Artigo
| Fe3O4@SIO2-OSO3H nanocomposite as an efficient catalyst for the preparation of tricarboxamides |
|
Mohammad Ali Ghasemzadeh*
Department of Chemistry, Qom Branch, Islamic Azad University - Qom, I. R., Iran Recebido em 29/04/2016 *e-mail: Ghasemzadeh@qom-iau.ac.ir In this research a highly efficient one-pot preparation of tricarboxamide derivatives via five-component reactions of isocyanides, aldehydes Meldrum's acid and 2equiv. of amines have been developed in the presence of Fe3O4@SiO2-OSO3H nanocomposite. Nano-Fe3O4 encapsulated-silica particles bearing sulfonic acid was readily recovered using an external magnet and could be reused several times without significant loss of reactivity. The catalyst was fully characterized by VSM, FT-IR, SEM, XRD, EDX and TEM analysis. INTRODUCTION Recently, to decrease waste and atom economy in the using raw materials, the purpose of technology and science has been modified toward more environmentally friendly, reusable catalysts and sustainable resources.1 During the last decades, magnetic nanoparticles (MNPs) have been intensively investigated because of their broad applications such as ferrofluids, digital media recording, targeted drug delivery, magnetic hyperthermia, magnetic resonance imaging, etc.2,3 Magnetite Fe3O4 nanoparticles (Fe3O4 NPs) as a class of nano-sized materials have received great attention due to their wide range of usages in various fields such as medical applications, drug delivery, remediation, catalyst, industry.4-6 The salient and significant property of magnetite nanoparticles is simple and facile separation of these catalysts from the reaction mixture using an external magnet. Coating magnetite nanoparticles with an amorphous silica layer is a useful and important approach in the growth of magnetite nanoparticle in various fields.7 Silica shell in nanostructures is chemically inert and has high stability against aggregation. In addition, the existing of silanol parts on the surfaces can easily be functionalized via the appropriate surface modifications which causing to attach of different functionalities.8 Furthermore, silanol groups on the surfaces of nanoparticles cause to protect of the related core toward oxidation and also various groups including guanidine,9 proline,10 sulfamic acid11 and sulfonic acid12 can attach to the outer shell. In recent years, nano-Fe3O4 encapsulated-silica particles bearing sulfonic acid (Fe3O4@SiO2-OSO3H) nanocomposites were applied as an effective nanocatalyst in the synthesis of 1,8-dioxo-octahydroxanthene,13 indazolo[2,1-b]phthalazine-triones and pyrazolo[1,2-b]phthalazine-diones,14 functionalized pyrimido[4,5-b]quinolines and indeno fused pyrido[2,3-d]pyrimidines15 and 3,4-dihydropyrimidinones/thiones.16 The multi-component coupling reactions (MCRs) are emerging as a valuable versatile for affording diverse organic structures for potential applications in medicinal and pharmaceutical chemistry.17 MCRs often adapt to the goals of green chemistry connected to economy of reaction approaches as well as the many particular principles of favorable chemical transformations.18-20 Due to of their benefits such as facile performance, environmentally friendly, fast and atom economic, MCRs interested great attention related to combinatorial chemistry.21 Tricarboxamide derivatives are a class of nitrogen heterocyclic compounds which have significant and well-reported biological activities that include neuroprotective,22 anti-diabetic,23 anti-bacterial,24 anti-carcinogenic25 and anti-tumor activities.26 Therefore, the importance of these constitutions has prompted researchers to develop more efficient pathways for the synthesis of these heterocycles compounds due to their significant biological and pharmaceutical properties. In continuation of our attempts on using heterogeneous nanocatalysts in multi-component reactions,27-31 herein we describe an efficient synthesis of functionalized tricarboxamide derivatives via five-component condensation reactions of isocyanides, aldehydes, Meldrum's acid and amines in the presence of magnetic Fe3O4@SiO2-OSO3H nanocomposites as catalyst under solvent-free conditions.
