Nota Técnica
| Assembly of low-cost lab-made photoreactor for preparation of nanomaterials |
|
Vera Katic; Pãmyla L. dos Santos; Joyce G. Gabriel; Acacia Adriana Salomão; Juliano Alves Bonacin*
Instituto de Química, Universidade Estadual de Campinas, 13083-970 Campinas - SP, Brasil Recebido em 31/05/2017 *e-mail: jbonacin@iqm.unicamp.br In this paper, a low-cost and versatile lab-made photoreactor was constructed for the preparation of nanomaterials such as gold nanoparticles and reduced graphene oxide. The power of the lab-made photoreactor can be easily adjusted according to the number of lamps turned on. Moreover, the lab-made photoreactor allows the utilization of different sources of irradiation with distinct wavelengths. Performance tests for production of gold nanoparticles showed reproducibility and equal efficiency as compared to the literature. Also, the photoreactor powered with UV-A and UV-C as sources of irradiation can be used for modulation of the reduction degree of graphene oxide. INTRODUCTION Photochemical methods for the preparation of nanomaterials have received great attention due to the possibility of temporal control, reaction rate modulation and spatial adjust. Also, the photochemical reactions can be changed stepwise even in mild condition.1 Gold nanoparticles (AuNP), for example, can be synthesized using tetrachloroauric(III) acid, IrgacureTM 2959 (photoinitiator) and UV light.2 This method allows the synthesis of unprotected AuNP in water with high reproducibility, high stability and a range of sizes controlled by the illumination intensity. Due to the reported features of these AuNP, it is possible to highlight their use in studies of SERS (Surface Enhanced Raman Scattering) effect, catalysis, photocatalysis and plasmonics. Also, the use of different wavelengths, in the photochemical synthesis, can lead to the formation of silver nanoparticles with different shape and size.3 This process occurs directly by irradiation of silver ions or nanometric spherical seed of AgNP.4 From the coordination chemistry point of view, the irradiation of nitroprusside under UV light leads to the production of Prussian blue nanoparticles and the modulation of the size can be performed by altering the light source and its intensity.5 Another example of the application of UV-light in materials is the modulation of the electrochemical properties of the graphene oxide through the control of reduction level which can be adjusted by the intensity and time of irradiation.6 Thereby, it is possible to make a fine and planned adjustment in the final properties of the graphene oxide, which is sometimes difficult to control using chemical methods. An overview of the main uses of light to produce or modulate properties of (nano)materials is found in the Figure 1.
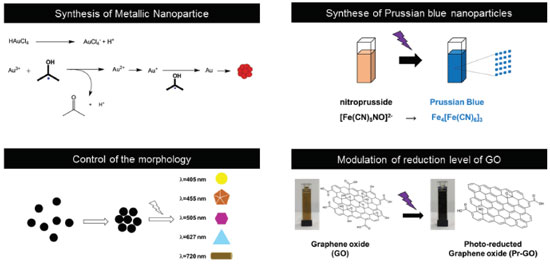 Figure 1. Overview of the main uses of light to produce (nano)materials. Figure adapted from references 2, 4-6
The use of light in chemistry goes beyond the preparation of materials and may be used in strategic areas ranging from medicine to energy. A simple UV-LED can be applied in generation of singlet oxygen and its quantification.7 Light can contribute to environment when used in systems of photodegrading of waste or polluting.8,9 Furthermore, light is converted directly in electricity10 or its energy can be stored regarding chemical bonding as in water splitting process.11 One of the strategies to work with light in preparation of nanomaterials is the utilization of the photoreactors. They offer the possibility to control the wavelength of light source, the power and the time of reaction. The atmosphere and the stirring can also be controlled and all process must be performed in closed system to protect the users from light exposure. A main disadvantage of photoreactors are their cost that is sometimes high for research laboratories. Due to the importance of the use of light in chemistry and material chemistry we are reporting how to construct a low-cost lab-made photoreactor.
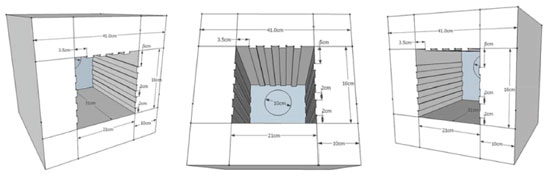
EXPERIMENTAL Design of the lab-made photoreactor The lamps used in photoreactor (PR) were UV-A (Sylvania, 8W) and UV-C (Sankyo Denki, 8W) with the approximate cost of US$ 15.00 each, including the socket. The lamps reactors Econoluz EC-1X8W were used in the PR with the approximate cost of US $ 6.00 each. The timer model CT4S-1P (Autonoics) had a cost of US$ 80.00. The electrical wires, seams, fan, metal plates and acrylics have an estimated cost of US$ 110.00. These reactions were designed to be performed in water. The photoreactor was designed using six steel plates arranged in a rectangular prism shape with dimensions described in Figure 2 . The frontal steel plate has an opening of 336.0 cm2 where a 31.0 cm wide reaction chamber was constructed.
