Artigo
| Binary systems of Brij® surfactants with Pluronic® F127 as griseofulvin carrier |
|
Ethanielda de L. Coelho1; Carolina L. de Moura1; Deyse de S. Maia1; Tamara G. de Araújo2; Francisco C. F. de França3; Nadja M. P. S. Ricardo1; Maria Elenir N. P. Ribeiro1,*; Nágila M. P. S. Ricardo1
1. Departamento de Química Orgânica e Inorgânica, Universidade Federal do Ceará, 60451-970 Fortaleza - CE, Brasil Recebido em 23/09/2016 *e-mail: elenir.ribeiro@ufc.br The binary mixtures of Brij® surfactant and Pluronic® F127 were studied as carriers of griseofulvin. The F127 systems and Brij® 78 (C18H37E20), Brij® 98 (C18H35E20) and Brij® 700 (C18H37E100) were prepared in the proportions 10/90, 30/70, 50/50 and 70/30, respectively. The characterizations were carried out by tube inversion, rheology, particle size, and theoretical cmcs. The griseofulvin solubilization tests were performed at 25 and 37 °C in micellar solutions of 1 wt. % and quantified by UV/Vis. According to the rheological data, the presence of F127 make the F127/Brij 78 70/30 and F127/Brij 98 70/30 mixtures thermoresponsive at concentrations of 30 and 35 wt.%, with transition fluid/gel in the range of 28-30 °C, ideal for topical use. All of the F127 / Brij 700 mixtures showed moderate distribution polydisperse (PDI < 0.4), as well as mixtures of F127 / Brij 98, with the exception of the mixture with ratio 30/70. Mixtures of F127 / Brij 78 had wide polydisperse distribution (PDI > 0.4). The optimal mixing for oral application would be of F127/Brij 78 because it exhibits greater solubilizing capacity (Scp) of griseofulvin at temperatures of 25 to 37 °C when compared to F127 alone. INTRODUCTION Studies show that theBrijs® surfactants have good characteristics assolubilizing agents for assisting in systems seeking to solubilize hydrophobic drugs. For example: niosomes (lamellar vesicles) were prepared from non-ionic Brijs® surfactants for transdermal drug delivery.1 In another study2 Brijs 78, 97 and 98 were used in the controlled release of Cyclosporin, a hydrophobic drug which acts as an immunosuppressant. Ribeiro et al.3 published results that reveal that the use the Brij 78 and Brij98 surfactants may increase up to 10 times, at 40 °C, the solubility of griseofulvin. These surfactants do not have thermoreversible characteristics, which would be optimal to assign the thermodynamic stability and gelling at specific temperatures. In order to achieve this, the thermoreversible Pluronic® F127 copolymer was added to the micellar system to obtain the synergistic effect. Pluronic® copolymers of ethylene oxide and propylene oxide, type EmPnEm, are available over a wide range of compositions and block lengths: we use the notation E = OCH2CH2 unit, P = OCH2CH(CH3) unit, and subscripts m and n to indicate number-average block lengths in repeat units. These copolymers are still used as drug carriers, for example, the Pluronic® F127 micellar systems have been utilized to enhance ocular delivery of the antibiotic ciprofloxacin.4In this study the investigated micellar formulations showed a significant improvement in ocular delivery of ciprofloxacin compared with that of commercial ciprofloxacin eye drops. The widespread use of Pluronic® F127 is mainly due to its thermoreversible property, either alone or in combination with other polymers or surfactants, and due to this property this copolymer has been proposed, in many studies, as an excipient of medications for topical use. Chavez et al.5 relates many uses of the F127 gels in pharmaceutical formulations, such as: good vehicle for topical and rectal administration of indomethacin, in prolonged anti-inflammatory and analgesic activities, useful for percutaneous delivery of fentanyl and as carriers for osseous graft materials. Surfactant mixtures were used in several studies to investigate the interaction in the aqueous medium or enhance their effects.6-10 Pinho et al.11 and Ribeiro et al.12 show studies that use the same blend of copolymers. The first one studied the gel properties and the last one the quercetin and rutin solubilization. The copolymer used in these studies contains ethylene oxide units and styrene oxide, E137S18E137, and E62P39E62 copolymer (Pluronic® F87). The results show that the system with proportion 80/20 has thermoresponsive characteristic, ideal for topical application, and larger values of the Scp than the system containing only the less hydrophilic copolymer block, for the above mentioned drugs, respectively. The aim of this work is to study