Perspect. 2003, 111, 1958. DOI: http://dx.doi.org/10.1289/ehp.6271 PMID: 14644673 15. Dewan, A.; Bhatnagar, V. K.; Mathur, M. L.; Chakma, T.; Kashyap, R.; Sadhu, H. G.; Sinha, S. N.; Saiyed, H. N.; J. Toxicol., Clin. Toxicol. 2004, 42, 363. DOI: http://dx.doi.org/10.1081/CLT-120039542 16. French, H.; Goebel, H.; US Pat. 2,799, 685, 1957. 17. http://chem.sis.nlm.nih.gov/chemidplus/rn/115-29-7 Accessed in March 25th 2016. 18. Schmidt, W. F.; Bilboulian, S.; Rice, C. P.; Fettinger, J. C.; McConnell, L. L.; Hapeman, C. J.; J. Agric. Food Chem. 2001, 49, 5372. DOI: http://dx.doi.org/10.1021/jf0102214 PMID: 11714330 19. Schmidt, W. F.; Hapeman, C. J.; Fettinger, J. C.; Rice, C. P.; J. Agric. Food Chem. 1997, 45, 1023. DOI: http://dx.doi.org/10.1021/jf970020t 20. Sutherland, T. D.; Horne, I.; Weir, K. M.; Russell, R. J.; Oakeshott, J. G.; Rev. Environ. Contam. Toxicol. 2004, 183, 99. PMID: 15369323 21. Keith, L.; Telliard, W.; Environ. Sci. Technol. 1979, 13, 416. DOI: http://dx.doi.org/10.1021/es60152a601 22. Verschueren, K.; Handbook of Environmental Data on Organic Chemicals, 2nd ed., Van Nostrand Reinhold Co.: New York, 1983. 23. Ernst, W. R.; Jonah, P.; Doe, K.; Julien, G.; Hennigar, P.; Environ. Toxicol. Chem. 1991, 10, 193. DOI: http://dx.doi.org/10.1002/etc.5620100112 24. Petri, D.; Glover, C. N.; Ylving, S.; Kolås, K.; Fremmersvik, G.; Waagbo, R.; Berntssen, M. H.; Aquat. Toxicol. 2006, 80, 207. DOI: http://dx.doi.org/10.1016/j.aquatox.2006.07.019 PMID: 17081631 25. Barcelo-Quintal, M. H.; Cebada-Ricalde, M. C.; Trejo-Irigoyen, A. R.; Rendon-Osorio, R. B.; Manzanilla-Cano, J. A.; J. Environ. Sci. Health, Part B 2008, 43, 120. DOI: http://dx.doi.org/10.1080/03601230701794992 26. Singh, S. K.; Pandey, R. S.; Indian J. Exp. Biol. 1990, 28, 953. PMID: 2279768 27. Sinha, N.; Narayan, R.; Saxena, D. K.; Bull. Environ. Contam. Toxicol. 1997, 58, 79. DOI: http://dx.doi.org/10.1007/s001289900303 PMID: 8952929 28. Sinha, N.; Adhikari, N.; Saxena, D. K.; Environ. Toxicol. Pharmacol. 2001, 10, 29. DOI: http://dx.doi.org/10.1016/S1382-6689(01)00066-7 PMID: 11382554 29. Turner, K. H.; Sharpe, R. M.; Rev. Reprod. 1997, 2, 69. DOI: http://dx.doi.org/10.1530/ror.0.0020069 PMID: 9414467 30. Chaudhuri, K.; Selvaraj, S.; Pal, A. K.; Mutat. Res. 1999, 439, 63. DOI: http://dx.doi.org/10.1016/S1383-5718(98)00174-0 PMID: 10029677 31. Paul, V.; Balasubramaniam, E.; Environ. Toxicol. Pharmacol. 1997, 3, 151. DOI: http://dx.doi.org/10.1016/S1382-6689(97)00009-4 PMID: 21781773 32. Walse, S. S.; Shimizu, K. D.; Ferry, J. L.; Environ. Sci. Technol. 