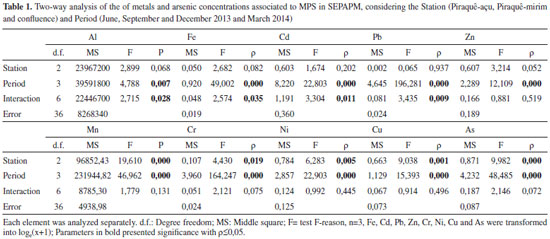
Artigo
| Geochemistry of suspended particulate matter in a tropical estuarine system, southeastern Brazil |
|
Sury de M. MonteiroI,*; Fabian SáII; Renato Rodrigues NetoIII
IInstituto de Geociências, Universidade Federal do Pará, 66075-110 Belém - PA, Brasil Recebido em: 10/11/2016 *e-mail: sury@ufpa.br The aim of this study is to assess the variation of the geochemistry of suspended particulate matter (SPM) in the tropical estuarine system composed of the adjacent Piraquê-Açu and Piraquê-Mirim (ES) rivers using SPM sampled with sediment traps (45 µm mesh) installed in the drainage channels. We collected SPM during different tidal stages and seasonal periods and analysed the metals using inductively coupled plasma mass spectrometry according to EPA 3051A, the mineralogy using X-ray diffraction, the particle size using laser ray diffraction, and organic matter (OM) using calcination. The traps collected enough SPM to perform analyses for each sampling period. The SPM consists of silt size particles, and the percentage of OM is between 20 and 40%. The particles are dominated by kaolinite and quartz, and gibbsite, haematite, goethite, and pyrite are also present. These materials are found in the Barreiras Formation, through which the drainage system cuts. The metal concentrations were higher in the summer: Al (3208.32 g kg-1), Pb (28.05 mg kg-1), Mn (676.35 mg kg-1), Cr (136.12 mg kg-1), and Cu (13.76 mg kg-1). The Piraquê-Açu River had higher metal concentrations than the Piraquê-Mirim River, and the geochemical indices of both rivers indicate that they naturally contribute to the estuarine system of the Piraquê-Açu and Piraquê-Mirim rivers (SEPAPM). However, anthropic interferences influence these indices at the confluence of the two channels. INTRODUCTION Estuaries are transitional environments that act as biogeochemical filters that retain dissolved and particulate material, including pollutants.1-4 Because they are largely influenced by hydrodynamic processes (waves, currents, and tides), estuaries distribute these elements and determine whether the system is a retainer or an exporter.5 Several studies have addressed the chemical composition and transport of suspended particulate matter (SPM; ≥ 0.45 µm) from estuarine regions to the adjacent coastal zones, mainly to quantify the continental contribution to the oceans.6-11 However, few studies have been conducted in estuaries located in small and medium river basins in tropical regions, especially in those where human impacts are not yet evident. Understanding the geochemistry of SPM and the processes that influence it in estuarine systems has become a challenge when considering conditioning parameters such as rainfall, river discharge, the concentrations of major and trace elements and the physicochemical variables that may modify the geochemical behaviour of the SPM. To address these issues, the estuarine system of the Piraquê-Açu and Piraquê-Mirim rivers (SEPAPM) was studied to 1) evaluate if the geochemistry of the SPM of these two adjacent fluvial channels with different drainages varies12,13 and 2) test if a sediment trap can be used to collect SPM considering the difficulty of sampling SPM with conventional methods, which require filtering many litres of water to acquire sufficient material for the geochemical analysis. Study area The SEPAPM is located in the municipality of Aracruz, northern Espírito Santo state (Brazil), and is fed by two hydrographic basins: the Piraquê-Mirim river basin, which has an area of 69 km2 and a flow of 2.59 m3 s-1, and the Piraquê-Açu river basin, which has an area of 379 km2 and a flow of 13.69 m3 s-1 (Figure 1). The confluence of the rivers is located approximately 4.5 km upstream from the mouth of the estuary, and the combined average annual flow of the two rivers is 14.5 m3 s-1.12,13
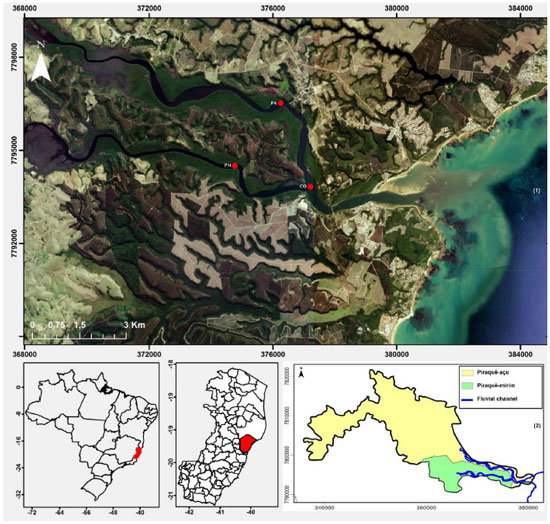 Figure 1. Location of the study area: (1) Estuarine System of Piraquê-açu and Piraquê-mirim rivers, Aracruz (ES) with sampling stations using sediment traps: Piraquê-açu (PA); Piraquê-mirim (PM); And Confluence (CO); (2) Hydrographic basins of the Piraquê-açu and Piraquê-mirim river
The region has a tropical coastal climate with an average annual rainfall of approximately 1,250 mm.14 It has two seasons: summer, which is characterised by the highest rainfall (October to March) with rainfall maxima between November and December (200-250 mm),12 and winter, which is characterised by lower rainfall (June to September) with the lowest rainfall indices between June and August (25-50 mm).12 The average temperatures range from 32.4 °C in the summer to 14.9 °C in the winter.15 This region contains undeveloped Quaternary deposits of the Barreiras Formation along narrow coastal plains, abrupt shorelines and active cliffs.16-18 The deposits provide different sedimentary distributions to the fluvial channels, including muddy facies rich in organic matter (OM) in regions of low hydrodynamics and sandy facies in regions with greater fluvial or marine influences.19,20 The bathymetric and geomorphological characteristics of the estuarine region are influenced by the tides and fluvial flow. The water depths range from 0 to 8 m with a maximum depth of 16.7 m.19 The region is dominated by a micro-tidal regime with ebb asymmetry and a maximum amplitude of 1.8 m in equinoctial periods. The intensities of the currents are controlled by the coastline, and the maximum velocity occurs during the ebb (0.8 m s-1). The maximum intensities of both rivers are similar: 0.7 m s-1 in the Piraquê-Açu River and 0.8 m s-1 in the Piraquê-Mirim River.21