The inner walls of the chamber were made of steel coated with a temperature resistant paint. As can be seen in Figure 3, a timer with a push button and an on/off switch were installed for time monitoring and start of the system, respectively. An acrylic plate of dimensions (30 x 24 cm) coated with black plastic film was used to cover the door to avoid light exposure by the user (Figure 4-A). A pilot lamp was fixed to indicate the on/off operation of the system. Lamps sockets for four lamps (8 W, 30 cm) were installed on each of the lateral and upper walls on the inner side of the chamber (Figure 4-B). Also, a handle was attached to the top of the reactor for handling and transport. (Figure 4-C).
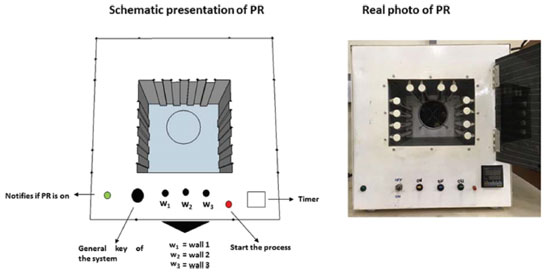 Figure 3. Scheme and digital photo of the lab-made photoreactor used for production of gold nanoparticles and photo-reduction of graphene oxide
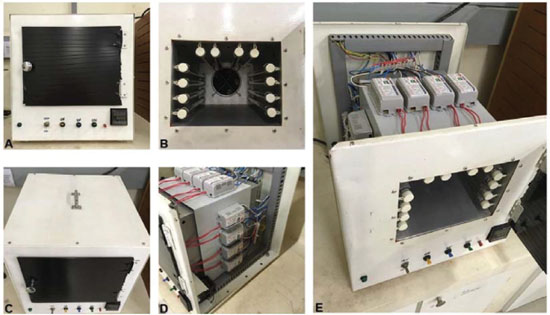 Figure 4. Digital photos of (A) frontal view of lab-made photoreactor with closed door and (B) with opened door, (C) upper view with the handle and (D, E) disassembled reactor showing the parallel arrangement of the reactors of the lamps
The electronic part was designed in such a way that each of the four lamp sets could be triggered individually or simultaneously via switches, modulating the intensity of the emitted light. The lamps reactors were linked in parallel way (Figure 4, D - E). The sockets of the lamps allowed them to be easily replaced with others of different wavelengths, such as UV-A and UV-C. The heat dissipation was performed by an 8 W fan cooler. A 220 V supply powered the whole system. The expert technicians of Institute of Chemistry (UNICAMP) have assisted in the assembly of the photoreactor. Materials Gold (III) chloride trihydrate (HAuCl4.3H2O) was purchased from Sigma-Aldrich. BASF S.A kindly provided photoinitiator IrgacureTM I-2959. Nacional de Grafite provided graphite flakes (Graflake 99580, 99.50% purity). Potassium permanganate (KMnO4), sulfuric acid (H2SO4 reagent grade, 95.0-98.0%) and hydrogen peroxide aqueous solution (H2O2, 30.0 wt%) were purchased from Synth, Brazil. All chemicals were acquired and used as received. Preparation method of gold nanoparticles The preparation of gold nanoparticle (GNP) following the procedure described in literature2. Briefly, 0.33 mmol L-1 gold (III) chloride trihydrate (HAuCl4.3H2O) was mixed with 1.0 mmol L-1 IrgacureTM (1-[4-(2-hydroxyethoxy)phenyl]-2-hydroxy-2-methyl- 1-propane-1-one) I-2959 in water. The mixture was added to 24-well plates and irradiated for 15 minutes in the photoreactor. The Sylvania UV-A lamps were used, since IrgacureTM I-2959 forms radicals upon excitation at λ = 350 nm. The well plates or cuvettes should be placed in the center of the photoreactor to guarantee the homogeneity of light, but the users can define a specific place according to their strategy. An important observation is to put the well plates in the same place to obtain reproducible results. We have obtained good results using a final volume of 50 mL for each batch. Photochemical reduction of graphene oxide Graphite oxide was synthesized using a modified Hummer's method6 and graphene oxide (GO) was obtained by exfoliation of graphite oxide using an ultrasonic bath during 10 minutes. Photochemical reduction of graphene oxide was performed under UV-A and UV-C lamps in the lab-made photoreactor by irradiation of GO (1.0 mg mL-1) for 12 h and 24 h in quartz cuvettes. The well plates also can be used for this purpose. We have obtained good results using quartz cuvettes and a volume of 3 mL per batch. Characterization Spectroradiometer LuzChem SPR-4002 was used to obtain the absorption spectra and power of the UV-A and UV-C lamps. UV-Vis spectra of the synthesized nanomaterials were recorded on a HP Agilent 8453 spectrophotometer.