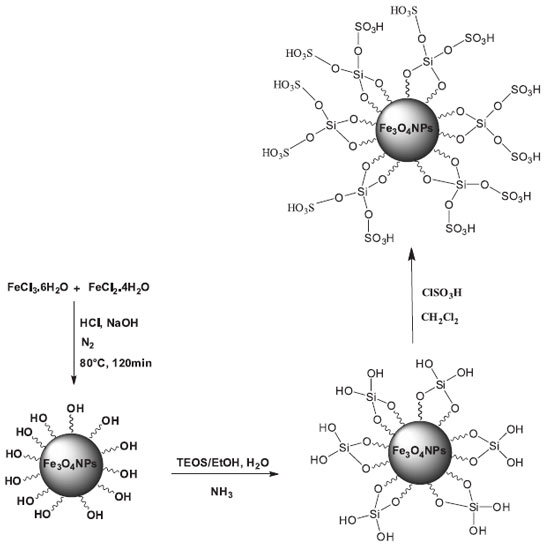
EXPERIMENTAL General Chemicals were purchased from the Sigma-Aldrich and Merck in high purity. All melting points are uncorrected and were determined in capillary tube on Boetius melting point microscope.1H NMR and 13C NMR spectra were obtained on Bruker 400 MHz spectrometer with DMSO-d6 as solvent using TMS as an internal standard. FT-IR spectrum was recorded on Magna-IR, spectrometer 550. The elemental analyses (C, H, N) were obtained from a Carlo ERBA Model EA 1108 analyzer. Powder X-ray diffraction (XRD) was carried out on a Philips diffractometer of X'pert Company with mono chromatized Cu Kα radiation (λ = 1.5406 Å). Microscopic morphology of products was visualized by SEM (LEO 1455VP). The mass spectra were recorded on a Joel D-30 instrument at an ionization potential of 70 eV. Transmission electron microscopy (TEM) was performed with a Jeol JEM-2100UHR, operated at 200 kV. Magnetic properties were obtained on a BHV-55 vibrating sample magnetometer (VSM) made by MDK-I.R.Iran. The compositional analysis was done by energy dispersive analysis of X-ray (EDX, Kevex, Delta Class I). Preparation of Fe3O4 nanoparticles Fe3O4 nanoparticles were prepared according to the procedure reported by Zhang et al.32 To a solution of FeCl2.4H2O (2.5 g) and FeCl3.6H2O (6 g) in 30 mL deionized water was added dropwise 1.0 mL of concentrated hydrochloric acid at room temperature. The solution was added in to 300 mL of 1.5 mol L-1 NaOH and then the solution was stirred vigorously at 80 ºC until precipitation. Afterwards, the prepared magnetic nanoparticles were separated magnetically, washed with deionized water and then dried at 70 ºC for 8 h. Preparation of Fe3O4@SiO2 nanocomposite The core-shell Fe3O4@SiO2 nanocomposite were prepared according to the previously reported method.33 Briefly 1g of Fe3O4 nanoparticles was treated with 0.5 mol L-1 HCl aqueous solution (25 mL) by sonication. After the treatment for 10 min, The magnetite particles were separated and washed with deionized water, and then homogeneously dispersed in the mixture of ethanol (60 mL), deionized water (100 mL) and concentrated ammonia aqueous solution (10 mL, 28 wt. %), followed by the addition of tetraethylorthosilicate (TEOS, 0.22 g, 0.144 mmol). After stirring at room temperature for 2 h, the Fe3O4@SiO2 nanocomposite were separated using an external magnet and washed with ethanol and water. Preparation of Fe3O4@SiO2-OSO3H nanocomposite Fe3O4@SiO2-SO3H MNPs were prepared according to a previously reported procedure by Kiasat and Davarpanah (Scheme 1).14 A suction flask equipped with a constant-pressure dropping funnel. The gas outlet was connected to a vacuum system through an adsorbing solution of alkali trap. Firstly, Fe3O4@SiO2 (1 g) was added to the flask and dispersed in dry CH2Cl2 (10 ml) by ultrasonic for 30 min. Subsequently, chlorosulfonic acid (1 ml) was added drop-wise to a cooled (ice-bath) solution of Fe3O4@SiO2 (1 g) over a period of 30 min at room temperature. After completion of the addition, the mixture was stirred for a further 6 h until to allow for the complete dissipation of HCl from the reaction vessel. The resulted MNPs were separated using an external magnet and washed with ethanol and water before been dried in an oven at 70 ºC to give Fe3O4@SiO2-OSO3H as a brown powder.