EXPERIMENTAL METHODS Considering the climate seasonality of the region and the influence of tides, SPM sampling was performed in the SEPAPM during the 2013/2014 hydrological cycle. The campaigns were carried out during the syzygy tide, during which the amplitude ranged between 1.40 and 1.60 m. Two campaigns were carried out during the winter (June and September 2013), and two were carried out during the summer (December 2013 and March 2014). The sampling network is composed of three fixed stations, which are located in the intermediate/lower section of the estuary. One is located in the Piraquê-Açu River (PA; 376,217.96 m E, 7,796,428.15 m S), one is located in the Piraquê-Mirim River (PM; 374,794. 85 m E, 7,794,496.52 m S), and one is located at the confluence of the rivers (CO; 377,366.13 m E, 7,793,553.61 m S) (Figure 1). Sampling of suspended particulate matter The SPM was collected with a system of ten traps attached to a vertical tower with 15 x 9 cm windows, which are covered by a polyester fabric shaped like a sieve with a 0.45 µm mesh to allow water to pass through and retain the SPM. The tower with the traps was installed on the side of a small vessel anchored at the sampling station. SPM sampling was performed during the flood and ebb (Figure 2). Moorings and anchors were used to keep the tower upright and pointing in the opposite direction to the current approximately 1 m below the surface and 1 m above the bottom to avoid sampling material carried by saltation.
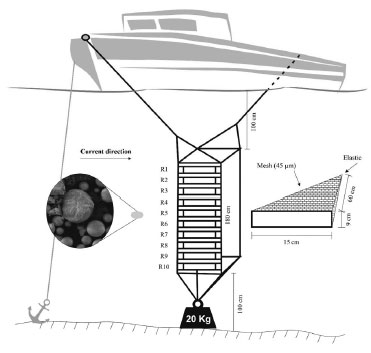 Figure 2. Scheme of MPS sampling in SEPAPM using sediment trap; polyester with 0.45 µm mesh are arranged from R1(1 m below the surface) to R10 (1 m above the bottom), oriented in the same direction as the tidal current
This method was designed to calculate the transport of sediment on beaches;22 however, in this study, we sought to only sample the SPM in the water column for geochemical analysis. Therefore, the sediment trap was installed based on approximate schedules for the three stations to standardise the sampling time. The structure was submerged for 2 h during each phase of the tide (flood and ebb). The order of sampling of the stations on consecutive days was as follows: Piraquê-Açu, confluence, and Piraquê-Mirim. After each sampling, the meshes were washed with purified water (18 MΩ cm) in a Milli-Q system (Millipore) to remove the retained SPM, which was stored under refrigeration until the analyses were performed. During the sampling period, measurements of the physical and chemical parameters of the water (surface and bottom), including temperature, pH, dissolved oxygen, and salinity, were performed with a calibrated portable multi-analyser (Horiba U-50) every hour during the tidal cycle (13 h). The SPM samples were lyophilised in the laboratory and sub-sampled for the metals, mineralogy, particle size and OM analyses. Metals and arsenic associated with SPM For the metals and arsenic analyses, SPM samples (25 mg) were digested in Teflon (X-press) tubes with 10 ml of concentrated nitric acid (according to the United States Environmental Protection Agency (EPA) method 3051A), and a weighing procedure was carried out in each phase (gravimetric method). The extracts were digested in a microwave system (Mars X-press, CEM) for 40 minutes (15 min ramp and 25 min hold) at a temperature of 185 ± 5 °C and a power of 1,600 W. After cooling for 2 h, the final extract was filtered on Whatman 40 paper. The extracts were analysed by inductively coupled plasma mass spectrometry (ICP-MS) on an Agilent model 7500 cx instrument. The accuracy of the methodology was evaluated through the analysis of a reference contaminated soil material (EnviroMAT Contaminated Soil SS2), which has certified values for all of the analysed elements. A multi-element solution was used as the internal standard to correct for interference effects. The accuracy of the analytical procedure was calculated using the Z score, which evaluates laboratory performance using certified reference material. This index showed that the concentrations obtained by the analytical method were satisfactory. The recovery percentages of the elements were 85.87% (Al), 96.63% (Mn), 93.97% (Fe), 91.71% (Cr), 96.76% (Ni), 97.83% (Zn), 99.97% (As), 99.50% (Cd) and 83.07% (Pb). Complementary analyses The samples were pulverised, and X-ray diffraction analyses were performed using a PANalytical model X'PERT PRO MPD (PW 3040/60) X-ray diffractometer with a PW 3050/60 goniometer (theta/theta) and a model PW3373/00 Cu anode ceramic X-ray tube (Kα1 1.540598 Å) with a long fine focus operating at 2200 W and 60 kV. The detector was an RTMS type X'Celerator. The data were recorded in the 2θ range of 5° to 75° and acquired using the X'Pert Data Collector software version 2.1a. The data were processed with the PANalytical X'Pert HighScore software version 2.1b. The generated diffractograms were processed with the APD software (PHILIPS) to scan the obtained records using the International Centre for Diffraction Data (ICCD) Powder Diffraction File (PDF) database for the identification of mineral phases. SPM particle size analysis was performed by laser diffraction using a laser particle analyser (Laser Diffraction, SALD 2101-Shimadzu). Before the grain size identification, each sample was immersed in H2O2 to remove the OM, and the residual sediments were ultrasonically separated at 200 J g -1 for approximately 1.5 min according to the method described for suspended fluvial sediments.23,24 The granulometric scale used the ranges of sand (2.0 - 0.0625 mm), silt (0.0625 - 0.0039 mm) and clay (0.0039 - 0.00006 mm),25 and the concentrations of OM in the SPM were obtained by calcination.26 Processing and interpretation of results The statistical tests of normality, bivariate correlation, analysis of variance (ANOVA) and principal component analysis (PCA) were applied. The geochemical indices Contamination Factor (CF) and Enrichment Factor (EF) were used based on the two regional base levels for the Piraquê-Açu River and the Piraquê-Mirim River.27.28 The CF is the ratio between the concentration of each element in the SPM and the estimated baseline level for the study area.29 The values range from no contamination (CF < 1) to high contamination (CF > 6). The FE indicates the enrichment of metal in the environment (1 ≤ FE < 50) and is often used to differentiate the source of the metal between natural and anthropogenic sources.30,31 Aluminium was used to normalise the concentrations of the metals and arsenic since aluminosilicate comprises the fine fraction (suspended) with which the metals are associated.32 The aluminium were determined from the mineralogical analyses.
