Artigo
| Processing of spent zinc-MnO2 dry cells in various acidic media |
|
Vinício Francisco IbiapinaI; Ulysses dos Santos FlorentinoI; Júlio Carlos AfonsoI,*; Valdir GanteII; Cláudio Augusto ViannaII; José Luiz MantovanoII
I Departamento de Química Analítica, Instituto de Química, Universidade Federal do Rio de Janeiro, 21941-909 Rio de Janeiro - RJ, Brasil Recebido em 30/08/2017 *e-mail: julio@iq.ufrj.br This paper describes a route for recovering manganese and zinc from spent zinc-MnO2 dry cells via acid leaching. Sulfuric, hydrofluoric and formic acids were used as leachants. Hydrogen peroxide was added as reductant, except for formic acid since it is itself a reductant. Experiments were run at 25-40 ºC for 1-3 h. Under the best optimal conditions, over 95 wt.% of zinc and manganese were leached irrespective of the leachant. Leaching of contaminants was strongly dependent on the leachant due to the insolubility of salts or complexing reactions. Zn(II) was best extracted with D2EHPA diluted in n-heptane at pH > 1, particularly from the leachates of weak acids. Mn(II) was much more co-extracted from sulfuric leachates, but was easily scrubbed with dilute leachant (~2 mol L -1). Zn(II) striping was possible using 5 mol L -1 H2SO4. Manganese was isolated as MnO2 carrying the leached contaminants. High-purity sodium salts of the anions of the leachants were recovered after slow evaporation of the final solution. INTRODUCTION In the last decades the consumption of batteries has increased because of their versatility, low maintenance, reduced cost and high demand from the electronics industry.1,2 The most commonly used types of batteries are the zinc-MnO2 dry cells (Leclanché and alkaline).3-6 They are usually chosen for objects where small quantities of power are required.7 These batteries are not rechargeable and usually run out rapidly.6,8 Disposal of spent zinc-MnO2 batteries has become an environmental challenge. Currently, a significant amount of spent zinc-MnO2 dry cells is still dumped in landfills or even incinerated.3,8-10 They constitute an important source of metal pollution in landfill leachates.1-4,11-13 Incineration releases toxic gases that must be cleaned by off-gas treatment systems; also, recyclable materials are lost.2 Recycling of spent zinc-MnO2 dry cells is the best choice to handle this residue from an environmental point of view, and has also become an urgent matter for resource saving.14-17 They have been regarded as a secondary source of zinc and manganese.9,15,18,19 Due to the rising demand and limited supply from natural sources, manganese and zinc have been listed among the strategic metals by many countries.20,21 Preliminary physical separation methods followed by pyro20,21 or hydrometallurgical treatments4,18,22-24 are applicable to the recovery of valuable metals from zinc-MnO2 dry cells. Pyrometallurgical routes have negative effects on the environment because of emissions, secondary waste streams, energy consumption and hazardous work environments.6,10 Hydrometallurgical routes tend to be less expensive and less energy consuming than pyrometallurgical methods.14,16,19 Sulfuric acid is by far the most used leachant.2,25-32 It provides high zinc recovery whereas most manganese remains in the insoluble residue (Mn(III) and Mn(IV) compounds). A reductant (such as hydrogen peroxide, sulfur dioxide, oxalic acid, carbohydrates etc.) is necessary to bring all manganese to 2+ state, which is soluble and stable in acidic medium.33,34 Hydrochloric acid has also been reported,19,35 but it is oxidized by Mn(III) and Mn(IV) producing dichlorine (Cl2), a toxic gas. Other reported draw-backs of the routes proposed so far are high recovery processing requirements, long time, low value-added products and high chemicals consumption.2,11,21 Therefore, new processes for recycling spent zinc-MnO2 dry cells need to be developed. Apparently, no mention has been made to hydrofluoric acid. Fluoride is a very hard base and forms very stable complexes with cations with noble gas-like configuration (the so-called hard acids). This is generally found in cations with a high charge and a small ionic radius, like Al3+ and Fe3+.36,37 This feature is of particular interest because iron frequently contaminates the acidic leachates, thus making Zn(II) recovery more difficult.17,38 This acid has already been investigated as a leachant for spent catalysts.39,40 Different from many carboxylic acids (citric, malic, succinic, oxalic, iminodiacetic, tartaric),5,21 formic acid has apparently not been tested yet. Like oxalic acid, the simplest aliphatic monocarboxylic acid is a strong reductant, but does not precipitate metal ions as does oxalate.36,37 Therefore, formic acid can act both as a leachant and as a reductant. It has been acknowledged as a versatile renewable reagent for green and sustainable chemical synthesis and processes.41 Leached Zn(II) can be separated from Mn(II) by chemical precipitation,22,24 solvent extraction (SX)2,25-31,42 or electrowinning.16,24 Zn(II) extraction using D2EHPA (di-(2-ethylhexyl)phosphoric acid, a cation exchanger) has been studied since the 1980s. In general, Zn(II) is preferably extracted over Mn(II) in sulfuric acid medium, but the extraction curves may be close to each other according to the experimental conditions.26,28,29,42 SX techniques have become essential to the hydrometallurgical processes due to the growing demand for high purity metals and the need to process low-grade ores with great complexity.42 The aim of this study was to carry out a complete investigation involving leaching of manganese and zinc from spent zinc-MnO2 dry cells under mild experimental conditions on lab-scale using a weak acid with reducing (formic acid) or complexing (hydrofluoric acid) properties. Sulfuric acid was employed for the sake of comparison. Experimental studies were carried out to assess the main factors that affect leaching efficiency. Zinc and manganese were separated by a combination of SX and precipitation techniques. The final solution was processed to recover the sodium salt of the anion of the leachant.