General procedure for the preparation of tricarboxamide derivatives A mixture of isocyanide (1 mmol), aromatic aldehyde (1 mmol), Meldrum's acid (1 mmol), aromatic amine (2 mmol) and Fe3O4@SiO2-OSO3H nanocomposite (0.003 g) were heated in a sealed tube at 50 ºC for appropriate times. Progress of the reaction was continuously monitored by TLC. After completion of the reaction, the mixture was cooled to room temperature and then was dissolved in methanol and then the nanocatalyst was separated using an external magnet. The solvent was evaporated under vacuum and the solid obtained recrystallized from EtOH to afford the pure tricarboxamides. All of the products were characterized and identified with m.p., 1H NMR, 13C NMR and FT-IR spectroscopy techniques. Spectral data of new compounds N2-cyclohexyl-2-(4-fluorophenyl)-N1,N'1-diphenylethane-1,1,2-tricarboxamide (5f): White solid; m.p 303-304 ºC; FT- IR (KBr): 3314 (NH), 3291 (NH), 1673 (CO), 1615 (C=C), 1462 (C=C) cm-1; 1H NMR (400 MHz, DMSO-d6) δ/ppm : 0.99-1.32 (m, 10 H, 5CH2), 3.27 (m, 1H, NCH), 4.01 (d, 1H, CH), 4.11 (d, 1H, CH), 6.91-7.63 (m, 14H, Ar), 8.41 (bs, 1H, NH), 10.12 (s, 2H, 2 NH); 13C NMR (100 MHz, DMSO-d6) δ/ppm : 173.1 (C=O), 169.4 (C=O), 149.1, 147.9, 144.6, 143.2, 131.3, 127.4, 121.2, 118.1, 64.3, 52.1, 48.1, 31.5, 30.4, 27.8. MS (EI) (m/z): 487 (M+); Anal. Calcd. mass fraction of elements, w/% for C29H30FN3O3 (Mr = 487.23) are: C 71.44; H 6.20; N 8.62; Found: C 71.32; H 6.26; N 8.69. 2-(4-cyanophenyl)-N2-cyclohexyl-N1,N'1-diphenylethane-1,1,2-tricarboxamide (5g): White solid; m.p 312-315 ºC; FT- IR (KBr): 3325 (NH), 3286 (NH), 2211 (CN), 1668 (CO), 1584 (C=C), 1453 (C=C) cm-1; 1H NMR (400 MHz, DMSO-d6) δ/ppm : 1.01-1.29 (m, 10 H, 5CH2), 3.31 (m, 1H, NCH), 4.03 (d, 1H, CH), 4.10 (d, 1H, CH), 6.95-7.42 (m, 14H, Ar), 8.38 (bs, 1H, NH), 10.09 (s, 2H, 2 NH); 13C NMR (100 MHz, DMSO-d6) δ/ppm : 172.8 (C=O), 168.1 (C=O), 149.2, 147.9, 145.1, 143.6, 131.3, 127.4, 120.8, 119.2, 116.5, 62.1, 51.9, 48.3, 30.8, 29.2, 27.6. MS (EI) (m/z): 494 (M+); Anal. Calcd. mass fraction of elements, w/% for C30H30N4O3 (Mr = 494.23) are: C 72.85; H 6.11; N 11.33; Found: C 72.97; H 6.16; N 11.19. N2-(tert-butyl)-2-(4-fluorophenyl)-N1,N'1-diphenylethane-1,1,2-tricarboxamide (5m): White solid; m.p 285-287 ºC; FT- IR (KBr): 3303 (NH), 3278 (NH), 1675 (CO), 1606 (C=C), 1458 (C=C) cm-1; 1H NMR (400 MHz, DMSO-d6) δ/ppm : 0.98-1.22 (s, 9 H, 3CH3), 3.87 (d, 1H, CH), 4.11 (d, 1H, CH), 7.07-7.64 (m, 14H, Ar), 8.27 (bs, 1H, NH), 10.23 (s, 2H, 2 NH); 13C NMR (100 MHz, DMSO-d6) δ/ppm : 176.2 (C=O), 172.5 (C=O), 151.2, 149.8, 144.1, 143.2, 131.1, 128.1, 120.7, 118.6, 64.1, 50.8, 42.9, 28.1. MS (EI) (m/z): 461 (M+); Anal. Calcd. mass fraction of elements, w/% for C27H28FN3O3 (Mr = 461.21) are: C 70.26; H 6.12; N 9.10; Found: C 70.42; H 5.98; N 9.03. N2-(tert-butyl)-2-(4-cyanophenyl)-N1,N'1-diphenylethane-1,1,2-tricarboxamide (5n): White solid; m.p 293-295 ºC; FT- IR (KBr): 3341 (NH), 3291 (NH), 2216 (CN), 1678 (CO), 1603 (C=C), 1483 (C=C) cm-1; 1H NMR (400 MHz, DMSO-d6) δ/ppm : 1.02-1.33 (m, 