RESULTS AND DISCUSSION The sediment trap was used in the SEPAPM in both analysed periods (winter and summer) to sample the materials for the geochemical analysis. The amount of SPM collected by the set of traps varied according to the tidal phase of the sampling period and the sampling station. The minimum amounts were collected during the flood tide phase (0.24, 0.17 and 0.36 g in the Piraquê-Mirim River, Piraquê-Açu River and confluence, respectively), and the maximum amounts were collected during the ebb phase (9.84, 1.85 and 5.39 g, respectively). In general, the maximum amounts of SPM were collected in the Piraquê-Mirim River, which had average retentions of 2.83 ± 0.09 g in the winter and 4.32 ± 0.07 g in the summer. The Piraquê-Açu River had average retentions of 1.32 ± 0.38 g in the winter and 0.87 ± 0.38 g in the summer, followed by the confluence (1.96 ± 1.07 g in the winter and 0.98 ± 0.59 g in the summer). Although the fluvial discharge of the Piraquê-Açu River is approximately five times greater than that of the Piraquê-Mirim River, the smaller size of the Piraquê-Mirim drainage basin allowed more effective transport of the particles, mainly during the onset of the rains in December. In addition, the tapered geomorphology and the erosive processes of the Piraquê-Mirim River33 are mainly influenced by the tide level, which leads to greater turbulence and the resuspension of the bottom sediments. These energy conditions favour SPM transport. Despite the differences in the SPM sampling during the different seasons, the traps collected sufficient amounts of materials for the geochemical and mineralogical analyses. Mineralogy, particle size and organic matter in suspended particulate matter SPM in estuarine regions can be characterised by complex mixtures, which are typically composed of four main components, silicates (mainly quartz), clay minerals, iron and manganese oxides and hydroxides, as well as organic particles such as microorganisms, diatoms, and plant debris.34.35 The mineralogy of the SPM of the SEPAPM is dominated by kaolinite and quartz and also includes gibbsite, haematite, muscovite, goethite, pyrite, magnetite, illite, anatase and halloysite. These minerals are consistent with the clay fraction of the Barreiras Group in the municipality of Aracruz ES, which is characterised by a predominance of kaolinite (870 g kg-1),36 gibbsite (50 g kg-1) and small amounts of quartz, anatase, mica,37,38 goethite, haematite and vermiculite.39 The predominance of quartz (SiO4) at all of the sampling stations and during the different seasons indicates strong chemical weathering in the area since quartz is a resistant mineral; it remains unaltered during chemical weathering reactions.40 Another possible factor is the intensity of the flows, which can affect the contents of the natural materials present in the waters, although not in a directly proportional way.41 Kaolinite (Al2[Si2 O5](OH)4) is commonly abundant in tropical regions42 and is one of the most typical and characteristic detrital clay minerals found in the sediments, which can be explained by its stability. Its presence indicates intense mineral alterations and favours the removal of Fe oxides.37 Kaolinite and Fe and Al oxides are important components for the retention of metals43 and are responsible for their low mobility and bioavailability in the environment.44,45 The common presence of illite (KxAl2[Si4-xAlxO10](OH)2), which is also a detrital mineral, may be due to its stability in alkaline water sediments, which indicates direct and mechanical alterations.46 Although these two clay minerals are generally deposited in the upper portion of estuaries as a result of salinity variations,47-49 such as in Guanabara Bay (Rio de Janeiro state; RJ),50 the Huelva estuary on the Spanish coast,51 the Capibaribe River (Pernambuco state; PE)52 and the Curimataú estuary (Rio Grande do Norte state; RN)6, their effective transport and dispersion in association with the SPM in the lower estuary should be considered. The presence of oxides (Fe, Al and Mg oxides, hydroxides, and oxyhydroxides) in tropical regions is due to crystalline minerals such as goethite and haematite.53 Goethite (α-FeOOH) is the most common iron oxide, and it may be associated with haematite (α-Fe2O3). Only γAl(OH)3 gibbsite, which is a natural crystalline aluminium oxide, was observed in the SEPAPM. It is very common in Brazilian latosols and argisols.42 Goethite, gibbsite, and kaolinite are typically found in tropical environments due to the intense chemical weathering caused by rain, drainage and topography.11 These factors favour the hydrolysis process and the leaching of several ions, such as Na, K, Ca, Mg and Sr, which are easily exported to the topographically lower regions and release smaller ions, such as Al, Ti, Si and Fe, that favour the formation of clay minerals. The presence of goethite in association with kaolinite is also influenced by laterite weathering of the Barreiras Formation, which is abundant in the SEPAPM region.37 In addition to the occurrence of minerals, variations in the OM content and sediments in the clay fraction54,55 also alter the metal concentrations associated with the SPM. In the SEPAPM, the granulometry of the SPM is characterised by the predominance of silt, and sand and clay are also present. The highest percentages of silt occur in the Piraquê-Mirim River (65.45% in the winter and 77.86% in the summer) and are associated with the highest percentages of OM (30.21% and 41.40%, respectively). The confluence and the Piraquê-Açu River are also dominated by silt as well as high sand contents (35.43 and 33.62%, respectively), which mainly occur in the summer. The OM percentages were also lower during the summer at these stations (20.96 and 25.69%, respectively) (Figure 3).