EXPERIMENTAL Materials Spent AA zinc-MnO2 dry cells (alkaline and Leclanché) were employed in this study. The AA format is the most current size employed in Brazil. Their expiration date was between March-July 2014. Alkaline and Leclanché dry cells were processed together. Twenty samples of each were manually dismantled (using gloves, glasses and dust masks). The active components (anode, cathode and electrolyte) were separated from other components such as plastic and paper films, ferrous and non-ferrous scraps and carbon rods. Samples were not calcined in order to recover carbon as insoluble matter. They were fed into a milling machine for size reduction (100% < 1 mm, 30 min). This mass was dried at 40 ºC for 24 h. HF (40 wt.%, ~20 mol L -1), H2SO4 (49 wt.%, ~9 mol L -1), HCOOH (formic acid) (88 wt.%, ~20 mol L -1) and H2O2 (30 wt.%, ~10 mol L -1) were used without further purification. The extractant D2EHPA (di-(2-ethylhexyl)phosphoric acid, Sigma-Aldrich) was used without further purification. n-Heptane (Sigma-Aldrich) was used as diluent. The salts ZnCl2∙6H2O, ZnSO4∙7H2O, ZnF2, Zn(HCOO)2∙2H2O, MnCl2∙4H2O, MnSO4∙H2O, MnF2 and Mn(HCOO)2∙xH2O were of analytical grade and used as received. Leaching procedure The experiments were run in triplicate and carried out in closed Teflon reactors under stirring (200 rotations per minute) for 1-3 h in a fume hood. Equal volumes of H2O2 and HF or H2SO4 or H2O and HCOOH were combined. The solid/liquid ratio was set at 100 g L -1 (10 g mol -1 HF or HCOOH; ~22 g mol -1 H2SO4; 20 g mol -1 H2O2). Initial temperature was 25 ºC. After adding the dried mass, temperature increased by 10-15 ºC after ~1 h in the presence of H2O2. Temperature decreased to 28-30 ºC at the end of the experiment. No thermal effect was observed when formic acid was the leachant. In this case, slow heating was used during 1 h to ~40 ºC, and then temperature was slowly decreased to ~30 ºC at the end of the experiment. Handling of acids (especially HF) and H2O2 was performed using appropriate personal protective equipment. The following equations describe the possible reactions of zinc metal and manganese/zinc oxides with values of ΔGº at 25 ºC:43
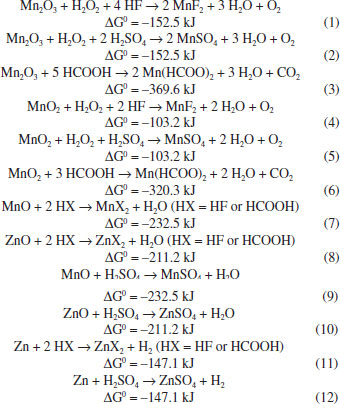 All calculated values of ΔGº are negative. It follows that the reactions occur with high probability in the direction of product formation under the temperature range used, as described earlier. The solid residue was separated from the leachate by filtration under vacuum. It was washed with water until pH 5.5. The washings were added to the leachate. The washed solid was dried at 150 ºC for 3 h, cooled down in a desiccator and weighed. The dried solids were then placed in ceramic crucibles and went through an oxidation step in air (600 °C, 3 h) in a furnace in order to eliminate carbon and other volatile components present. The gaseous effluent of the furnace was passed through distilled water at 25 ºC. The roasted mass was cooled down in the furnace and weighed. Procedure for solvent extraction (SX) All SX experiments were performed at 25 ºC. The aqueous/organic (A/O) phase ratio was set at 1 v/v. D2EHPA concentration varied from 3 to 16 vol.