9 H, 3CH3), 3.91 (D, 1H, CH), 4.11 (d, 1H, CH), 6.98-7.53 (m, 14H, Ar), 8.22 (bs, 1H, NH), 10.14 (s, 2H, 2 NH); 13C NMR (100 MHz, DMSO-d6) δ/ppm : 171.1 (C=O), 168.4 (C=O), 148.8, 147.5, 145.1, 142.4, 129.1, 128.6, 123.7, 111.4, 115.1, 63.9, 51.1, 38.8, 28.2. MS (EI) (m/z): 468 (M+); Anal. Calcd. mass fraction of elements, w/% for C28H28N4O3 (Mr = 468.22) are: C 71.78; H 6.02; N 11.96; Found: C 71.67; H 5.92; N 12.14. Recycling and Reusing of the Catalyst After completion of the reaction, the obtained product was dissolved in methanol and then the nanocatalyst was separated by an external magnet, then the nanocatalyst were washed three to four times with methanol and ethyl acetate and then dried overnight in an oven at 50 ºC. To investigate lifetime and level of recoverability of the Fe3O4@SiO2-OSO3H MNPs, the model study was carried out several times using recycled magnetite nanocomposite. The summarized results of Table 1 show that the recovered catalyst could be used for five successive runs with a slightly decreased in activity.
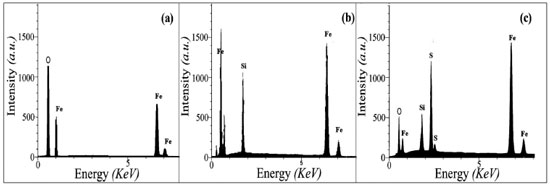
RESULTS AND DISCUSSION In the initial experiments Fe3O4@SiO2-SO3H nanocomposites was prepared and characterized by EDX, SEM XRD, FT-IR VSM and TEM analysis. The chemical purity of the samples as well as their stoichiometry was tested by EDX studies. The EDX spectrum given in Figure 1a shows the presence of Fe and O as the only elementary components of Fe3O4 NPs. EDX spectrum Fe3O4@SiO2 (Figure 1b) shows the elemental compositions are (Fe, O and Si) of core-shell nanocomposites. EDX spectrum of Fe3O4@SiO2-OSO3H (Figure 1c) shows the elemental compositions are (Fe, Si, O and S) of magnetic nanocomposites.
Scanning electron microscopy (SEM) is a useful tool for determining the size distribution, particles shape, and porosity. It has been a primary tool for characterizing the surface morphology and fundamental physical properties of the surface. According to Figure 2b, Fe3O4@SiO2 nanocomposites still keep the morphological properties of Fe3O4 (Figure 2a) except for a slightly larger particle size and smoother surface, while silica are uniformly coated on the Fe3O4 particles to form silica shell compared to the Fe3O4@SiO2, The SEM image shown in Figure 2c demonstrates that Fe3O4@SiO2-OSO3H nanocomposite are nearly spherical with about 30 nm in size.
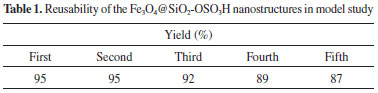
The structure of Fe3O4 (a), Fe3O4@SiO2 (b) and Fe3O4@SiO2 sulfonic acid (c) were analyzed by X-ray diffraction (XRD) spectroscopy (Figure 3). XRD diagram of the bare Fe3O4 NPs displayed patterns consistent with the patterns of spinel ferrites described in the literature (Figure 3).11 The same peaks were observed in the both of the Fe3O4@SiO2 and Fe3O4@SiO2 sulfonic acid XRD patterns, indicating retention of the crystalline spinel ferrite core structure during the silica-coating process. The average MNPs core diameter of Fe3O4, Fe3O4@SiO2 and Fe3O4@SiO2 sulfonic acid were calculated to be about 18, 25 and 32 nm, respectively from the XRD results by Scherrer's equation.