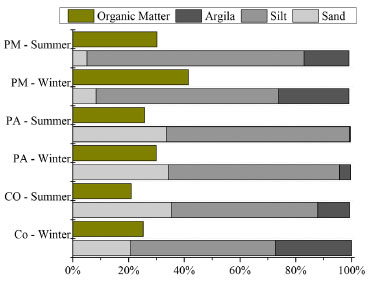 Figure 3. Particle size and Organic Matter associated with SPM during sum mer and winter (March and September, respectively), In the sampling stations: Confuence (CO), Piraquê-açu (PA) and Piraquê-mirim (PM)
These sedimentological characteristics of the SPM are related to the fraction of the bottom sediment near the sampling station. The highest percentages of mud are located in the Piraquê-Mirim sampling region, and those of fine and very fine sand are located in the Piraquê-Açu River and confluence.19,33 The confluence also has the lowest percentages of OM (5 - 10%) due to its larger marine domain,20,28 whereas the two river courses have higher percentages of OM (10 - 20%),19 which may be due to the contributions of terrestrial plants and phytoplankton.28 Physicochemical parameters The mean salinity of the water column in the SEPAPM increased from 29.38 ± 2.1 to 34.32 ± 1.1 psu as the rainfall decreased. The salinity minima (25.66 ± 1.5 psu in March and 25.82 ± 1.9 psu in December) occurred in the Piraquê-Açu River, whereas the maxima (36.96 ± 0.6 psu in September) occurred at the confluence during the winter, when the intrusion of marine waters increased. The marine influence also favoured the increase of the pH values, which reached their maxima in December, especially in the confluence (8.36 ± 0.1) and Piraquê-Mirim River (8.20 ± 0.2). The mean water temperature varied from 23.46 ± 0.6 °C in June to 26.63 ± 1.16 °C in March, and there were no differences between the sampling stations. The concentrations of dissolved oxygen were highest in June (8.33 mg L-1). They decreased slightly when the estuarine waters became less saline; however, they remained saturated throughout the analysed period at the three sampling stations. Metals and arsenic associated with SPM The metals and arsenic associated with the SPM varied significantly (ρ < 0.05) as functions of both the sampling station and the seasonal period. All of the metals varied significantly, but only Ni, Zn and As showed distinct seasonal variations as demonstrated by Tukey's multiple comparison tests. The highest mean concentrations of these metals occurred in the winter (13.08 mg kg-1, 103.00 mg kg-1 and 31.35 mg kg-1, respectively), which were two times higher than the concentrations in the summer. Fe (4,712.57 mg kg-1) and Cd (0.17 mg kg-1) also had higher concentrations in the winter, but they did not have such significant seasonal variations. The other metals had higher concentrations in the summer, with the maximum Al (3,208.32 g kg-1) and Pb (28.05 mg kg-1) concentrations occurring in December and the maximum Mn (676.35 mg kg- 1), Cr (136.12 mg kg-1) and Cu (13.76 mg kg-1) concentrations occurring in March. The analysis of the variance between the sampling stations showed that only Mn, Cr, Ni, Cu and As varied significantly (ρ < 0.05); however, there were no variations in these elements between the Piraquê-Açu and Piraquê-Mirim rivers. Tukey's comparisons indicate that the concentrations of Mn and Ni varied between the estuarine confluence region and the two rivers, whereas Cu only varied between the confluence and the Piraquê-Mirim River, and As and Cr varied between the confluence and the Piraquê-Açu River. Pb was the only element that showed no variation (ρ = 0.99) between the three stations. The estuarine confluence had the highest concentrations of metals except for Al and Pb, which had their maximum concentrations in the Piraquê-Açu and Piraquê-Mirim rivers, respectively. The combination of the water supplies of these two rivers, the influence of the tidal currents and the penetration of the marine waters due to the estuarine confluence results in the greater transport and/or remobilisation of the chemical species associated with the SPM, which causes the increases in their concentrations. The comparison between the two river channels indicates that the Piraquê-Açu station had the highest concentrations of Al (30.90 g kg-1), Fe (40.54 g kg-1), Mn (451.09 mg kg-1), Ni (8.13 mg kg-1), Cu (10.16 mg kg-1), Zn (78.07 mg kg-1) and Cd (0.10 mg kg-1), whereas Cr (72.48 mg kg-1), As (22.26 mg kg-1) and Pb (16.27 mg kg-1) were highest at the Piraquê-Mirim station. The concentrations of metals and arsenic are plotted against the sampling periods in Figure 4, and the analyses of variance between the stations, periods and their interactions are shown in Table 1.