%. pH of the leachate changed from its original value to 4 by adding 6 mol L -1 NaOH. The system was shaken for 10 min. Phase separation was achieved in ~10 min. The experiments were carried out in triplicate. The distribution ratio, DM(II), (M = Zn, Mn) is defined as the ratio of metal ion concentration in organic phase to the metal ion concentration in aqueous phase at reaction equilibrium. Experiments were carried out to assess the influence of Mn(II) on Zn(II) extraction by D2EHPA . A 0.3 mol L -1 Mn(II) salt of the anion of the leachant was contacted with 6 vol.% D2EHPA in n-heptane. The organic phase was then contacted with a 0.3 mol L -1 Zn(II) salt of the same anion. The pH of the aqueous phases was adjusted to the same value as that of the corresponding leachates by adding the appropriate amount of concentrated acid leachant. The experimental conditions were the same of the extraction process. The best conditions to remove Mn(II) and Zn(II) from the organic phase were also investigated. The organic solutions were contacted with aqueous solutions of acid leachants. The experimental conditions were similar to those of the extraction process. Precipitation of Mn(II) The raffinate was added dropwise to 6 mol L -1 NaOH containing 3 mol L -1 H2O2 under stirring (200 rpm) at 25 ºC. Final pH was set at about 10:

At pH above 9 this precipitate is readily oxidized by H2O2:36,37
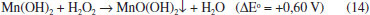
The precipitate was filtered and washed with water (5 mL g -1), dried at 150 ºC for 3 h and weighed. Recovery of sodium salts The final solution contains basically Na + and OH - ions and the anion of the leachant (SO42-, F - or HCOO -). The corresponding acid leachant was carefully added to adjust the pH to the theoretical equilibrium pH of the saturated solution of the salt (Na2SO4 - 7; NaF - 8.5; HCOONa - 9.0).36,44 The treated solution was then slowly evaporated at 60-70 ºC (without stirring). A white crystalline solid was obtained. The solids were weighed and kept in tightly closed containers. Analytical methods The solids, after milling and drying the active components, the insoluble matter isolated after leaching, the ash isolated after calcining the insoluble matter and the solids obtained during the separation procedure were weighed in an analytical balance (Scientech SA 120) and analyzed by x-ray fluorescence (Shimadzu XRF 800HS). Crystalline phases in the solid samples were identified by X-ray powder diffraction (XRPD, Shimadzu model XRD 6000) by continuous scanning method at 20 mA and 40 kV, using Cu Kα as the radiation source. Metal ion concentrations in the leachates were determined by atomic absorption spectrometry (Perkin Elmer AAS 3300). pH measurements were conducted using a combination of a glass electrode and a Ag/AgCl reference electrode (Orion 2AI3-JG).
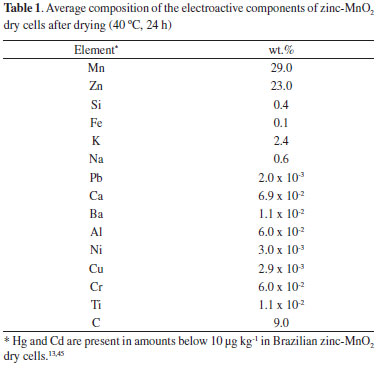
RESULTS AND DISCUSSION Composition of the dried electroactive components Table 1 presents the average composition of the solid after milling and drying the active components of spent zinc-MnO2 dry cells. It must be emphasized that lead has been found in detectable amounts only in Brazilian Leclanché cells.13,22,45
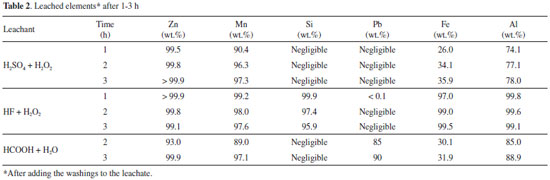
Leaching results The reproducibility of leaching was determined to be about ± 4%. Manganese and zinc were leached with high yields (> 95 wt.%, Table 2) after 1 h (HF) or 3 h (HCOOH and H2SO4). These results are comparable to the best ones reported in the literature20 using strong acids. Leaching with H2SO4 was somewhat longer than the average time reported in the literature,5,7,14,16 but temperature was higher in such studies (40-90 ºC) than in the present work and carbon was not eliminated.