 Figure 3. XRD patterns of (a) Fe3O4, (b) Fe3O4@SiO2 and (c) Fe3O4@SiO2-OSO3H MNPS
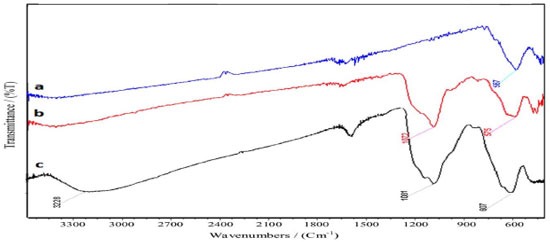
The FT-IR spectra of Fe3O4 nanoparticles, Fe3O4@SiO2, and Fe3O4@SiO2 sulfonic acid are shown in Figure 4. The FT-IR analysis of the Fe3O4@SiO2 and Fe3O4@SiO2 sulfonic acid exhibits two basic characteristic peaks at ~3300 cm-1 (O-H stretching) and 580 cm-1 (Fe-O vibration).34 The band at 1081 cm-1 comes from the Si-O-Si group. The presence of the sulfonyl group of Fe3O4@Silica sulfonic acid is confirmed by 1217 cm-1 and 1124 cm-1 bands, which were covered by a stronger absorption of Si-O bonds at 1081 cm-1.11 A wide bond at 2500-3409 cm-1 is due to the stretching of OH groups in the SO3H.
The magnetic properties of the uncoated magnetic iron oxide (Fe3O4), Fe3O4@SiO2, and Fe3O4@SiO2-SO3H were measured by vibrating sample magnetometer, VSM, at room temperature (Figure 5). In Figure 5, the hysteresis loops that are characteristic of superparamagnetic behavior can be clearly observed for all the nanoparticles. Superparamagnetism is the responsiveness to an applied magnetic field without retaining any magnetism after removal of the applied magnetic field. From M versus H curves, the saturation magnetization value (Ms) of uncoated Fe3O4 NPs was found to be 48.12 emu g-1. For Fe3O4@SiO2 and Fe3O4@SiO2-SO3H, the magnetization obtained at the same field were 38.16 and 35.82 emu g-1, respectively, lower than that of uncoated Fe3O4. These results indicated that the magnetization of Fe3O4 decreased considerably with the increase of SiO2 and SO3H. This is mainly attributed to the existence of nonmagnetic materials on the surface of the nanoparticles.
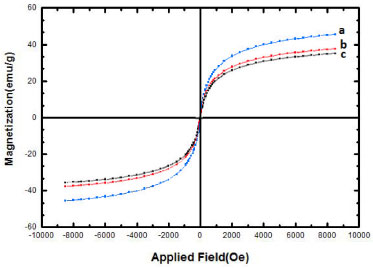 Figure 5. VSM magnetization curves of the (a) Fe3O4, (b) Fe3O4@SiO2 and (c) Fe3O4@SiO2-OSO3H MNPs
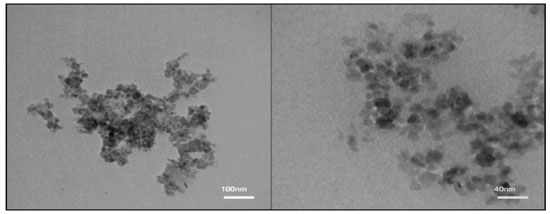
The size and morphology of Fe3O4@SiO2-SO3H nanocomposites were analyzed by Transmission electron microscopy (TEM) (Figure 6). The results show that these nanocatalysts consist of spherical particles with the crystallite size about 30 nm, confirming the results calculated from Scherrer's formula based on the XRD pattern.
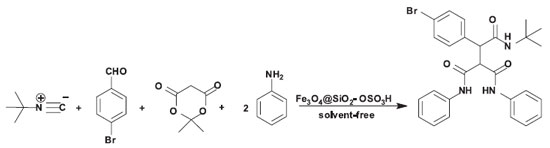
Initially, to optimize the reaction conditions, the reaction of 4-bromobenzaldehyde, Meldrum's acid, tert-butyl isocyanide and aniline was selected as a model reaction (Scheme 2).