2002, 36, 4846. DOI: http://dx.doi.org/10.1021/es0256257 PMID: 12487308 33. Lee, H. K.; Moon, J. K.; Chang, C. H.; Choi, H.; Park, H. W.; Park, B. S.; Lee, H. S.; Hwang, E. C.; Lee, Y. D.; Liu, K. H.; Kim, J. H.; Drug Metab. Dispos. 2006, 34, 1090. DOI: http://dx.doi.org/10.1124/dmd.105.009134 PMID: 16581944 34. Awasthi, N.; Ahuja, R.; Kumar, A.; Soil Biol. Biochem. 2000, 32, 1697. DOI: http://dx.doi.org/10.1016/S0038-0717(00)00087-0 35. Walse, S. S.; Scott, G. I.; Ferry, J. L.; J. Environ. Monit. 2003, 5, 373. DOI: http://dx.doi.org/10.1039/B212165D PMID: 12833978 36. Sutherland, T. D.; Horne, I.; Lacey, M. J.; Appl. Environ. Microbiol. 2000, 66, 2822. DOI: http://dx.doi.org/10.1128/AEM.66.7.2822-2828.2000 PMID: 10877774 37. Awasthi, N.; Manickam, N. N.; Kumar, A.; Bull. Environ. Contam. Toxicol. 1997, 59, 928. DOI: http://dx.doi.org/10.1007/s001289900571 PMID: 9400664 38. Martens, R. Appl. Environ. Microbiol. 1976, 31, 853. PMID: 7195 39. Miles, J. R. E.; Moy, P.; Bull. Environ. Contam. Toxicol. 1979, 23, 13. DOI: http://dx.doi.org/10.1007/BF01769908 PMID: 497418 40. Kshemkalyani, S. B.; Vasudevan, P.; Patki, A. H.; Naik, R. B.; Rahalkar, S. B.; Francis, R. P.; Patel, G. S.; Indian J. Environ. Health 1987, 29, 148. 41. Katayama, A.; Matsumura, F.; Environ. Toxicol. Chem. 1993, 12, 1059. DOI: http://dx.doi.org/10.1002/etc.5620120612 42. Kullman, S. W.; Matsumura, F.; Appl. Environ. Microbiol. 1996, 62, 593. 43. Beck, E. W.; Johnson, J. C.; Woodham, D. B.; Leuck, D. B.; Dawsey, L. H.; Robbins, J. E.; Bowman, M. C.; J. Econ. Entomol. 1966, 59, 1444. DOI: http://dx.doi.org/10.1093/jee/59.6.1444 PMID: 5976124 44. Dorough, H. W.; Huhtanen, K.; Marshall, T. C.; Bryant, H. E.; Pestic. Biochem. Physiol. 1978, 8, 241. DOI: http://dx.doi.org/10.1016/0048-3575(78)90022-6 45. Trajkovska, S.; Mbaye, M.; Gaye, S. M. D.; Aaron, J. J.; Chevreuil, M.; Blanchoud, H.; Anal. Bioanal. Chem. 2009, 394, 1099. DOI: http://dx.doi.org/10.1007/s00216-009-2783-z PMID: 19387620 46. Stoker, C.; Repetti, M. R.; García, S. R.; Zayas, M. A.; Galoppo, G. H.; Beldoménico, H. R.; Luque, E. H.; Muñoz-de-Toro, M.; Chemosphere 2011, 84, 311. DOI: http://dx.doi.org/10.1016/j.chemosphere.2011.04.013 PMID: 21531435 47. Wang, X.; Kennedy, K.; Powell, J.; Keywood, M.; Gillett, R.; Thai, P.; Bridgen, P.; Broomhall, S.; Paxman, C.; Wania, F.; Mueller, J. F.; Environ. Sci.: Process Impacts 2015, 17, 525. PMID: 25592874 48. Zhang, X.; Meyer, T.; Muir, D. C.; Teixeira, C.; Wang, X.; Wania, F.; Environ. Sci.: Process Impacts 2013, 15, 2304. PMID: 24158382 49. Potter, T. L.; Hapeman, C. J.; McConnell, L. L.; Harman-Fetcho, J. A.; Schmidt, W. F.; Rice, C. P.; Schaffer, B.; Sci. Total Environ. 