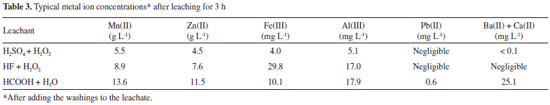
Iron and aluminum were especially leached by HF probably due to formation of complexes36,44 such as [AlF6]3- and [FeF6]3-. Calcium, barium and lead were not found in HF and H2SO4 leachates because their fluorides/sulfates are insoluble or very sparingly soluble in water.36,37,44 Soluble silicon (as [SiF6]2- ions) was found when HF + H2O2 was the leachant.36,37,44 Table 3 presents the concentration of leached species after adding the washing waters. All solutions were pale pink, which is typical of Mn2+aq..36,44 Iron and aluminum were present in very low amounts. Other elements found (< 0.1 mg L -1) were nickel, silicon, copper and chromium. The concentrations shown in Table 3 vary according to the amount of water used to wash the insoluble matter (H2SO4, 80 mL g -1 dried electroactive components; HF, 25 mL g -1; HCOOH, 20 mL g -1). The average pH was 0.9 (H2SO4) and 1.1 (HF and HCOOH). Mn2+ ions are the stable aqueous species over a broad redox potential (Eh) range.33,34
Analysis of the insoluble matter after leaching The amount of insoluble matter (Table 4) increased following HF < H2SO4 < HCOOH. After calcination, the mass loss was essentially the same (~8.5 wt.%), in agreement with the carbon content (~9 wt.%, Table 1), except for formic acid, where mass loss was much higher. The pH of the aqueous solution of the gaseous effluent produced during calcination was always acid and increased accordingly: HCOOH (5.8) < H2SO4 (6.2) ≈ HF (6.3). After adding acetic acid (elimination of CO2) no precipitate or turbidity (CaF2 or BaSO4) was found after adding Ca(NO3)2 or Ba(NO3)2 to the corresponding solutions from the experiments with HF and H2SO4. The acidity came mainly from CO2(aq.). The lowest pH value found for HCOOH suggests an additional source of CO2: the leachant itself. This point requires further investigation, but it is likely that some acid (and/or the formate anion) was strongly adsorbed on carbon; the acid is easily thermally decomposed under oxidant atmosphere:36
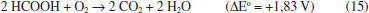
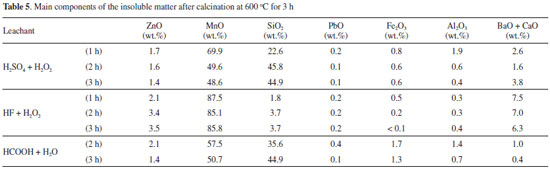
The chemical composition of the insoluble matter after calcination is presented in Table 5. Manganese is the most abundant element. The high amounts of alkali-earth metals in the insoluble matter after experiments with HF and H2SO4 and the low amount of silicon in the experiments with HF correlate with the insolubility of CaF2/BaF2 and CaSO4/BaSO4 in water and the presence of SiF62- ions in the leachate, respectively.
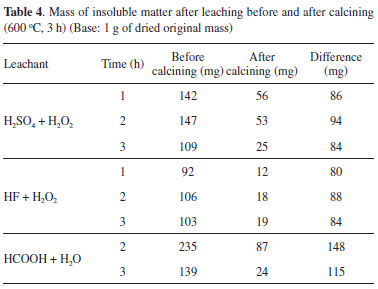
The diffractograms of the insoluble matter show the presence of manganese oxides: Mn3O4 and Mn2O3 (HCOOH, Figure 1A); Mn3O4 (HF, Figure 1B); Mn2O3 (H2SO4, Figure 1C). Mn3O4 is the expected phase when manganese oxides are heated in air at about 500 ºC,46 but tends to be slowly oxidized to Mn2O3 above 560 ºC.24
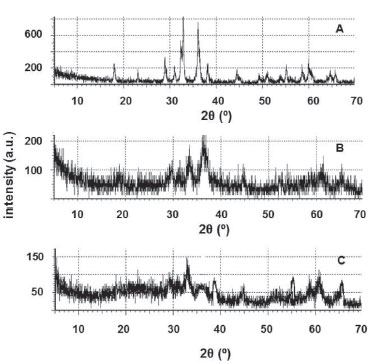 Figure 1. XRPD patterns of the insoluble residue in water after leaching with HCOOH + H2O (A), HF + H2O2(B) and H2SO4+ H2O2(C) followed by calcination at 600 ºC for 3 h
SX of Zn(II) Influence of extractant concentration on Zn(II) extraction In these experiments, the pH of the leachate was not modified. The reproducibility was determined to be within ±5%. Zn(II) extraction increased with the increase of the extractant concentration regardless of the leachant (Figure 2). This result is in agreement with literature data for D2EHPA.25,27,28,31,42
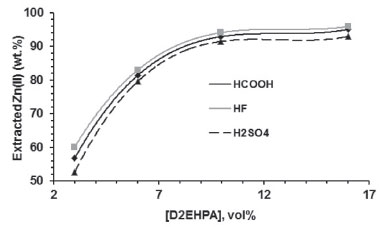 Figure 2. Influence of D2EHPA concentration on Zn(II) extraction in the pH of the leachates (one stage, A/O = 1 v/v, 25 ºC)
The graph log D as a function of log [D2EHPA] (Figure 3) shows that the slope is close to 2 for all leachants. The extraction reaction is well established in sulfuric medium:

 Figure 3. Graph of log D as a function of log [D2EHPA] for Zn(II) extraction in the pH of the leachates (one stage, A/O = 1 v/v, 25 ºC)
Therefore, this reaction is also valid for HF and HCOOH. A net effect of Eq. 16 is the increase of the acidity of the raffinate. Experimental data confirm that the raffinate is more acidic than the original leachate: 0.74 (H2SO4), 0.92 (HF) and 0.95 (HCOOH). Zn(II) was best extracted with D2EHPA from the leachates of the weak acids: HF > HCOOH > H2SO4 (Figure 3). Release of acidity (Eq. 16) to the aqueous phase tends to reduce the extraction rate, but the H3O + ions can restore the original weak acid:36,37
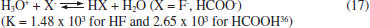
thus removing some of the acidity released by Eq. 16. In general, Zn(II) extraction with D2EHPA at pH 1 is not so effective. Typical pH0.5 values (equilibrium pH at which 50% metal extraction occurs) for Zn(II) are between 0.8-1.3.25,27,30,31 Furthermore, Zn(II) concentration (Table 3) was lower than in most literature studies (usually above 10 g L -1). Mn(II) extraction The increase of extractant concentration also increased Mn(II) extraction (Figure 4). In contrast to Zn(II), Mn(II) was especially extracted from the sulfuric leachates, but was hardly extracted in the presence of HF.