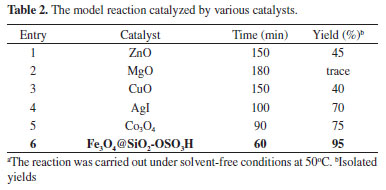
The reaction conditions were optimized on the basis of the solvent, catalyst, and different temperatures for synthesis of tricarboxamide (5a). To show the advantage of the current approach in comparison to other catalysts, the model reaction was carried out using various nanocatalysts such as ZnO, MgO, CuO, AgI, Co3O4 and Fe3O4@SiO2-SO3H. From the results provided in Table 2, it was clear that nano-Fe3O4 encapsulated-silica particles bearing sulfonic acid afforded the corresponding tricarboxamide (5a) in excellent yield and short reaction time (60 min in 95 % yield).
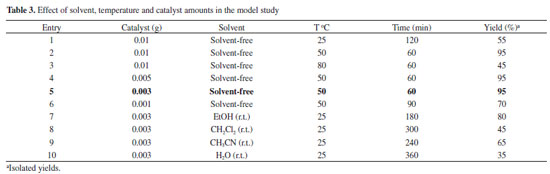
During the optimization of reaction conditions the influence of solvent was studied when the model reaction was carried out using Fe3O4@SiO2-OSO3H MNPs by different solvents and also solvent-free conditions. We found that the best result was obtained when the reaction was carried out under solvent-free conditions at 50 ºC. Increasing temperature showed unsatisfactory effect on the yield and reaction time (Table 3).
The reaction conditions were also investigated with different catalyst loading, revealing that 0.003 g of the catalyst provided the best results in terms of reaction time, economy of catalyst charge, and reaction yield (Table 3, entry 5). As shown in Table 3 increasing the amount of catalyst does not improve the yield of the product any further, whereas decreasing the amount of catalyst leads to decrease in the product yield. Hence, the optimum concentration of Fe3O4@SiO2-OSO3H was chosen 0.003 g in the model reaction.
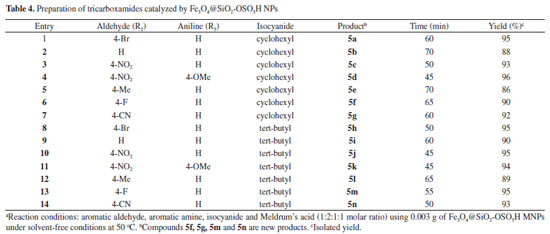
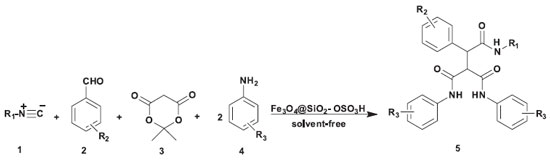
To study the scope and limitations of multi-component synthesis of tricarboxamide, we next utilized a diversity of aldehydes and amines to investigate five-component reactions under the optimized conditions (Scheme 3). As shown in Table 4, aldehydes with both electron-withdrawing and electron donating groups were effectively produced the corresponding products. In addition aryl amines with various substituents afforded tricarboxamide derivatives in excellent yields and short reaction times. Comparison between tert-butyl isocyanide and cyclohexyl isocyanide shows that multi-component reactions of isocyanides, aldehydes Meldrum's acid and amines was smoothly proceed using tert-butyl isocyanide.
CONCLUSIONS In summary, we have described a highly effective, mild and green approach for the preparation of tricarboxamide using Fe3O4@SiO2-OSO3H nanocomposites under solvent-free conditions at 50 ºC. The products were obtained in excellent yields and short reaction times via one-pot pseudo-five component reactions of isocyanides, aldehydes Meldrum's acid and 2 equiv. of amines. The salient properties of the applied nanocatalyst are simple work-up procedure, ease of separation, and recyclability of the magnetic nanocatalyst.
ACKNOWLEDGEMENTS This work is funded by the Research Affairs Office of the Islamic Azad University, Qom Branch, Qom, I. R. Iran [grant number 2014-13929].