2014, 468, 505. DOI: http://dx.doi.org/10.1016/j.scitotenv.2013.08.070 PMID: 24055666 50. Kong, L.; Zhu, S.; Zhu, L.; Xie, H.; Su, K.; Yan, T.; Wang, J.; Wang, J.; Wang, F.; Sun, F.; J. Environ. Sci. (China) 2013, 25, 2257. DOI: http://dx.doi.org/10.1016/S1001-0742(12)60288-5 PMID: 24552054 51. Varayoud, J.; Monje, L.; Bernhardt, T.; Muñoz-de-Toro, M.; Luque, E. H.; Ramos, J. G.; Reprod. Toxicol. 2008, 26, 138. DOI: http://dx.doi.org/10.1016/j.reprotox.2008.08.004 PMID: 18790044 52. Narayan, S.; Dani, H. M.; Misra, U. K.; J. Biochem. Toxicol. 1989, 4, 205. DOI: http://dx.doi.org/10.1002/jbt.2570040402 PMID: 2561290 53. Narayan, S.; Dani, H. M.; Misra, U. K.; J. Environ. Sci. Health, Part B 1990, 25, 243. DOI: http://dx.doi.org/10.1080/03601239009372688 54. Narayan, S.; Dani, H. M.; Misra, U. K.; J. Environ. Sci. Health, Part B 1990, 25 259. DOI: http://dx.doi.org/10.1080/03601239009372687 55. Rousseau, J.; Cossette, L.; Grenier, S.; Martinoli, M. G.; Gen. Comp. Endocrinol. 2002, 126, 175. DOI: http://dx.doi.org/10.1006/gcen.2002.7789 PMID: 12030773 56. Ayub, S.; Verma, J.; Das, N.; Int. Immunopharmacol. 2003, 3, 1819. DOI: http://dx.doi.org/10.1016/j.intimp.2003.08.006 PMID: 14636831 57. Pal, R.; Ahmed, T.; Kumar, V.; Suke, S. G.; Ray, A.; Banerjee, B. D.; Indian J. Exp. Biol. 2009, 47, 723. PMID: 19957884 58. Ahmed, T.; Tripathi, A. K.; Ahmed, R. S.; Das, S.; Suke, S. G.; Pathak, R.; Chakraboti, A.; Banerjee, B. D.; J. Biochem. Mol. Toxicol. 2008, 22, 299. DOI: http://dx.doi.org/10.1002/jbt.20240 PMID: 18972393 59. Choudhary, N.; Sharma, M.; Verma, P.; Joshi, S. C.; J. Environ. Biol. 2003, 24, 305. PMID: 15259607 60. Uboh, F. E.; Asuquo, E. N.; Eteng, M. U.; Toxicol. Ind. Health 2011, 27, 483. DOI: http://dx.doi.org/10.1177/0748233710387011 PMID: 21543461 61. Ozmen, O.; Sahinduran, S.; Mor, F.; Pancreas 2010, 39, 367. DOI: http://dx.doi.org/10.1097/MPA.0b013e3181bd95d6 PMID: 19959968 62. Ozdem, S.; Nacitarhan, C.; Gulay, M. S.; Hatipoglu, F. S.; Ozdem, S. S.; Toxicol. Ind. Health 2011, 27, 437. DOI: http://dx.doi.org/10.1177/0748233710388450 PMID: 21245203 63. Silva de Assis, H. C.; Nicaretta, L.; Marques, M. C.; Crestani, S.; Soares, K. C.; Olmedo, D.; Dalsenter, P. R. Bull. Environ. Contam. Toxicol. 2011, 86, 368. DOI: http://dx.doi.org/10.1007/s00128-011-0227-x PMID: 21340455 64. Conacher, H. B. S.; Mes, J.; Food Addit. Contam. 1993, 10, 5. DOI: http://dx.doi.org/10.1080/02652039309374125 PMID: 8504874 65. Silva, M. H.; Carr, W. C.; Regul. Toxicol. Pharmacol. 2010, 56, 18. DOI: http://dx.doi.org/10.1016/j.yrtph.2009.08.015 PMID: 19733202 66. Wang, Y.; Guo, S.; Xue, R.; Qi, S.; Xu, Y.; Xue, B.; Yuan, D.; Environ. Monit. Assess. 