the polymeric micellar systems formed by binary Pluronic® F127 (E98P67E98) and surfactants Brij®78 (C18H37E20), Brij® 98 (C18H35E20) and Brij® 700 (C18H37E100) by applying their mixtures in the solubilization of the griseofulvin drug (Figure 1) and the characterization of thermoreversible gels, targeting improvements in oral and topical application of the formulation. Griseofulvin is an antifungal antibiotic, which is poorly water-soluble and is a suitable standard for testing micellar hosts for solubilization/carrier of a range of other hydrophobic drugs.3 Zhong et al. reports that this drug could inhibit the growth of cancerous cells K562 in a dose dependent manner with a mean (SD) inhibitory concentration of 50%.13
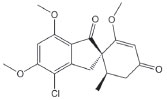 Figure 1. Chemical structure of griseofulvin
EXPERIMENTAL Materials Griseofulvin (molecular weight 352.8 g mol-1) was obtained from Sigma-Aldrich (Poole, Dorset, UK) and was used in the form of a finely ground (1 mm2 mesh) powder. The differential scanning calorimetry indicated a crystalline form with a melting point of 220.4 °C and an enthalpy of fusion of 115.6 J g−1. There was no detectable transition consistent with a glassy component. Brij® 78, Brij® 98 and Brij® 700 samples were obtained from Sigma-Aldrich (Poole, Dorset, UK) and were used as received. The ratio of weight-average to number average molar massvalues, Mw/Mn, was determined by gel permeation chromatography (GPC) in the method previously described.3 Pluronic® F127 Triblock copolymer, a product of BASF Corp. purchased from Sigma, was used. The number-average molar mass supplied with the sample was 12,500 g mol-1. The weight to number average molar mass ratio, Mw/Mn = 1.20 was determined by gel permeation chromatography in a method previously described.14 Rheometry Samples were prepared in small vials and stored for at least 1 week at 5 °C to reach equilibrium. A strain-controlled ARES rheometer (TA Instruments) with cone-and-plate geometry (diameter 50 mm, angle 0.04 rad) and with Peltier control of plate temperature (±0.1 °C) was used in oscillatory-shear mode at frequency of 1 Hz to determine elastic (G') and loss (G'') moduli as the samples were heated from 5 °C at 1 °C min-1. The strain amplitude was held at a low value (A = 0.5%), thus ensuring that measurements of G' and G'' were in the linear viscoelastic region. A solvent trap maintained a solvent-saturated atmosphere around the cell, but evaporation of solvent from the edge of the cone-and-plate cell limited measurements to temperatures larger than 80-85 °C. The temperature at which G' increased was sharp enough to overcome the values G'' (loss modulus or viscous) featured at the top of the gel training to the system being studied. Each experiment was repeated three times. Gel boundary by tube inversion Onset temperatures of mobile-immobile boundaries were measured to ±1 °C by enclosing samples of aqueous solutions (0.5 g) in small tubes and observing while heating them slowly (0.1 °C min-1) in a water bath through the temperature range 5-90 °C. Gelation was recognized by immobility of the solution when the tube was inverted at intervals of 1 °C. Solubilization of griseofulvin In the solubilization experiment, a 10 cm3 portion of a stock solution of 1 wt. % copolymer or binary mixture solution was added to finely-ground (1 mm2 mesh) griseofulvin powder (0.02 g). The system was stirred at 25 °C for 72 h before being filtered (0.45 µm Millipore) to remove any non-solubilized drug. The sample was then diluted with methanol to enable analysis by UV/Vis spectroscopy. The water content after dilution was low enough to allow the calibration for methanol solutions to be used without correction. After dilution, the samples were held at 37 °C for 72 h before analysis and three measurements were carried out and the results averaged. A UV/Vis spectrometer (Hitachi U-2000) was calibrated by recording the absorbance (wavelength range 200-350 nm) of methanol solutions of griseofulvin (2-20 mg L−1) against a blank solvent. The wavelength with the highest absorbance value was 292 nm. Micelle size The hydrodynamic radii of the surfactant mixtures micelles were determined using a Nano Zetasizer, Malvern, Zetasizer Nano ZS (ZEN 90). Measurements were made using copolymer recovered from the solubilization studies by freeze-drying and redissolved in Milli-Q water. Solutions of 1wt.% copolymer and their mixtures at 25 °C were investigated using 30 scans with 60 s acquisition time allowed for each scan.