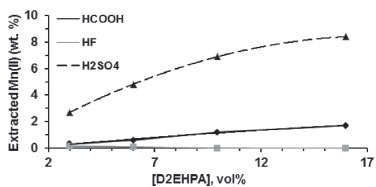 Figure 4. Effect of D2EHPA concentration on Mn(II) extraction in the pH of the leachates (one stage, A/O = 1 v/v, 25 ºC)
A greater amount of Mn(II) was extracted from its 0.3 mol L -1 salts (Table 6) than from the corresponding leachates (Figure 5), but the extraction followed the same trend: H2SO4 >> HCOOH > HF. From data in Table 6, more than 90 wt.% of Mn(II) in the organic phase was displaced by 0.3 mol L -1 aqueous Zn(II) in all experiments. These results confirm a preferential extraction of Zn(II) over Mn(II) by D2EHPA under our experimental conditions.
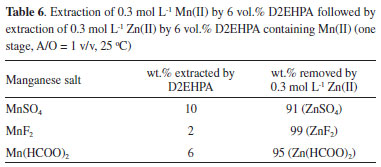
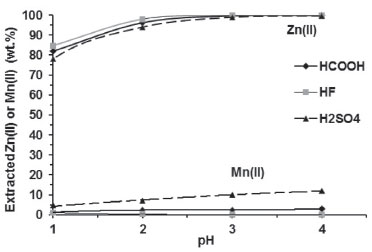 Figure 5. Influence of acidity on Zn(II) and Mn(II) extraction with 6 vol.% D2EHPA (one stage, A/O = 1 v/v, 25 ºC)
Influence of leachate pH on Zn(II) and Mn(II) extraction D2EHPA concentration was fixed at 6 vol.%. Zn(II) extraction increased as the pH of the leachants increased (Figure 5) regardless of the leachant. This is the normal behavior found in sulfuric medium.25,27,30,31 More than 95 wt.% of Zn(II) was extracted in one stage at pH 2 (HF and HCOOH) and 2.5 (H2SO4). More Mn(II) was extracted as pH increased (H2SO4 >> HCOOH > HF), also in agreement with literature data.2 Removal of Mn(II) and Zn(II) from the organic phase Mn(II) was easily scrubbed (> 99.5 wt.%) in one stage using a dilute leachant (1-2 mol L -1, Figure 6). A similar result has already been reported in the literature.26 Zn(II) was not removed. The aqueous solution was added to the raffinate.