2011, 180, 489. DOI: http://dx.doi.org/10.1007/s10661-010-1801-0 PMID: 21107999 67. Pathak, R.; Suke, S. G.; Ahmed, R. S.; Tripathi, A. K.; Guleria, K.; Sharma, C. S.; Makhijani, S. D.; Mishra, M.; Banerjee, B. D.;. Bull. Environ. Contam. Toxicol. 2008, 81, 216. DOI: http://dx.doi.org/10.1007/s00128-008-9459-9 PMID: 18488129 68. Blanco-Coronado, J. L.; Repetto, M.; Ginestal, R. J.; Vicente, J. R.; Yelamos, F.; Lardelli, A.; Clin. Toxicol. 1992, 30, 575. 69. Karatas, A. D.; Aygun, D.; Baydin, A.; Singapore Med. J. 2006, 47, 1030. PMID: 17139397 70. Sood, A. K.; Yadav, S. P.; Sood, S.; Indian J. Med. Sci. 1994, 48, 68. PMID: 8045634 71. Spencer, P. S.; Schaumburg, H. H.; Chlorinated cyclodienes. In: Spencer, P. S., Scheumburg, H. H., Ludolph, A. C., eds.; Oxford University Press: Oxford, 2000. 72. Dalvie, M. A.; Africa, A.; Solomons, A.; London, L.; Brouwer, D.; Kromhout, H.; J. Environ. Sci. Health, Part B 2009, 44, 271. DOI: http://dx.doi.org/10.1080/03601230902728351 73. Ely, T. D.; MacFarlane, J. W.; Galen, W. P.; Hine, C. H.; J. Occup. Med. 1967, 9, 35. DOI: http://dx.doi.org/10.1097/00043764-196702000-00001 PMID: 5226634 74. Scremin, O. U.; Chialvo, D. R.; Lavarello, S.; Berra, H. H.; Lucero, M. A.; Neurotoxicology 2011, 32, 31. DOI: http://dx.doi.org/10.1016/j.neuro.2010.12.001 PMID: 21144862 75. Aleksandrowicz, D. R.; Arch. Toxicol. 1979, 43, 65. DOI: http://dx.doi.org/10.1007/BF00695875 PMID: 533363 76. Shemesh, Y.; Bourvine, A.; Gold, D.; Bracha, P.; J. Toxicol., Clin. Toxicol. 1988, 26, 265. DOI: http://dx.doi.org/10.3109/15563658809000353 77. Boereboom, F. T.; van Dijk, A.; van Zoonen, P.; Meulenbelt, J.; J. Toxicol., Clin. Toxicol. 1998, 36, 345. DOI: http://dx.doi.org/10.3109/15563659809028031 78. Lo, R. S. K.; Chan, J. C. N.; Cockram, C. S.; Lai, F. M. M.; Clin. Toxicol. 1995, 33, 67. 79. Galatone, V.; Annex E of the Stockholm Convention pursuant to Article 8 of the Convention, Stockolm, Sweden, 2009. 80. Sang S, Petrovic S. Endosulfan - A Review of its toxicity and its effects on the endocrine system, WWF: Canada, 1999. 81. Lebailly, P.; Vigreux, C.; Lechevrel, C.; Ledemeney, D.; Godard, T.; Sichel, F.; LeTalaër, J. Y.; Henry-Amar, M.; Gauduchon, P.; Cancer Epidemiol., Biomarkers Prev. 1998, 7, 929. 82. Venegas, W.; Zapata, I.; Carbonell, E.; Marcos, R.; Teratog., Carcinog., Mutagen. 1998, 18, 123. DOI: http://dx.doi.org/10.1002/(SICI)1520-6866(1998)18:3<123::AID-TCM3>3.3.CO;2-G 83. Falck, G. C. M.; Hirvonen, A.; Scarpato, R.; Saarikoski, S. T.; Migliore, L.; Norppa, H.; Mutat. Res., Fundam. Mol. Mech. Mutagen. 1999, 441, 225. 84. Scarpato, R; Landini, E.; Migliore, L.; Mutat. Res., Fundam. Mol. Mech. Mutagen. 