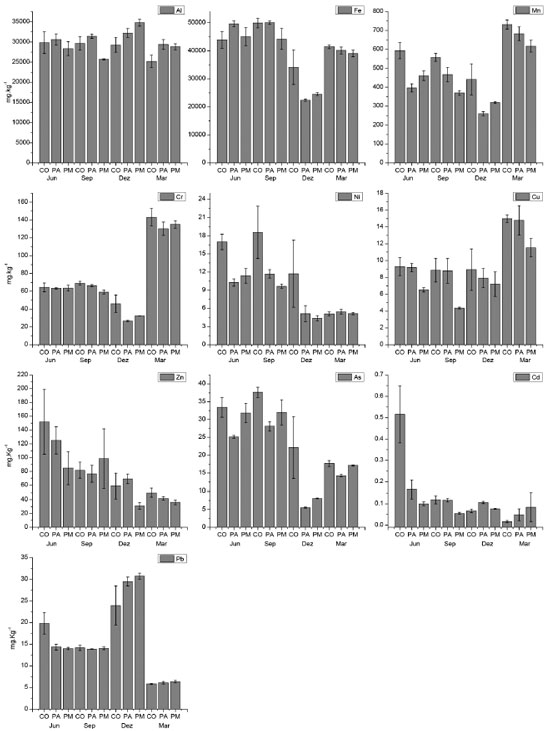 Figure 4. Metals and arsenic (mg Kg-1) associated with SPM during the winter (June and September) and summer (December and March) in the SEPAPM sampling stations: Confluence (CO), Piraquê-açu (PA) and Piraquê-mirim (PM)
Among the analysed metals, Al (29.59 g kg-1) and Fe (40.36 g kg-1) had concentrations below the levels considered to be natural (71.00 and 40.00 g kg-1, respectively).56 However, the concentration of Mn (490.92 mg kg-1) exceeded the natural value (131.69 mg kg-1),56 and the local base levels ranged between 306.46 and 412.22 mg kg-1 at Piraquê-Açu and Piraquê-Mirim, respectively.26,28 Despite these differences, the presence of these metals can be considered to be due to the presence of minerals such as quartz, magnetite, kaolinite, pyrite, haematite and goethite. In addition, although biotite ("iron mica") was not identified, it is present in the Barreiras Group in this region and contains high levels of Al (18%) and Fe (25%),37 which could contribute to the high natural concentrations of these metals. The elements As, Cr, Pb, Ni and Cu had lower concentrations in both rivers than the background values of the region. However, Zn had higher concentrations (78.07 mg kg-1) than both the baseline concentration (68.85 mg kg-1)26,28 and the tidal plain sediment in the Piraquê-Açu River (56.00 mg kg-1).57 The control of some elements can be demonstrated by the significant inter-metal correlations shown in the PCA, which explained 72.54% of the data and indicated the formation of four groups: Mn, Cr and Cu; As and Fe; Cd, Zn and Ni; and Pb alone. The higher loads of PC1 (40.99%) represent the relationships between Fe, Mn, Cr, Cu and As, which are related to the natural flow of elements adsorbed to the SPM,58 the continental sources from the weathering of rocks and soils around the drainage basin59 or the influences of similar biogeochemical processes,60 whereas the higher loads of Cd, Ni, Pb and Zn for PC2 (31.55%) demonstrate the absence of a common source and confirm the anthropogenic influences. Thus, the source and control of the natural concentrations of the elements may be associated with the natural lithologic conditions of the Barreiras Formation, which comprises sandstones and siltstones in the region surrounding the estuary.61 However, the mobility of these metals in saline environments such as the SEPAPM (20 - 30 psu) can also be influenced by Mn/Fe oxides and the OM content.62,63 Mn oxides are efficient sorbents of metals due to their small size and high specific surface area.64-66 In this case, the saturation of dissolved oxygen in the SEPAPM can also influence the content of the metals associated with the SPM, which has been verified in other river systems under oxide conditions, where Cd, Zn and Ni were associated with Mn/Fe oxides.67 For example, Pb and Cu exhibit low mobility due to their affinity with the OH- (hydroxyl) functional group present on the surfaces of kaolinite, oxide, oxyhydroxides, and Fe and Al hydroxides,68,69 which are found in the region and whose concentrations are controlled by erosion and natural leaching. 70 The control of Pb mobility is also associated with pH and high OM contents,71 as observed in the SEPAPM, which has alkaline pH values (mean of 7.77) and OM contents between 20.97 and 41.42%. These conditions may favour the formation of insoluble compounds. In addition, in natural waters with pH ~7, colloidal particles are negatively charged and can adsorb and fix the metals to the SPM.72,73 The availability of the metals can also be reduced by their association with the high molecular weight compounds of the OM in the solid phase.74 The seagrass region of the SEPAPM, which is populated by mangroves, is one of the main sources of OM in the sediment in both rivers, which has a high abundance of organic compounds, such as taraxerol and β-amerin.75 These organic compounds may reduce the availability of Cu and Pb. The lower concentrations of Cu in Piraquê-Mirim (7.40 mg kg-1) than in Piraquê-Açu and the confluence (10.16 and 10.51 mg kg-1, respectively) may be indicative of this influence of the OM on the adsorption of Cu. The source of As may be related to the mineralogical composition of the region,76 which has high concentrations of As in the sedimentary matrix of the estuaries, the continental shelf and the Barreiras Formation of Espírito Santo. Thus, the high background levels of As are the main reason for the high concentrations. The correlation of As with Fe (oxides) is also strong, both in sediment28 and in SPM (ρ = 0.85). In this case, the Fe oxides and hydroxides are mainly responsible for the adsorption of As to the particulate material77 and control its retention in the particulate phase,77,78 as has been observed in other estuarine systems.8,79 Despite the strong natural contributions, the effects of anthropic activities in the drainage basin should not be ruled out. These activities, which are the result of the chemical, metallurgical and domestic sewage industries, can also directly influence the high concentrations of Zn and Cd in the SPM of the Piraquê-Açu River. In addition, in the estuarine region, the eucalyptus monoculture implemented in the 1960s80 contributes to this enrichment due to the direct application of chemical products such as pesticides and fertilisers,81-83 which consist of N:P:K (5:33:6), borax and zinc sulphide84 because the soils in the Espírito Santo state generally contain low levels of Zn (22.61 mg kg-1).85 The use of mineral and organic fertilisers, limestone and pesticides in agriculture is considered to be a source of metals for the environment, especially when crops are developed near fluvial or estuarine courses,86-88 as is the case of the Piraquê-Açu and Piraquê-Mirim drainage basin.89 In this case, the use of natural and soluble phosphates may contribute to the introduction of Cd, Cu, Cr, Ni, Pb and Zn;90 nitrogen fertilisers and limestone contribute approximately 1% Cu and Pb, and pesticides affect the contents of As, Cu and Zn.91 The comparison between the concentrations of the analysed chemical species indicates that the SEPAPM has similar or lower concentrations than other estuarine regions,6-11 which suggests that the natural influences are generally more significant than the anthropic contributions. The interactions between chemical species and salinity, OM, pH and oxides are also important, as has been observed in other estuarine environments.92 However, there are no interference patterns of these parameters with the concentrations of the metals in the SPM. Due to their complexity, these interactions should be studied in more detail in both the SPM and the background sediment. Geochemical indices The SPM contamination levels in the SEPAPM indicate that only Mn (1.47) and Zn (1.11) are classified as moderately contaminated. The EF values indicate that the metals are slightly enriched, except for As, Ni and Cd, which are not enriched in the SPM of the SEPAPM. However, this enrichment is associated with the mineralogical composition of the SPM because the natural enrichment of most metals (values between 0.5 and 2.029,93,94) is considered to be caused by variations of the climate and the local geology, except for Mn (EF = 2.36), which may be affected by anthropogenic factors or biological processes. The EF values at the sampling stations formed three groups.95-97 Cd was the only non-enriched element (EF ≤ 1), and Pb and Cu were enriched (1 < EF < 2) at all three stations. Mn, Cr and Zn were highly enriched (EF ≥ 2) only at the estuarine confluence, whereas As was also enriched at the confluence but did not exceed its natural value (EF ≤ 2) (Figure 5). In addition, the confluence station was more enriched than the two river courses, which may be caused by biological or anthropic influences, atmospheric contributions, urban and agricultural runoff and the drainage of industrial effluents carried by the two drainage basins to this region. However, the Piraquê-Açu basin may be most responsible for carrying metals to the confluence since it is more enriched in metals than the Piraquê-Mirim basin.