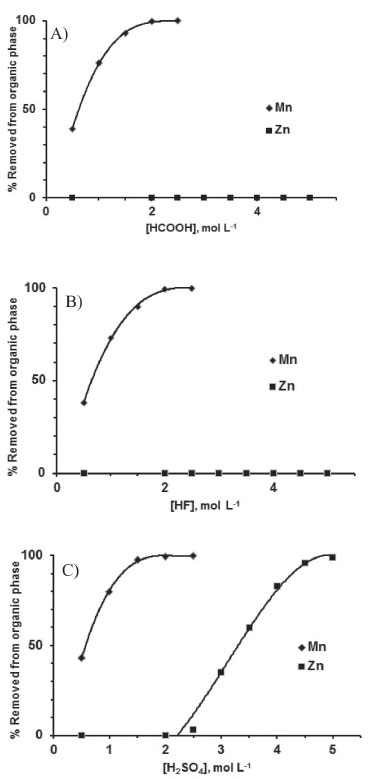 Figure 6. Removal of Zn(II) and Mn(II) from the organic phase with aqueous leachants at variable concentrations (one stage, A/O = 1 vol./vol., 25 ºC)
In contrast to Mn(II), the weak acids were not able to remove Zn(II) from the organic phase irrespective to their concentration. 2 mol L -1 H2SO4 began to strip it.2,26,27,31 More than 99.5 wt.% was stripped in one stage using 5 mol L -1. H2SO4 concentration should not surpass 8 mol L -1 to avoid emulsification. Manganese was not detected in the aqueous solution. Mn(II) precipitation Manganese dioxide comprises over 98.5 wt% of the solid precipitated at pH ~9. The impurities come from the remaining Zn(II) (0.2-0.5 wt.%), the other leached elements (Fe, Al - 0.4-0.5 wt.%) and, occasionally, Pb, Ca, Ba and Si (0.3 wt.% maximum). Zn(II), Al(III) and Pb(II) form complexes with hydroxide ions at high pH.36,37,44 At pH ~9 such reactions were avoided, which led to the precipitation of these elements with manganese. Its diffractogram shows that the solid containing manganese is amorphous. Recovery of the sodium salt of the anion of the leachant The diffractograms shown in Figure 7 contain peaks corresponding to a single anhydrous salt. XRF data show the absence of zinc, manganese and other metals found in the leachates (they were precipitated with manganese). This is of special importance for the recovery of high value-added salts (NaF and HCOONa). After drying the solids at 150 ºC for 3 h, the mass loss was lower than 3 wt.%, thus confirming that all salts were anhydrous.
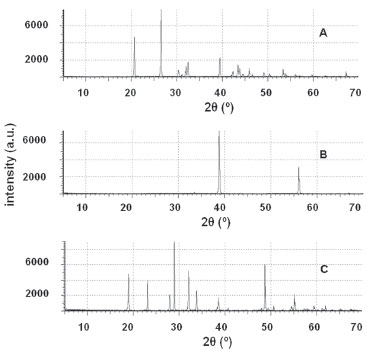 Figure 7. XRPD patterns of the solids recovered after slow evaporation of the final solution. The peaks represent HCOONa (A), NaF (B) and Na2SO4(C)
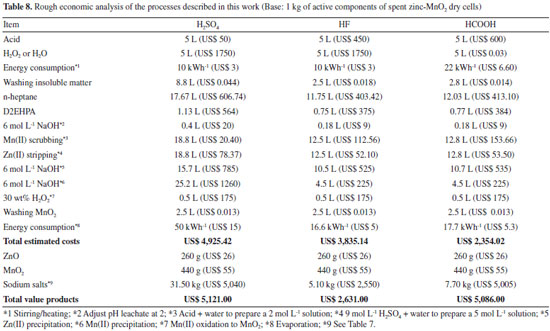
Rough economic analysis No detailed study on economics was performed, but some insight can be obtained based on the market value of the reactants, energy, water and products. The present average prices (for the same purity grade) in Brazil for bulk quantities are:47 49 wt.% (9 mol L -1) H2SO4, US$ 10 L -1; 40 wt.% (20 mol L -1) HF, US$ 90 L -1; 88 wt.% (20 mol L -1) HCOOH, US$ 120 L -1; 30 wt.% (~10 mol L -1) H2O2, US$ 350 L -1; D2EHPA, US$ 500 L -1; n-heptane, US$ 103 L -1; 6 mol L -1 NaOH, US$ 50 L -1; water, US$ 5 m -3 (including taxes); energy, US$ 0.30 kWh -1 (including taxes); Na2SO4 (> 99.5 wt.%), US$ 160 kg -1; NaF (> 99.5 wt.%), US$ 500 kg -1; HCOONa (> 99.5 wt.%), US$ 650 kg -1; ZnO (99.5 wt.%), US$ 100 kg -1; MnO2 (technical grade, 95-98 wt.%), US$ 125 kg -1. Carbon was not included in this study because of its very low price (~US$ 0.10 kg -1) and the low amount recovered (84-148 g kg -1 active components, Table 4). Table 8 presents the estimated costs and the market value of the products obtained in this study (base: 1 kg active components, 6 vol.% D2EHPA as extractant, three uses of the extractant solution and Zn(II) stripping with 5 mol L -1 H2SO4).