RESULTS AND DISCUSSION The lab-made photoreactor was powered by UV-A or UV-C for the syntheses of nanomaterials. The emission spectra of UV-A and UV-C spectra are presented in the Figure 5.
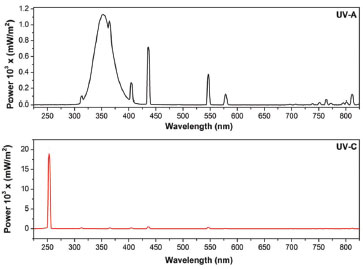 Figure 5. Emission spectra of UV-A and UV-C lamps
The UV-A lamp emits in a broad region between 300 and 850 nm, with the maximum at 350 nm. The UV-C lamp emits the most of its total irradiation at 254 nm. Scaiano et al.2 reported a synthesis of unprotected aqueous gold nanoparticles prepared in minutes with a commercial photoreactor (Luzchem LZC-4V or LZC-ORG). By comparison, we have synthesized gold nanoparticles (AuNP) using the same procedure in our lab-made photoreactor. AuNP were synthesized with photoinitiator IrgacureTM I-2959 in excess to generate enough acetone ketyl radicals to reduce tetrachloroaurate and form nanoparticle.12 Extinction spectra from Figure 6 shows surface plasmon band with an extinction peak close to 530 nm indicating the size close to 12 nm, which has been confirmed by TEM microscopy (an Inset of Figure 6). It was possible to synthesize reproducible gold nanoparticles in a very short period (15 minutes of irradiation) with efficiency comparable to commercial photoreactors, but with a final cost very lower than those available in the market.
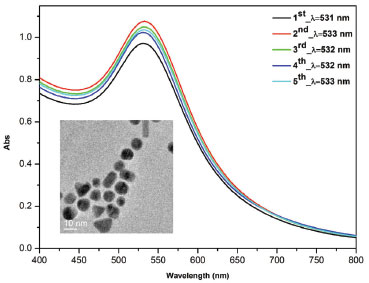 Figure 6. Extinction spectra of AuNP synthesized five times with a 5 minutes interval between each synthesis. The AuNP were prepared with 0.33 mmol L-1 HAuCl4 and 1.0 mmol L-1 Irgacure 2959 in 24-well plates at 15 minutes UV-A exposure. Insert: TEM images of AuNP
Another example of use of the lab-made photoreactor is related to the photochemical reduction of graphene oxide. This process is simple and effective in tuning the percentage of oxygenated groups and consequently the physical and chemical properties of the reduced graphene oxide (rGO). The photoreduction can be accompanied by changes in the electronic spectrum of the graphene oxide. As shown in Figure 7 , the electronic spectrum of the GO has two characteristic bands in UV-Vis region. The first one at 234 nm is assigned to p→π* (transitions of aromatic C=C bonds) and the second one is a shoulder at 310 nm assigned to n→π* (transition of C=O bonds).13 When GO is reduced to rGO, the band at 234 nm undergoes a red-shift and the shoulder at 310 nm disappears.