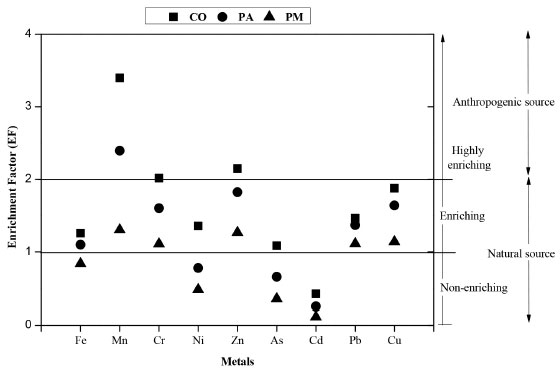 Figure 5. Enrichment Factor of metals and arsenic associated with SPM in SEPAPM sampling stations: Confluence (CO), Piraquê-açu (PA) and Piraquê-mirim (PM)
The comparison between the concentrations of the analysed chemical species indicates that the SEPAPM has similar or lower concentrations than other estuarine regions,6-10 which indicates that this system still responds mainly to natural changes.
CONCLUSION The use of SPM traps in the SEPAPM during different seasons facilitated the collection of sufficient material for geochemical analyses, and it was not necessary to filter many litres of water samples in the laboratory. Considering the low material load transported by the SEPAPM, this method was efficient and allowed the geochemistry of the SPM to be studied at different time scales, including in different tidal phases (flood and ebb) and seasons of the year (summer and winter). The mineralogy of the SPM carried by the Piraquê-Açu and Piraquê-Mirim rivers predominantly consists of kaolinite and quartz, and gibbsite, muscovite and pyrite are also present. The mineralogy reflects the influence of the geology of the drainage basins, which cut through the Barreiras Formation. Although this natural contribution is supported by the geochemical indices, human interferences are indicated by the high concentrations of metals and arsenic associated with the SPM at the confluence of the two adjacent fluvial channels. The SPM geochemistry of this region is predominantly influenced by the extensive drainage network of the Piraquê-Açu River, including its high fluvial flow and the different physicochemical characteristics, mainly during the summer. However, the advection of metals and arsenic associated with the SPM from the confluence of the channels predominantly affects the geochemistry of the Piraquê-Mirim River.
ACKNOWLEDGEMENTS We acknowledge the Fundaçao de Amparo a Pesquisa e Inovaçao do Espírito Santo (Foundation for Research and Innovation of Espírito Santo; FAPES) for financing the Project "Geochemical Processes in the APA Costa de Algas of Santa Cruz and surroundings in Aracruz, ES".