RESULTS AND DISCUSSION Gel characterisation Figure 2 illustrates the temperature dependence of the dynamic modulus measured at a frequency of 1 Hz. These results were used to confirm the hard gel boundary and to locate a region of mobile fluid with a raised modulus and all solutions are characterized by rigid gels at certain temperatures. The mixed micellar systems are thermoreversible due to the presence of Pluronic® F127 at the micelles. This phenomenon of gelation (liquid-to-gel transition) is to be expected since there is a rise in the temperature, which according to Bohorquez et al.15 this is associated with a decrease in the critical micelle concentration and an increase in the number of aggregation, whereas Wanka et al.16 justified this phenomenon due to the dehydration of the groups of polyoxide of propylene in the core micelle.
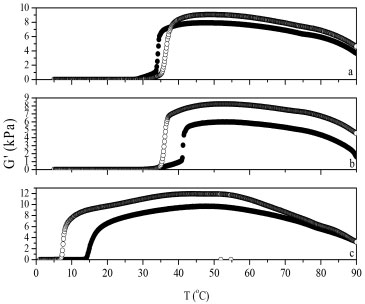 Figure 2. Temperature dependence of the elastic modulus (f=1, A= 0.5%) for aqueous solutions of copolymer F127 and surfactants Brij 78, 98 and 700 in 70/30, represented respectively by a, b and c, in the concentrations of 30 and 35 wt.% indicated by closed and open circle, respectively
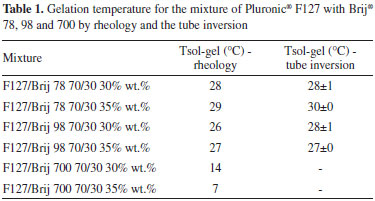
The temperature of fluid/gel transition for F127/Brij 78 70/ 30 and F127/Brij 98 70/30 systems at 30 wt. % occurs at 28 and 29 °C and 35 wt. % at 26 and 27 °C, respectively. This behaviour is important when the goal is to use gels for topical applications. At room temperature the solution has the characteristics of a fluid and of a gel at body temperature, however, for F127/Brij700 systems at the same concentrations, the fluid/gel transition is very low, at 14 and 7 °C respectively, and not suitable for topical applications as can be seen in Figure 2. Sharma et al.17 showed that Pluronic® F127 copolymer forms gels at 30 wt.% at temperatures below 13 °C. By comparing these results, it can be concluded that the addition of surfactants Brijs 78 and 98 increases the fluid-gel transition temperature, due to the increase of the hydrophobic block portion present in these surfactants, causing saturation on the micellar core, decreasing the cmc and delaying the micelle organization. For the mixture containing Brij 700, this behaviour was blocked by the broad hydrophilic portion of the surfactant containing 100 ethylene oxide units, which increased the hydration of the micelle corona and decreased the aggregation number. A tube inversion experiment was performed, on the systems containing Brij 78 and 98, at concentrations of 30 and 35 wt.% to confirm the temperature of fluid/gel transition. The temperature of the fluid/gel transition for the mixtures containing Brij 78 30/35 wt. % and Brij 98 30/35 wt. % occur at 28 and 30 °C and at 28 and 27 °C respectively. The results of rheology and the tube inversion experiments are listed in Table 1. The experimental error between rheology results and the tube inversion was of ±2 °C.
Micelle size and cmc theoretical Figure 3 shows the variation of the average hydrodynamic diameter depending on the percentage of F127 in aqueous solutions of 1 wt. %. The hydrodynamic diameters of F127/Brij binary mixtures are larger than the hydrodynamic diameter of the isolated Brijs®.3 Therefore, although there is an increase of the radii of the micellar mixtures, this is accompanied by an increase of F127 concentration, promoting a decrease in the solubility of griseofulvin, which can be seen in the solubilization of griseofulvin section.