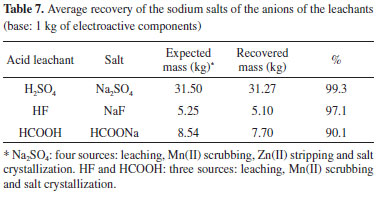
The revenue of the process decreased following the order HCOOH > H2SO4 > HF >. Although formic acid was the most expensive acid employed, the absence of H2O2 as co-reactant during leaching greatly reduced the cost of the leachant. Despite its losses (Table 7) the recovery of high purity grade sodium formate also increased the revenue because this product presents the highest market value among all salts. The process using H2SO4 (the cheapest acid) also presented favorable revenue. However, the high water consumption during washing of the insoluble residue increased the costs for Zn(II) extraction, Zn(II) stripping, Zn(II) precipitation and Mn(II) precipitation, thus reducing its overall revenue. The process using HF appears not to be viable. The leachant containing HF + H2O2 was the most expensive and the amount of recovered NaF (Table 7), despite its high commercial value, did not compensate the costs involving the use of this acid. The contribution of recovered ZnO and MnO2 to the revenue was very low. Although the contribution of carbon to the revenue was nil, its elimination via controlled burning (before leaching) would impact costs due to high energy consumption.20,24 If MnO2 is purified to a grade > 99 wt.% its price increases 5-8 fold in comparison to the technical grade product. On the other hand, if impurities were brought into the sodium salts, their market value would decrease by a factor of 10-15, making all processes not economically viable. At present, considering other costs (labor, equipment etc.), it is likely that the revenue will partially come from money (price surcharge) that consumers will pay for recycling batteries.24
CONCLUSIONS Over 95 wt.% of zinc and manganese were leached from the electroactive components of spent zinc-MnO2 dry cells under mild experimental conditions in the presence of a weak acid and a reductant. Formic acid effectively served the dual role of leachant and reductant as HF or H2SO4 + H2O2 mixtures. Leaching was fastest in the presence of hydrofluoric acid. Precipitation and complexation reactions influenced leaching of minor elements present in the electroactive components. The insoluble matter corresponded to carbon and non-leached elements except for formic acid, where an additional volatile mass was found. More than 95 wt.% of Zn(II) was extracted by D2EHPA in one stage (6 vol.%, A/O = 1 v/v, 25 ºC) at pH 2 following the order HF > HCOOH > H2SO4. Mn(II) extraction from leachates of weak acids was the lowest. Therefore, hydrofluoric or formic acids are alternative leachants for processing spent zinc-MnO2 dry cells. The effect of pH and D2EHPA concentration on Zn(II) extraction were the same regardless of the leachant. Extracted Mn(II) was easily scrubbed with 2 mol L -1 leachant. Zn(II) stripping was only possible using a strong acid (5 mol L -1 H2SO4). High purity crystalline sodium salts of the anions of the leachants were obtained after precipitation of Mn(II) and pH adjustment of the final solution followed by slow evaporation. Recovery of these salts reduced the amount of final wastes.
SUPPLEMENTARY MATERIAL Figure 1S presents the general scheme for the recovery of zinc and manganese from spent zinc-MnO2 dry cells after acid leaching in the presence of a reductant. It is available for download at http://quimicanova.sbq.org.br in pdf format with free access.
ACKNOWLEDGEMENTS V. F. Ibiapina and U. S. Florentino acknowledge PIBIC/CNPq-UFRJ for the fellowship. We are grateful to CNPq for financial support.