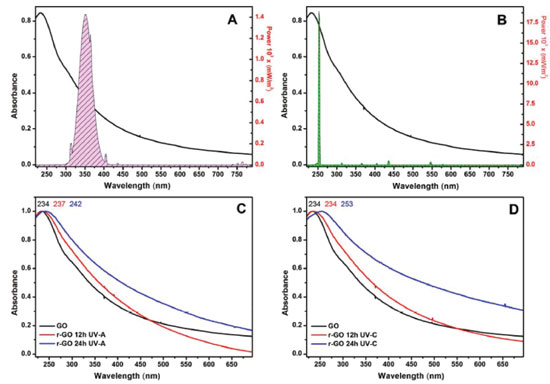 Figure 7. The observed differences between electronic spectra of graphene oxide after irradiated by (A) UV-A lamps and (B) UV-C lamps. UV-Vis absorption spectra of GO before and after 12h and 24h of irradiation with (C) UV-A lamps and (D) UV-C lamps
The reduction degree increases with the irradiation time and is more pronounced for the irradiation by UV-C lamps. Upon excitation by light with enough energy to overcome the value of the bandgap of graphene oxide, electron-hole pairs are generated in the conduction and the valence bands of GO, respectively. The main process observed in the irradiation of GO by UV light is the reduction of GO nanosheets by the photogenerated electrons. On the other hand, the holes produced by light contribute to the oxidation of the C skeleton to CO2, but in minor proportion than the reduction.6,14 The excitation with highly energetic light (UV-C) leads to the formation of a greatly reduced material and reduction level of GO can be controlled by setting the parameter such as time of irradiation, power of the lamp and wavelength.
CONCLUSION We have described a procedure to assemble a lab-made photoreactor with low cost and similar efficiency when compared with commercial one. The pieces and electronic components can be found easily on the market, and an electronic technician or expert in electronics can set up a photoreactor. The size and power of the equipment will depend on the proposed use. The described photoreactor is suitable for synthesis of metallic nanoparticles, as gold nanoparticles, using environmentally friendly approach. The photoreactor also is suitable for controlling the photoreduction level of graphene oxide under mild conditions.
ACKNOWLEDGEMENTS The authors acknowledge: i) Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - grant#459923/2014-5); ii) Fundo de Apoio ao Ensino, à Pesquisa e à Extensão - Universidade Estadual de Campinas (FAEPEX-UNICAMP) grant#2163/15); iii) Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP - grant#2012/18433-8 and FAPESP - grant#2013/22127-2); iv) Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; v) The expert technicians of the Institute of Chemistry - UNICAMP; vi) Paulo Henrique Moda and Rafael T. Mattos from the BASF S. A. by provide kindly the IrgacureTM; vii) Dr. Christopher D. McTiernan and Prof. Tito Scaiano for receiving Prof. Juliano A. Bonacin in his laboratory in a short visit; viii) Brazilian Nanotechnology National Laboratory (LNNano) for TEM measurements (process#TEM-17349).
REFERENCES 1. Scaiano, J. C.; Billone, P.; Gonzalez, C. M.; Marett, L.; Marin, M. L.; McGilvray, K. L.; Yuan, N.; Pure Appl. Chem. 2009, 81, 635. 2. McGilvray, K. L.; Decan, M. R.; Wang, D.; Scaiano, J. C.; J. Am. Chem. Soc. 2006, 128, 15980. 3. Jin, R.; Science 2001, 294, 1901. 4. Stamplecoskie, K. G.; Scaiano, J. C.; J. Am. Chem. Soc. 2010, 132, 1825. 5. dos Santos, P. L.; Dissertação de Mestrado, Universidade Estadual de Campinas, Brasil, 2015. 6. dos Santos, P. L.; Timm, R. A.; Kubota, L. T.; Bonacin, J. A.; ChemistrySelect 2016, 1, 1168. 7. Bonacin, J. A.; Engelmann, F. M.; Severino, D.; Toma, H. E.; Baptista, M. S.; J. Braz. Chem. Soc. 2009, 20, 31. 8. Chen, D.; Appl. Catal., B 1999, 23, 143. 9. Gelover, S.; Mondragón, P.; Jiménez, A.; J. Photochem. Photobiol. Chem. 2004, 165, 241. 10. Correa-Baena, J.-P.; Abate, A.; Saliba, M.; Tress, W.; Jesper Jacobsson, T.; Grätzel, M.; Hagfeldt, A.; Energy Environ. Sci. 2017, 10, 710. 11. Sousa, S. F.; Patrocinio, A. O. T.; Quím. Nova 2014, 37, 886. 12. Gachard, E.; Remita, H.; Khatouri, J.; Keita, B.; Nadjo, L.; Belloni, J.; New J. Chem. 1998, 22, 1257. 13. Dreyer, D. R.; Park, S.; Bielawski, C. W.; Ruoff, R. S.; Chem. Soc. Rev. 2010, 39, 228. 14. Matsumoto, Y.; Koinuma, M.; Ida, S.; Hayami, S.; Taniguchi, T.; Hatakeyama, K.; Tateishi, H.; Watanabe, Y.; Amano, S.; J. Phys. Chem. C 2011, 115, 19280. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access