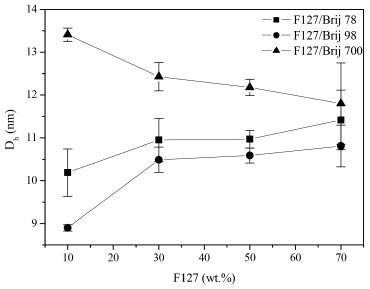 Figure 3. Plot of F127 concentration versus Hydrodynamic Diameter at 25 °C for F127/Brij 78, F127/Brij 98 and F127/Brij 700 mixtures
The difference of Dh for the systems with and without encapsulated drugs is smaller than 1 nm, as seen by Wei et al.18 Therefore, micelles of binary mixtures of Pluronics® form particles smaller than 200 nm, which is a great advantage for use in pharmacological applications. The larger radius found for solutions containing Brij® 700 micelles is consistent with the fact that their longest polyoxyethylene block contributes to a thicker micelle corona.3 When there is only 30% of Brij in the binary mixture, this difference is not clear due to the low concentration of Brij in the nanoparticles. The mixtures F127/Brij 700 show an opposite behaviour of F127/Brij 78 and F127/Brij 98 mixtures as the F127 concentration increases (Figure 3). In the F127/Brij 700 mixtures, the hydrodynamic diameter of particles decreases by increasing concentration of F127, as in the systems formed by mixed micelles obtained of the mixture F127/ SDS in the study proposed by Mondal et al.19 Pellosi et al.20 observed in their studies of pluronic mixed micelles that, in mixtures containing F127/P123 there was an inverse behaviour: the hydrodynamic diameter of particles increases when the concentration of F127 decreases. The first behaviour can be explained by considering that the sizes of the hydrophilic parts of the F127 are much higher than those of the Brij 78 and Brij 98. There are less attractive interactions between the hydrophilic parts of F127 and Brij 78 and Brij 98 respectively when the concentration of F127 increases. In other words, what dominates are the repulsive forces between the hydrophilic parts in the corona due to the higher degree of hydration under the same conditions which leads to an increase in size when the concentration of F127 increases. The second behaviour can be explained considering that both sizes of the hydrophilic blocks of F127 and Brij 700 are relatively large, resulting in greater attractive interaction between the hydrophilic parts of F127 and Brij 700 as the F127 concentration increases which cause the repulsive forces of these hydrophilic groups to decrease in the corona, due to the lower degree of hydration, which causes the contraction of the size when the F127 concentration increases. The polydispersity value is dimensionless and represents the distribution of particle size. If the value is closer to zero, the particle size population will be more homogeneous. Otherwise, if closer to 1 (one) the more heterogeneous will be the particle size. The F127/ Brij 78 mixtures with and without griseofulvin in all concentrations showed widely polydisperse distribution with PDI> 0.4 and intermediate hydrodynamic diameters such as observed by Basalious et al.7 in their studies of pluronics mixed micelles whose PDI of all formulations showed a wide range of values from 0.35 to 0.79. The mixtures F127/ Brij 98 10/90, 50/50 and 70/30 without griseofulvin exhibited moderately polydisperse distribution with PDI between 0.1 - 0.4 and smaller hydrodynamic diameters. However, the F127/Brij 98 30/70 mixtures without griseofulvin also presented a type of widely polydisperse distribution (PDI = 0.452). The mixtures F127/ Brij 700 without griseofulvin in all concentrations also exhibited a type of moderately polydisperse distribution and greater hydrodynamic radii. The same was observed for the F127/ Brij 700 30/70, 50/50 and 70/30 mixtures with griseofulvin, while the F127/Brij 700 10/90 mixture with griseofulvin revealed a type of strictly monodisperse distribution with PDI 0.075 (Figure 4). The F127/Brij 98 mixtures at all concentrations without griseofulvin showed lower hydrodynamic diameters because the hydrophobic block of Brij 98 has a double bond which generates a curve in the chain of this hydrophobic block. The mixtures of F127/ Brij 78 at all concentrations without griseofulvin showed intermediate hydrodynamic diameters because the molecules of Brij 78 have hydrophobic block of the same size of the hydrophobic block of Brij 98 without the double bond, hence, their chains are more extended and therefore, with values of hydrodynamic diameters slightly larger than F127/ Brij 98 mixtures. These are intermediate values because they are smaller than the values of F127/ Brij 700 mixtures that present in all concentrations without griseofulvin larger hydrodynamic diameters and the hydrophilic block of Brij 700 is greater than the Brij 78 and Brij 98, respectively.