REFERENCES AND NOTES 1. Biswas, R. K.; Karmakar, A. K.; Kumar, S. L.; Waste Manage. 2016, 51, 174. 2. Biswas, R. K.; Karmakar, A. K.; Kumar, S. L.; Waste Manage. 2016, 51, 149. 3. Xará, S. M.; Delgado, J. N.; Almeida, M. F.; Costa, C. A.; Waste Manage. 2009, 29, 2121. 4. Ippolito, N. M.; Belardi, G.; Medici, F.; Piga, L.; Waste Manage. 2016, 51, 182. 5. Sobianowska-Turek, A.; Szczepaniak, W.; Zabłocka-Malicka, M.; J. Power Sources. 2014, 270, 668. 6. Owais, A.; Gepreel, M. A. H.; Ahmed, E.; Hydrometallurgy 2015, 157, 60. 7. Karnchanawong, S.; Limpiteeprakan, P.; Waste Manage. 2009, 29, 550. 8. Xará, S. M.; Almeida, M. F.; Costa, C.; Waste Manage. 2015, 43, 460. 9. Belardi, G.; Medici, F.; Piga, L.; J. Power Sources. 2014, 248, 1290. 10. Deep, A.; Sharma, A. L.; Mohanta, G. C.; Kumar, P.; Kim, K. H.; Waste Manage. 2016, 51, 190. 11. Ma, Y.; Cui, Y.; Zuo, X.; Huang, S.; Hu, K.; Xiao, X.; Nan, J.; Waste Manage. 2014, 34, 1793. 12. Komilis, D.; Bandi, D.; Kakaronis, G.; Zouppouris, G.; Sci. Total Environ. 2011, 409, 2555. 13. Câmara, S. C.; Afonso, J. C.; da Silva, L. I. D.; Domingues, N. N.; Alcover Neto, A.; Quim. Nova 2012, 35, 82. 14. Buzatu M.; Săceanu, S.; Petrescu, M. I.; Ghica, G. V.; Buzatu, T.; J. Power Sources 2014, 247, 612. 15. Chen, S.; Guo, G.; Liu, F.; Solid State Ionics 2014, 261, 59. 16. Cruz-Díaz, M. R.; Torres, Y. A.; Caballero, F.; Lapidus, G. T.; González, I.; J. Power Sources 2015, 274, 839. 17. Nogueira, C. A.; Margarido, F.; Hydrometallurgy 2015, 157, 13. 18. Kim, T. H.; Senanayake, G.; Kang, J. G.; Sohn, J. S.; Rhee, K. I.; Lee, S. W.; Shin, S. M.; Hydrometallurgy 2009, 96, 154. 19. Baba, A. A.; Adekola, A. F.; Bale, R. B.; J. Hazard. Mater. 2009, 171, 838. 20. Sayilgan, E.; Kukrer, T.; Civelekoglu, G.; Ferella, F.; Akcil, A.; Veglio, F.; Kitis, M.; Hydrometallurgy 2009, 97, 158. 21. Xin, B.; Jiang, W.; Aslam, H.; Zhang, K.; Liu, C.; Wang, R.; Wang, Y.; Bioresour. Technol. 2012, 106, 147. 22. Quintanilha, C. L.; Afonso, J. C.; Vianna, C. A.; Gante, V.; Mantovano, J. L.; J. Power Sources 2014, 248, 596. 23. Rácz, R.; Ilea, P.; Hydrometallurgy 2013, 139, 116. 24. Ferella, F.; Michelis, I.; Veglio, F.; J. Power Sources. 2008, 183, 805. 25. Balesini, A. A.; Razavizadeh, H.; Zakeri, A.; J. Chem. Eng. 2011, 8, 43. 26. Haghighi, H. K.; Moradkhani. D.; Salarirad, M. M.; Hydrometallurgy, 2015, 154, 9. 27. Gharabaghi, M.; Irannajad, M.; Azadmehr, A. R.; Physicochem. Probl. Miner. Process. 2013, 49, 233. 28. Vahidi, E.; Raschi, F.; Moradkhami, D.; Miner. Eng. 2009, 22, 204. 29. Lum, K. H.; Stevens, G. W.; Kentish, S. E.; Hydrometallurgy 2014, 142, 108. 30. Larsson, K.; Ekberg, C.; Odegaard-Jensen, A.; Hydrometallurgy 2012, 129-130, 35 31. Jha, M. K.; Kumar V.; Jeong, J.; Lee, J. C.; Hydrometallurgy 2012, 111-112, 1. 32. El Dessouky, S. I.; El-Nadi Y. A.; Ahmed, I. M.; Saad, E. A.; Daoud, J. A.; Chem. Eng. Process. 2008, 47, 177. 33. Takeno, N.; Atlas of Eh-pH diagrams, intercomparison of thermodynamic databases. National Institute of Advanced Industrial Science and Technology: Tokyo, 2005. 34. Hem, J. D.; Chemical Equilibria and Rates of Manganese Oxidation, The US Government Printing Office: Washington, 1963. 35. Li, Y.; Xi, G.; J. Hazard. Mater. 2005, B127, 244. 36. Lurie, J.; Handbook of Analytical Chemistry, 3rd ed., Mir: Moscow, 1978, chap. 3, 6 and 10. 37. Feigl, F.; Spot Tests in Inorganic Analysis, Elsevier: Amsterdam, 1958, chap. 3. 38. Formanek, J.; Jandova, J.; Capek, J.; Hydrometallurgy 2013, 138, 100. 39. Lima, T. S.; Campos, P. C.; Afonso, J. C.; Hydrometallurgy 2005, 80, 211. 40. Pinheiro, A. A. S.; Lima, T. S.; Campos, P. C.; Afonso, J. C.; Hydrometallurgy 2004, 74, 77. 41. Liu, X.; Li, S.; Liu, Y.; Cao, Y.; Chin. J. Catal. 2015, 36, 1461. 42. Gouvea, L. R.; Morais, C. A.; Miner. Eng. 2010, 23, 492. 43. Roine, A.; HSC Chemistryr ver. 6.1, Outotec Research Oy, Finland, 2010. 44. Greenwood, N. N.; Earnshaw, A.; Chemistry of the Elements, 2nd ed., Elsevier Butterworth-Heinemann: London, 2010, chap.10. 45. Silva, B. O.; Câmara, S. C.; Afonso, J. C.; Neumann, R.; Alcover Neto, A.; Quim. Nova 2011, 34, 812. 46. Alguacil, F. J.; Martinez, S.; J. Chem. Technol. Biotechnol. 2001, 76, 298. 47. Average prices were obtained from quotations provided by five different chemical suppliers in July 2017. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access