REFERENCES 1. Zhang, Y.; Zhao, Y.; Xia, C.; J. Mol. Catal. A: Chem. 2009, 306, 107. 2. Raj, K.; Moskowitz, R.; J. Magn. Magn. Mater. 1990, 85, 233. 3. Pankhurst, Q. A.; Connolly, J.; Jones, S. K.; Dobson, J.; J. Phys. D: Appl. Phys. 2003, 36, R167. 4. Lu, A.-H.; Salabas, E. L.; Schuth, F.; Angew. Chem. Int. Ed. 2007, 46, 1222. 5. Perez, J. M.; Nat. Nanotechnol. 2007, 2, 535. 6. Sun, S. H.; Murray, C. B.; Folks, D. L.; Moser, A.; Science 2000, 287, 1989. 7. Kim, J.; Lee, J. E.; Lee, J.; Yu, J. H.; Kim, B. C.; An, K.; Hwang, Y.; Shin, C-H.; Park, Je-G.; Kim, J.; Hyeon, T.; J. Am. Chem. Soc. 2006, 128, 688. 8. Shen, H.; Chen, J.; Dai, H.; Wang, L.; Hu, M.; Xia, Q.; Ind. Eng. Chem. Res. 2013, 52, 12723. 9. Atashkar, B.; Rostami, A.; Tahmasbi, B.; Catal. Sci. Technol. 2013, 21, 44. 10. Yang, H.; Li, S.; Wang, X.; Zhang, F.; Zhong, X.; Dong, Z.; Ma, J.; J. Mol. Catal. A: Chem. 2012, 363- 364, 404. 11. Kassaee, M. Z.; Masrouri, H.; Movahedi, F.; Appl. Catal. A: Gen. 2011, 395, 28. 12. Nemati, F.; Heravi, M. M.; Saeedirad, R.; Chin. J. Catal. 2012, 33, 1825. 13. Naeimi, H.; Nazifi, Z. S.; J. Nanopart. Res. 2013, 15, 2026. 14. Kiasat, A. R.; Davarpanah, J.; J. Mol. Catal. A: Chem. 2013, 373, 46. 15. Nemati, F.; Saeedirad, R.; Chin. Chem. Lett. 2013, 24, 370. 16. Kiasat, A. R.; Davarpanah, J.; Res. Chem. Intermed. 2015, 41, 2991. 17. Ulaczyk-Lesanko, A.; Hall, D. G.; Curr. Opin .Chem.Biol. 2005, 9, 266. 18. Domling, A.; Ugi, I.; Angew. Chem. Int. Ed. 2000, 39, 3168. 19. Zhang, X. N.; Li, Y. X.; Zhang, Z. H.; Tetrahedron, 2011, 67, 7426. 20. Mohammadi Ziarani, G.; Moradi, R.; Lashgari, N.; Badiei, A.; Abolhasani Soorki, A.; Quim. Nova 2015, 38, 1167. 21. Schätz, A.; Reiser, O.; Stark, W. J.; Chem. Eur. J. 2010, 16, 8950. 22. Faden, A. I.; Movsesyan, V. A.; Knoblach, S. M.; Ahmed, F.; Cernak, I.; Neuropharmacology, 2005, 49, 410. 23. Tang, X.; Fan, L.; Yu, H.; Liao, Y.; Yang, D.; Chin. J. Org. Chem. 2009, 29, 595. 24. Strom, K.; Sjogren, J.; Broberg, A.; Schnurer, J.; Appl. Environ. Microbiol. 2002, 68, 4322. 25. Cui, C.; Kakeya, B. H.; Osada, H.; Tetrahedron 1997, 53, 59. 26. Wang, J. L.; Liu, D.; Zheng, Z. J.; Proc. Natl. Acad. Sci. U.S.A. 2000, 97, 7124. 27. Safaei-Ghomi, J.; Taheri, M.; Ghasemzadeh, M. A.; Org. Prep. Proced. Int. 2010, 42, 485. 28. Ghasemzadeh, M. A.; Safaei-Ghomi, J.; J. Chem. Res. 2014, 38, 313. 29. Ghasemzadeh, M. A.; Abdollahi-Basir, M. H.; Babaei, M.; Green Chem. Lett. Rev. 2015, 8, 40. 30. Ghasemzadeh, M. A.; Safaei-Ghomi, J.; Zahedi, S.; J. Serb. Chem. Soc. 2013, 78, 769. 31. Ghasemzadeh, M. A.; Safaei-Ghomi, J.; Acta Chim. Slov. 2015, 62, 103. 32. Lu, H. Y.; Yang, S. H.; Deng, J.; Zhang, Z. H.; Aust. J. Chem. 2010, 63, 1290. 33. Xu, X. Q., Deng, C. H., Gao, M. X., Yu, W. J., Yang, P. Y., Zhang, X. M., Adv. Mater. 2006, 18, 3289. 34. Lin, Y.; Chen, H.; Lin, K.; Chen, B.; Chiou, C.; J. Environ. Sci. 2011, 23, 44. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access