1996, 372, 195. DOI: http://dx.doi.org/10.1016/S0027-5107(96)00139-X 85. Scarpato, R.; Migliore, L.; Angotzi, G.; Fedi, A.; Miligi, L.; Loprieno, N.; Mutat. Res., Genet. Toxicol. Environ. Mutagen. 1996, 367, 73. DOI: http://dx.doi.org/10.1016/0165-1218(95)00071-2 86. Antherieu, S.; Ledirac, N.; Luzy, A. P.; Lenormand, P.; Caron, J. C.; Rahmani, R.; J. Cell. Physiol. 2007, 213, 177. DOI: http://dx.doi.org/10.1002/jcp.21108 PMID: 17503468 87. IPCS. 2000. Poisons Information Monograph 576 Endosulfan. International Programme On Chemical Safety, World Health Organisation, Geneva. http://www.inchem.org/documents/pims/chemical/pim576.htm Accessed in Feb 14th 2016. 88. Soto, A. M.; Chung, K. L.; Sonnen, S. C.; Environ. Health Perspect. 1994, 102, 380. DOI: http://dx.doi.org/10.1289/ehp.94102380 PMID: 7925178 89. Preziosi, P.; Pure Appl. Chem. 1998, 70, 1617. DOI: http://dx.doi.org/10.1351/pac199870091617 90. Zhu, Z.; Edwards, R. J.; Boobis, A. R.; Toxicol. Lett. 2008, 181, 93. DOI: http://dx.doi.org/10.1016/j.toxlet.2008.07.006 PMID: 18675332 91. Li, X. R.; Zhang, S.; Safe, S.; J. Steroid Biochem. Mol. Biol. 2006, 98, 122. DOI: http://dx.doi.org/10.1016/j.jsbmb.2005.08.018 PMID: 16413991 92. Bonefeld-Jorgensen, E. C.; Grunfeld, H. T.; Gjermandsen, I. M.; Mol. Cell. Endocr. 2005, 244, 20. DOI: http://dx.doi.org/10.1016/j.mce.2005.01.013 93. Ibarluzea, J. J.; Fernandez, M. F.; Santa Marina, L.; Olea, S. M. F.; Rivas, A. M.; Aurrekoetxea, J. J.; Enposito, J.; Lorenzo, M.; Torne, P.; Villalobos, M.; Pedraza, V.; Sasco, A. J.; Olea, N.; Cancer, Causes Control, Pap. Symp. 2004, 15, 591. DOI: http://dx.doi.org/10.1023/B:CACO.0000036167.51236.86 94. Rupa, D.; Reddy, P.; Reddi, O.; Environ. Res. 1991, 55, 123. DOI: http://dx.doi.org/10.1016/S0013-9351(05)80168-9 PMID: 1868815 95. ATSDR. 2000; Toxicological Profile for Endosulfan, Agency of Toxic Substances and Disease Registry, Atlanta, USA. http://www.atsdr.cdc.gov/toxprofiles/tp41.html, Acessed in February 2016. 96. Lemaire, G.; Balaguer, P.; Michel, S.; Rahmani, R.; Toxicol. Appld. Pharmacol. 2005, 202, 38. DOI: http://dx.doi.org/10.1016/j.taap.2004.06.004 97. Gupta, P. K.; Chandra, S. V.; Bull. Environ. Contam. Toxicol. 1975, 14, 513. DOI: http://dx.doi.org/10.1007/BF01683364 PMID: 1203562 98. Gupta, P. K.; Chandra, S. V.; Bull. Environ. Contam. Toxicol. 1977, 18, 378. DOI: http://dx.doi.org/10.1007/BF01683436 PMID: 907863 99. Gupta, P. K.; Bull. Environ. Contam. Toxicol. 1976, 15, 708. DOI: http://dx.doi.org/10.1007/BF01685621 PMID: 938763 100. Gupta, P. K.; Murthy, R. C.; Chandra, S. V.; Toxicol. Lett. 1981, 7, 221. 