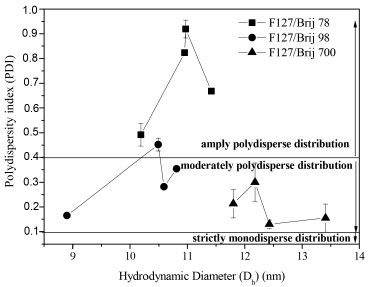 Figure 4. Plot of Hydrodynamic Diameter versus Polydispersity Index for F127/Brij 78, F127/Brij 98 and F127/Brij 700 mixtures
From the molecular-thermodynamic theory it is possible to predict the cmc of a mixture of surfactants A and B. For this, the following expression is used to relate the cmc of a binary mixture of surfactants (cmcmix) using the cmc of the pure surfactants (cmcA and cmcB).21 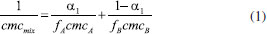 A represents copolymer F127 and B the Brij® surfactants, α1 is mass fraction of copolymer F127, fA and fBis the activity coefficients of the surfactants. It was considered fa and fA= fB=1 because the solutions are very dilute, 1 wt.%. The critical micelle concentration of F127 and Brij 78, 98 and 700 and their mixtures were calculated and plotted in Figures 5 and 1S at 25 and 37 °C respectively. The cmc values of Brij 78, 98 and 700 and F127 were obtained from another research that used fluorescence spectrophotometry and UV/Vis spectrophotometry methods.3,14
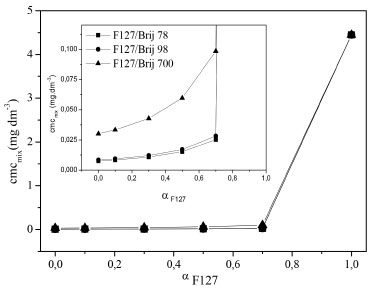 Figure 5. Plots of mass fraction of F127 versus theoretical critical micelle concentration of F127/Brij 78, F127/Brij 98 and F127/Brij 700 mixtures at 25 °C
It can be seen in Figures 5 and 1S that the cmc of the mixtures of Brij and F127 are much lower than the cmc of the pure copolymer F127. This is expected and reported by Oliveira et al.22 This behaviour is positive because previous studies report that low cmc values are important in the solubilization of drugs, because it increases the stability of their solutions in micelles after its dilution in blood.23-26 Solubilization of griseofulvin One alternative is to alter the composition of the micelle core seeking the increased solubility and therefore its action in the human body. The solubilization in binary micellar systems has frequently been used with the objective of achieving the synergistic effect.27-29 In these studies the synergistic effect was positive because it increased the solubility of hydrophobic drugs and assigned some systems thermoreversible characteristics. The solubilizing capacity values (Scp) of copolymer and surfactant solutions with griseofulvin was obtained by UV/Visible. The results are expressed in milligrams of solubilized drug per gram of copolymer and surfactant in solution (Scp/ mg g-1). The values of the solubility in water of the drug were subtracted from S because Scp=S - S0. The values of S0 for griseofulvin were 1.34 and 1.83 mg dl-1 to 25 and 37 °C respectively, similar to Ribeiro et al.3 The values of Scp obtained for Brij 78, Brij 98, Brij 700 and F127 and their mixtures, F127/Brijs (78, 98 and 700) 10/90, 30/70, 50/50 and 70/30, are shown in Figures 6 and 7.