REFERENCES 1. Andrews, E.; Samways, G.; Shimmield, B.; Sci. Total Environ. 2008, 405, 1. 2. Cave, R.; Andrews, E.; Jickells, T.; Coombes, G.; Estuarine, Coastal Shelf Sci. 2005, 62, 547. 3. Andrews, E.; Burgess, D.; Cave, R.; Coombes, G.; Jickells, D.; Parkes, J.; Turner, K.; Sci. Total Environ. 2006, 371, 19. 4. Monbet, P.; Mar. Chem. 2006, 98, 59. 5. Dyer, R. Em Sediment transport process in estuaries; Perillo, E., ed.; Elsevier Science: Amsterdan, 1995, cap. 14. 6. Garlipp, B.; Tese de Doutorado, Universidade Federal do Rio Grande do Norte, Brasil, 2006. 7. Carvalho, V.; Salomao, B.; Molisani, M.; Rezende, E.; Lacerda, D.; Sci. Total Environ. 2002, 284, 85. 8. Hatje, V.; Birch, F.; Hill, M.; Estuarine, Coastal Shelf Sci. 2001, 53, 63. 9. Duquesne, S.; Newton, C.; Guisti, L.; Marriott, B.; Stärk, J.; Bird, J.; Environ. Pollut. 2006, 143, 187. 10. Jonas, C.; Millward, E.; Mar. Pollut. Bull. 2010, 61, 52. 11. Kessarkar, M.; Shynu, R.; Rao, P.; Chong, F.; Narvekar, T.; Zhang, J.; Environ. Monit. Assess. 2013, 185, 4461. 12. Leite, A.; Dissertaçao de Mestrado, Universidade Federal do Espírito Santo, Brasil, 2012. 13. Barroso, F.; Martins, O.; Léllis, S.; Santana, E. Em Integrated river basin management: incorporating coastal zone issues; Bilibio, C.; Hensel, O.; Selbach, F., eds.; Fundaçao Universidade Federal do Pampa: Jaguarao, 2012. 14. Mello, R.; Viola, R.; Curi, N.; Silva, M.; Rev. Bras. Cienc. Solo. 2012, 36, 1878. 15. Feitoza, R.; Castro, L. L. F. de; Resende, M.; Zangrande, B.; Stocking, M.; Borel, A.; Fulin, A.; Cerqueira F.; Salgado, S.; Feitoza, N.; Tock A.; Filho, N.; ITC J. 1997, 3/4, 1. 16. Dominguez, L. Em The coastal zone of Brazil; Dillenburg, R.; Hesp, A., eds.; Springer-Verlag: Berlin, 2009, cap. 2. 17. Martin, L.; Suguio, K.; Flexor, M.; Archanjo, D.; An. Acad. Bras. Cienc. 1996, 68, 389. 18. Albino, J.; Girardi, G.; Nascimento, A.; Em Erosao e progradaçao do litoral do Espirito Santo; Muehe, D., ed.; Ministério de Meio Ambiente: Brasília, 2006. 19. Silva, E.; Dissertaçao de Mestrado, Universidade Federal do Espírito Santo, Brasil , 2012. 20. Silva, E.; Menandro, S.; Nascimento, A.; Quaresma, S.; Bastos, C.; Rev. Bras. Geof. 2014, 32, 301. 21. Monteiro, M.; Tese de Doutorado, Universidade Federal do Espírito Santo, Brasil , 2015. 22. Kraus, C.; Dean, L.; Em Longshore sediment transport rate distributions measured by trap; Kraus, C., ed.; American Society of Civil Engineers: New York, 1987. 23. Lunardi, B.; Dissertaçao de Mestrado, Universidade Federal do Rio Grande do Sul, Brasil, 2002. 24. Poleto, C.; Bortoluzzi, C.; Merten, H.; Resumos do 7° Simpósio Nacional de Engenharia de Sedimentos, Porto Alegre, Brasil, 2006. 25. Wentworth, K.; J. Geol. 1922, 30, 377. 26. Kralik, M.; Appl. Geochem. 1999, 14, 807. 27. Costa, S.; Tese de Doutorado, Universidade Federal do Espírito Santo, Brasil , 2014. 28. Costa, S.; Grilo, F.; Woll, A.; Thompson, A.; Sá, F.; Neto, R.; Reg. Stud. Mar. Sci. 2016, 6, 49. 29. Förstner, U.; Contaminated Sediments, 1th ed., Springer-Verlag: Heidelberg, 1989. 30. Zahra, A.; Hashmi, Z.; Malik, N.; Ahmed, Z.; Sci. Total Environ. 2014, 470-471, 925. 31. Chen, W.; Kao, M.; Chen, F.; Dong, D.; Chemosphere 2007, 66, 1431. 32. Windom, L.; Schropp, J.; Calder, D.; Ryan, D.; Smith, Jr., R. G.; Burney, C.; Lewis, G.; Rawlinson, H.; Environ. Sci. Technol. 1989, 23, 314. 33. Silva, E.; Quaresma, S.; Bastos, C.; J. Sediment. Res. 2013, 83, 994. 34. Ramamoorthy, S.; Rust, R.; Environ. Geol. 1978, 2, 165. 35. Hiller, S.; Sci. Total Environ. 2001, 265, 281. 36. Melo, F.; Tese de Doutorado, Universidade Federal de Viçosa, Brasil, 1998. 37. Melo, F.; Novais, F.; Schaefer, R.; Fontes, F.; Singh, B.; Rev. Bras. Cienc. Solo 2002, 26, 29. 38. Duarte, N.; Curi, N.; Pérez, V.; Kämpf, N.; Claessen,C.; Pesqui. Agropecu. Bras. 2000, 35, 1237. 39. Jacomine, T.; Reuniao técnica sobre solos coesos dos tabuleiros costeiros, Cruz das Almas, Brasil, 1996. 40. Deer, A.; Howie, A.; Zussman, J.; Minerais constituintes das rochas, 2th ed., Fundaçao Calouste Gulbenkian: Lisboa, 2000. 41. Ortiz, S.; Dissertaçao de Mestrado, Universidade Federal do Rio Grande do Sul, Brasil, 1999. 42. Fontes, F.; Camargo, A.; Sposito, G.; Sci. Agric. 2001, 58, 627. 43. Naidu, R.; Summer, E.; Harter, D.; Environ. Geochem. Health 1998, 20, 5. 44. Pierzynski, M.; Schwab, P.; J. Environ. Qual. 1993, 22, 247. 45. Sobrinho, B.; Velloso, X.; Costa, M.; Oliveira, C.; Rev. Bras. Cienc. Solo 1998, 22, 345. 46. Millot, G.; Géologie des argiles, 1th ed., Masson et Cie: Paris, 1964. 