101. Miller, L. L.; Gefell, D.; Avallone, A.; Llados, F.; Toxicological profile for endosulfan. U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry: Atlanta, 2000. 102. Izmerov, N. F.; Sanotsky, I. V.; Sidorov, K. K.; GKNT Moscow 1982, 72. 103. Melnikov, N. N.; Chemistry of Pesticides, 1st ed., Springer-Verlag: New York, 1971. 104. Agricultural Research Service, USDA Information Memorandum, 1966, 20, 9. 105. Dzwonkowska, A.; Hübner, H.; Arch. Toxicol. 1986, 58, 152. DOI: http://dx.doi.org/10.1007/BF00340974 PMID: 3964078 106. Truhaut, R.; Gak, J. C.; Graillot, C.; Eur. J. Toxicol. Environ. Hyg. 1974, 7, 159. 107. Perkow, W.; Wirksubstanzen der Pflanzenschutz und Schadlingsbekampfungsmittel, Verlag Paul Parey: Berlin, 1971-1976. 108. Schafer, E. W.; Toxicol. Appl. Pharmacol. 1972, 21, 315. DOI: http://dx.doi.org/10.1016/0041-008X(72)90151-2 PMID: 5027965 109. Grimmett, W. G.; Dzendolet, I.; Whyte, I.; J. Toxicol., Clin. Toxicol. 1996, 34, 447. DOI: http://dx.doi.org/10.3109/15563659609013817 110. Doman, I.; Magy. Allatorv. Lapja 1971, 26, 342. 111. Utklev, H. E.; Westbye, C.; Nor. Veterinaertidsskr. 1971, 83, 31. 112. Lajmanovich, R. C.; Cabagna, M.; Peltzer, P. M.; Stringhini, G. A.; Attademo, A. M.; Mutat. Res. 2005, 587, 67. DOI: http://dx.doi.org/10.1016/j.mrgentox.2005.08.001 PMID: 16150634 113. Hack, R.; Ebert, E.; Leist, K. H.; Food Chem. Toxicol. 1995, 33, 941. DOI: http://dx.doi.org/10.1016/0278-6915(95)00063-8 PMID: 7590542 114. Reuber, M. D.; Sci. Total Environ. 1981, 20, 23. DOI: http://dx.doi.org/10.1016/0048-9697(81)90034-6 PMID: 7280658 115. Ferdousi, Z.; Islam, M. S.; Khan, M. Z. H. J. Korean Soc. Appl. Biol. Chem. 2008, 51, 294. DOI: http://dx.doi.org/10.3839/jksabc.2008.051 116. Fransson-Steen, R.; Flodstrom, S.; Warngard, L.; Carcinogenesis 1992, 13, 2299. 117. Dubois, M,; Pfohl-Leszkowicz, A.; De Waziers, I.; Kremers, P.; Environ. Toxicol. Pharmacol. 1996, 1, 249. 118. Warngard, I.; Bager, Y.; Kato, Y.;, Kenne, K.; Ahlborg, U. G.; Arch. Toxicol. Suppl. 1996, 18, 149. PMID: 8678790 119. Singh, N.; Sharma, A.; Dwivedi, P.; Patil, R.; Kumar, M.; J. Appl. Toxicol. 2007, 27, 143. DOI: http://dx.doi.org/10.1002/jat.1185 PMID: 17186572 120. Singh, N. D.; Sharma, A. K.; Dwivedi, P.; Patil, R. D.; Kumar, M.; J. Appl. Toxicol. 2006, 27, 589. DOI: http://dx.doi.org/10.1002/jat.1242 PMID: 17429798 121. Khan, P. K.; Sinha, S. P.; Mutagenesis 1996, 11, 33. DOI: http://dx.doi.org/10.1093/mutage/11.1.33 PMID: 8671712 122. Dalsenter, P. R.; de Araújo, S. L.; de Assis, H. C.; Andrade, A. J.; Dallegrave, E.; Hum. Exp. Toxicol. 2003, 22, 171. 123. Lubick, N.; Science 2010, 328, 1416. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access