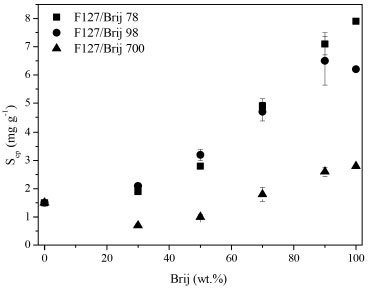 Figure 6. Solubility capacity (Scp) variation on the basis of the percentage of Brij in the mixture at 25 °C
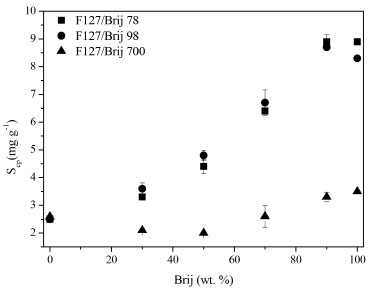 Figure 7. Solubility capacity (Scp) variation on the basis of the percentage of Brij® in the mixture at 37 °C
By analysing Figures 6 and 7, it can be seen that the temperature increases and the values in Scp increase as well. This behaviour is observed in studies using copolymers with poly(oxyethylene) as the hydrophilic block. This is because the rise in temperature reduces the solubility of poly (oxyethylene) in water and so increases the extent of micellization of its copolymers.3,12,30 Another observation is that mixtures containing Brij 700 have a lower solubilization capacity than the blends containing Brij 78 and Brij 98 by factors in the range of 2-3. This is because the molecules of Brij 700 have 80 more polyoxyethylene units: possibly the present effect arises from improved shielding of the micelle core by the longer polyoxyethylene blocks.3 Studies related to the solubilization the griseofulvin, rutin and quercetin in binary copolymers mixtures show that the best mixture is that of the maximum proportion of the hydrophobic copolymer, particularly when such copolymers have blocks of different hydrophobicities (F87 and E137S18E137).10 The addition of Brij® surfactants in the mixtures resulted in an increase in solubility of the hydrophobic griseofulvin drug. In another publication using the same method of this study,14 the value of Scp obtained for the F127 was low: 1.5 mg g-1 at 25 °C, whereas under the same conditions, the mixtures in our research, the Scp values reached a maximum of 7.1 mg g-1 (F127/Brij 78 10/90) at 25 °C and at 37 °C this value is 8.9 mg g-1 (F127/Brij 78 10/90).
CONCLUSIONS The binary mixtures of Pluronic® F127 and Brij® surfactant showed the characteristic of the formation of thermoresponsive gel observed in F127, especially in mixtures F127/Brij 78 70/30 and F127/Brij 98 70/30 in 30 and 35% concentration at temperatures near body temperature (26-30 °C) as shown in the data obtained by tube inversion and rheology, allowing such systems to have fluid characteristics at room temperature (≈ 25 °C) and a hard gel characteristic at body temperature (≈ 37 °C). The mixtures have low cmc values for the isolated polymers and consequently to their binary mixtures, as shown in the theoretical calculation. In the particle size studies, the binary systems showed moderate and full distribution in dilute aqueous solutions with a particle size smaller than 20 nm, an advantage for pharmacological applications. The above-mentioned mixtures have the potential for intravenous/oral (for aqueous formulation) and topical/subcultaneous (for gels) hydrophobic drug delivery, as demonstrated by the tests performed on diluted and concentrated aqueous solutions, with and without the antifungal drug griseofulvin as the model drug, respectively.
SUPPLEMENTARY MATERIAL Some images of the systems used in this study are available in http://quimicanova.sbq.org.br in the form of PDF files with free access.
ACKNOWLEDGEMENTS N.M.P.S.R. acknowledges for the support from MCTI/CNPq/Universal 456725/2014-5 (Grant number: 304392/2013-8), INCT-NanoBioSimes and Central Analítica UFC/SisNANO and CAPES (M.E.N.P.R.). The authors thank to Dr. R.S. Araújo for micelle size measurements carried out at Chemistry Laboratory of Chemistry Technology (LTC), IFCE - Campus Fortaleza, Fortaleza - CE, Brazil.