47. Whitehouse, G.; Jeffrey, M.; Debbrecht, D.; Clays Clay Miner. 1958, 7, 1. 48. Gibbs, J.; J. Sediment. Petrol. 1983, 53, 237. 49. Chamley, H.; Clay Sedimentology, 5th ed., Springer: Berlin, 1989. 50. Faria, M.; Sanchez, B.; An. Acad. Bras. Cienc. 2001, 73, 121. 51. Caliani, F.; Munoz, R.; Galán, E.; Sci. Total Environ. 1997, 198, 181. 52. Brayner, M.; Barbosa, F.; Silva, P.; Melo, V.; J. Phys. IV 2003, 107, 221. 53. Childs, W.; Aust. J. Soil Res. 1981, 19, 175 54. Chesworth, W.; Em Geochemistry of micronutrients; Mortvedt T.; Cox R.; Shuman, M.; Welch M., eds.; Madison: WI, 1991. 55. Soares, R.; Tese de Doutorado, Universidade de Sao Paulo, Brasil, 2004. 56. Turekian, K; Carr, H.; Geol. Soc. Am. Bull. 1960, 72, 175. 57. Souza, B.; Dissertaçao de Mestrado, Universidade Federal do Espírito Santo, Brasil , 2009. 58. Ruiz, F.; Mar. Pollut. Bull. 2001, 42, 482 59. Maanan, M.; Zourarah, B.; Carruesco, C.; Aajjane, A.; Naud, J.; J. Afr. Earth Sci. 2004, 39, 473. 60. Beltrame, O.; Marco, G.; Marcovecchio, E.; Sci. Total Environ. 2014, 485-486, 604. 61. Moraes, R.; Contribuiçao para a gestao da zona costeira do Brasil: elementos para uma geografia do litoral brasileiro, 2th ed., Annablume: Sao Paulo, 2007. 62. Perin, G.; Fabris, R.; Manente, S.; Wagener, R.; Hamacher, C.; Scotto, S.; Water Res. 1997, 31, 3017. 63. Hatje, V.; Barros, F.; Mar. Pollut. Bull. 2012, 64, 2603. 64. McKenzie, M.; Aust. J. Soil Res. 1980, 18, 61. 65. Turner, A.; Rawling, C.; Water Res. 2001, 35, 4379. 66. Turner, M.; Martino, M.; Roux, L.; Environ. Sci. Technol. 2002, 36, 4578. 67. Salomons, W.; Rooij, M.; Kerdijik, H.; Bril, J.; Hydrobiologia 1987, 149, 13. 68. Smith, A.; Means, L.; Chen, A.; Alleman, B.; Chapman, C.; Tixier Jr., J. S.; Brauning, E.; Gavaskar, R.; Royer, D. Em Remedial options for metals-contaminated sites. CRC Press: Boca Raton, 1995. 69. Forstner, U.; Hydrobiologia 1987, 149, 221. 70. Pedrozo, M.; Lima, V.; Ecotoxicologia do cobre e seus compostos, 1th ed., Centro de Recursos Ambientais: Salvador, 2001. 71. Kabata-Pendias, A.; Pendias, H.; Trace elements in soil and plants, 3th ed., CRC Press: Boca Raton , 2001. 72. Manahan, E.; Environmental chemistry, 3th ed., CRC Press: Boca Raton ,1994. 73. Hatje, V.; Payne, M.; Hill, M.; McOrist, G.; Birch, F.; Szymczak, R.; Environ. Int. 2003, 29, 619. 74. Gambrell. P.; Environ. Qual. 1994, 23, 883. 75. Grilo, F.; Tese de Doutorado, Universidade Federal do Espírito Santo, Brasil , 2014. 76. Mirlean, N.; Medeanic, S.; Garcia, A.; Travassos, P.; Baish, P.; Cont. Shelf Res. 2012, 35, 129. 77. Bostick, C.; Chen, C.; Fendorf, S.; Environ. Sci. Technol. 2004, 38, 3299. 78. Dixit, S.; Hering, G.; Environ. Sci. Technol. 2003, 37, 4182. 79. Leblanc, A.; Schroeder, A.; Hydrobiologia 2008, 604, 123. 80. Loureiro, K.; Rev. Agora 2006, 3, 1. 81. Forstner, U.; Wittman, G.; Metal Pollution in the Aquatic Environment, 7th ed., Springer-Verlag: New York. 1979. 82. Jiméneza, S.; Páez-Osunab, F.; Ruiz-Fernández, C.; Environ. Pollut. 2003, 125, 423. 83. Silva, S.; Vitti, C.; Quim. Nova 2008, 31, 6. 84. Pinto Jr., J. E.; Jacob, S.; IPEF 1979, 7, 1. 85. Paye, S.; Mello, V.; Abrahao, P.; Filho I.; Dias, P.; Castro, O.; Melo, B.; França, M.; Rev. Bras. Cienc. Solo 2010, 34, 6. 86. Fu, J.; Zhao, P.; Luo, P.; Liu, S.; Kyzas, Z.; Luo, Y.; Zhao, Y.; An, Q.; Zhu, L.; J. Hazard. Mater. 2014, 270, 102. 87. Reis, A.; Parker, A.; Alenvoao, A.; Appl. Geochem. 2014, 44, 69. 88. Duarte, B.; Caçador, I.; Mar. Pollut. Bull. 2012, 64, 2109. 89. Silva, W. A. da.; Resumos do 7° Simpósio Brasileiro de Geografia Física Aplicada, Natal, Brasil. 2007. 90. Freitas, E. V. de S.; Nascimento, A.; Goulart, F.; Silva, S.; Rev. Bras. Cienc. Solo 2009, 33, 1899. 91. Guilherme, G.; Marques, J.; Pierangeli, P.; Zuliani, Q.; Campos, L.; Marchi, G.; Em Elementos-traço em solos e sistemas aquáticos; Vidal-Torrado, P.; Alleoni, F.; Cooper, M.; Silva, P.; Cardoso, J., eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, 2005, cap. 9. 92. Gerringa, A.; Hummel, H.; Moerdijk-Poortvliet, W.; J. Sea Res. 1998, 40, 193. 93. Sposito, G.; The chemistry of soils, 2th ed., Oxford University Press: New York, 1989. 94. Grousset, E.; Quetel, R.; Thomas, B.; Donard, X.; Lambert, E.; Guillard, F.; Monaco, A.; Mar. Chem. 1995, 48, 291. 95. Delgado, J.; Nieto, M.; Boski, T.; Estuarine, Coastal Shelf Sci. 2010, 88, 71. 96. Magesh, S.; Chandrasekar, N.; Vetha, D.; Estuarine, Coastal Shelf Sci. 2011, 92, 618. 97. Mil-Mans, M.; Stevens,L.; Abrantes, F.; Cato, I.; Cont. Shelf Res. 2006, 26, 1184. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access