REFERENCES 1. Aldén, M.; Tegenfeldt, J.; Sjokvist, E.; Int. J. Pharm. 1992, 83, 47. 2. Medarevíc. D.; Kachrimanis, K.; Djuríc, Z.; Ibríc, S.; Eur. J. Pharm. Sci. 2015, 78, 273. 3. Ribeiro, M. E. N. P.; Moura, C. L.; Vieira, M. G. S.; Gramosa, N. V.; Chaibundit, C.; Mattos, M. C.; Attwood, D.; Yeates, S. G.; Nixon, S. K.; Ricardo, N. M. P. S.; Int. J. Pharm. 2012, 436, 631. 4. Pawar, J. N.; Shete, R. T.; Moravkar, G. K. K. M.; Javeer, S. D.; Jaiswar, D. R.; Amin, P. D.; Asian J. Pharm. Sci. 2016, 11, 385. 5. Vieira, A. C. C.; Fontes, D. A. F.; Chaves, L. L.; Alves, L. D. S.; Neto, J. L. F.; Soares, M. F. R.; Sobrinho, J. L. S.; Rolim, L. A.; Neto, P. J. R.; Carbohydr. Polym. 2015, 130, 133. 6. Tallury, P.; Randall, M.K.; Thaw, K.L.; Preisser, J. S.; Kalachandra, S.; Dent. Mater. 2007, 23, 977. 7. Basalious, E. B.; Shamma, R. N.; Int. J. Pharm. 2015, 493, 347. 8. Rangabhatla, A. S.; Tantishaiyakul, V.; Oungbho, K.; Boonrat, O.; Int. J. Pharm. 2016, 499, 110. 9. Daí, W.G.; Dong, L.C.; Li, S.; Deng, Z.; Int. J. Pharm. 2008, 31, 355. 10. Escobar-Chavéz, J. J.; Lópaz-Cervantes, M.; Naik, A.; Kalia, Y. N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A.; J. Pharm. Pharm. Sci. 2006, 9, 339. 11. Pinho, M. E. N.; Costa, F. de M. L. L.; Filho, F. B. S.; Ricardo, N. M. P. S.; Yeates, S. G.; Attwood, D.; Booth, C.; Int. J. Pharm. 2007, 328, 95. 12. Ribeiro, M. E. N. P.; Vieira, I. G. P.; Cavalvante, I. M.; Ricardo, N. M. P. S.; Attwood, D.; Yeates, S. G.; Booth, C.; Int. J. Pharm. 2009, 378, 211. 13. Zhong, N.; Chen, H.; Zhao, Q.; Wang, H.; Yu, X.; Eaves, A. M.; Sheng, W.; Miao, J.; Cui, F.; Wang, J.; Curr. Ther. Res. 2010, 71, 384. 14. Dutra, L. M. U.; Ribeiro, M. E. N. P.; Cavalcante, I. M.; Brito, D. H. A.; Semiao, L. M.; Silva, R. F.; Fechine, P. B. A.; Yeates, S. G.; Ricardo, N. M. P. S.; Polim.: Cienc. Tecnol. 2015, 25, 433. 15. Bohorquez, M.; Koch, C.; Trygstad, T.; Pandit, N.; J. Colloid Interface Sci. 1999, 216, 34. 16. Wanka, G.; Hoffmann, H.; Ulbricht, W.; Macromolecules 1994, 27, 4145. 17. Sharma, P. K.; Bhatia, S. R.; Int. J. Pharm. 2004, 278, 361. 18. Wei, Z.; Hao, J.; Yuan, S.; Li, Y.; Juan, W.; Sha, X.; Fang, X.; Int. J. Pharm. 2009, 376, 176. 19. Mondal, R.; Ghosh, N.; Mukherjee, S.; J. Phys. Chem. 2016, 120, 2968. 20. Pellosi, D. S.; Tessaro, A. L.; Moret, F.; Gaio, E.; Reddi, E.; Caetano, W.; Quaglia, F.; Hioka, N.; J. Photochem. Photobiol. 2016, 314,143. 21. Puvvada, S.; Blankschtein, D.; J. Phys. Chem. 1992, 96, 5567. 22. Oliveira, S. A.; Moura, C. L.; Cavalcante, I. M.; Lopes, A. A.; Leal, L. K. A. M.; Gramosa, N. V.; Ribeiro, M. E. N. P.; França, F. C. F.; Yeates, S. G.; Ricardo, N. M. P. S.; J. Braz. Chem. Soc. 2015, 11, 2195. 23. Kulthe, S. S.; Inamdar, N. N.; Choudhari, Y. M.; Shirolikar, S. M.; Borde, L. C.; Mourya, V. K.; Colloids Surf., B 2011, 88, 691. 24. Yagui, C. O. R.; Pessoa-Jr, A.; Tavares, L. C.; J. Pharm. Pharm. Sci. 2005, 8, 147. 25. Djordjevic, J.; Barch, M.; Uhrich, K. E.; Pharm. Res. 2005, 22, 24. 26. Torchilin, V. P.; Cell. Mol. Life Sci. 2004, 61, 2549. 27. Pujol, M. O.; Coleman, D. J. L.; Allen, C. D.; Heidenreich, O.; Fulton, D. A.; J. Controlled Release 2013, 172, 939. 28. Negm, N. A.; Sabagh, A. M. E.; Quim. Nova 2011, 34, 1007. 29. Zhang, I.; Lam, Y. M.; J. Nanosci. Nanotechnol. 2006, 6, 1. 30. Crothers, M.; Zhou, M.; Zho, Z.; Ricardo, N. M. P. S.; Yang, Z.; Taboada, P.; Chaibundit, C.; Attwood, D.; Booth, C.; Int. J. Pharm. 2005, 293, 91. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access





