Artigo
| Adenanthera pavonina galactomannan for controlled delivery of rutin - a preliminary study |
|
Karine A. NobreI; Carlos E. A. SoaresII; Icaro G. P. VieiraIII; Raimundo R. de AlmeidaI; Renato de A. MoreiraIV; Tamara G. de AraújoV; Maria E. N. P. RibeiroI,*; Nágila M. P. S. RicardoI
I. Departamento de Química Orgânica e Inorgânica, Centro de Ciências, Universidade Federal do Ceará, 60455-760 Fortaleza - CE, Brasil Recebido em 02/11/2017 *e-mail: elenir.ribeiro@ufc.br Improving the development of modified release matrix systems strictly depends on selecting an appropriate agent capable of controlling the drug's release. In this study, Adenanthera pavonina galactomannan was investigated as a potential basis for developing a controlled release of rutin. This galactomannan was extracted, purified and characterized by GPC, NMR and FT-IR spectroscopy. The mannose/galactose ratio (M/G) of 1.46 was calculated by the relative area from signals of the anomeric region of 1H-NMR spectrum. The main chain of this galactomannan consists of β(1→4)-D-mannose and α(1→6)-D-galactose linked units (1H-decoupled 13C NMR, 13C DEPT 135º NMR and HSQC spectra). The sustained-release tablets containing rutin and galactomannan were compared with the extensively investigated hydrophilic matrix HPMC. Drug release analysis revealed similar release mechanism for both matrices. A new formulation for controlled drug delivery from water-swellable matrix systems is proposed. INTRODUCTION Controlled release dosage forms have made significant progress in terms of clinical efficacy and benefits to patients due to a number of decreased drug doses, fewer side effects and reduced toxicity.1 Considerable attention has been focused on hydrophilic polymers in drug delivery systems,2 in which mechanisms of diffusion, swelling and erosion influence the drug release rate.3,4 Controlled administration of drugs from swelling matrix (hydrogel) systems has been used in the pharmaceutical industry for more than 40 years. Such delivery systems are widely used to control drug release because of their low cost, ease of manufacture, favourable in vivo performance and their use in controlling the release of drugs with a wide range of physico-chemical properties.5-7 Natural gums (galactomannans) may also be modified to have materials tailored for drug delivery systems and thus may compete with the available synthetic biodegradable excipients. A study with galactomannan from fenugreek8 showed that it is an excellent candidate to be used for industrial purposes. This galactomannan depicted excellent functional attributes which included oil-holding capacity, foaming capacity, swelling index and foaming stability. SEM depicted a smooth and glittered surface which is the most important feature for film-making. Another study showed that nanoparticles form galactomannan could be a safe and promising tool for ocular drug delivery.9 A polysaccharide and its sulphated A. pavonina derivative have been evaluated for anti-HSV and poliovirus type 1 (PV-1) activity in HEp2 cell cultures.10 The sulphated compound inhibited HSV-1 infection at different replication stages, and thus represents a valuable compound for preclinical studies in anti-herpetic therapy.10 The sulphated compound also inhibited PV in more than one replication step as an antiviral action mechanism. The authors therefore selected the compound as a potential candidate for further development for infection control. Based on this, the judicious combination of polymers in certain concentrations may constitute the matrix of a very promising drug delivery system.11 Rutin belongs to the bioflavonoid category and is a powerful antioxidant with pharmacological benefits including anti-inflammatory, antiallergic, neuroprotective, antiulcer behaviour in vivo and antiproliferative and antimutagenic activity in vitro.12 Despite its beneficial effects, it suffers from the problem of low aqueous solubility (125 mg L-1) which is responsible for low oral bioavailability. Thus, many strategies have been used to overcome this characteristic such as pH sensitive hydrogel.13 In this study, galactomannan sustained-release rutin tablets were compared to the hydrophilic matrices of hydroxypropylmethylcellulose (HPMC), a polymer which has been extensively investigated to control the drug release of the dosage forms due to biocompatibility, biodegradability and safety.1 The in vitro drug, rate of release, as well as the release mechanism in the developed matrix systems were observed. The characterization of galactomannan from A. pavonina was done using a unique NMR study and FT-IR spectroscopy technique.
EXPERIMENTAL Materials Rutin has a molecular weight of 610.5 g mol-1 and was supplied by Flora Brasil Ltda. Mature seeds from Adenanthera pavonina (L.) were collected at Campus do Pici, Universidade Federal do Ceará, Fortaleza - CE, Brazil, during the months of November and December in 2008. Galactomannan was isolated from Adenanthera pavonina seeds and used as polymeric carriers. Microcrystalline cellulose, magnesium stearate and colloidal silicon dioxide, all of USP grade, were supplied by DEG, Brazil. Hydroxypropylmethylcellulose (Methocel K4M CR) was supplied by Colorcon Limited, USA. Galactomannan extraction The process of galactomannan isolation from the endosperm of A. pavonina seeds was based on the method reported by Vieira et al.14 100 g of seeds were boiled in distilled water (for 20-30 min) to inactivate endogenous enzymes and hydrate the endosperm. The seeds were thereafter kept at room temperature for about 24 h or until they reached about double their original size. The hulls and germs were disposed of and the endosperms were removed manually. About 10 g of endosperms were used to extract the galactomannans using hot distilled water (at 85-100 °C). The endosperms were kept in the hot water for about 3 h to liberate the polysaccharides. Next, the viscous solution was filtered with nylon net to remove any impurities or large particles. The procedure was repeated several times until the gel was workable. The viscous solution was filtered again using celite and then lyophilized. Molecular weight determination The elution volumes were determined by Gel Permeation Chromatography (GPC) using a Shimadzu LC-10AD chromatograph with an RID-10A refractive index detector at room temperature. The analysis was performed with an ultrahydrogel linear column (7.8 mm x 300 mm), flow rate of 0.5 mL min-1, polysaccharide concentration of 0.1% (w/v) dissolved in water and 0.1 mol L-1 NaNO3 was used as eluent. The sample volume injected was 20 µL and the molecular weight was determined using a calibration curve with pullulan standards (Shodex Denko®; Mw of 5.9 x 103 to 7.88 x 105 g mol-1). Nuclear Magnetic Resonance Spectroscopy (NMR) NMR analysis was based on the method reported by Vieira et al.14 A galactomannan sample was dissolved (20 mg mL-1) in D2O (99.96D) at 80-85 °C. The spectra were obtained at 85 °C using a Bruker Spectrospin Avance DRX-500 spectrometer, with an inverse-detection multinuclear probe equipped with z-shielded gradient coils, and a Silicon Graphics computer. 1H NMR spectra were obtained using acquisition and delay times of 1.0 s and a flip angle of 90° to achieve the conditions for quantitative analysis. Peak integrals were calculated using the Bruker software. For 13C NMR, as well as the basic 1-D spectrum, a Distortionless Enhancement by Polarization Transfer (DEPT 135) spectrum was recorded in order to determine the hydrogenation of each carbon; the acquisition and delay times were 1.0 s. A 1H-13C Heteronuclear Single-Quantum Coherence (HSQC) 2-D spectrum was obtained using the pulse program supplied with the apparatus. FT-IR spectroscopy The Fourier Transform Infrared spectrum (FT-IR) was recorded with a Shimadzu IR spectrophotomer (model 8300) between 400 and 4000 cm-1. The sample was analyzed as KBr pellets. The experiment was repeated two times. Matrix tablet preparation The best result for the manufacture of prolonged release tablets containing Galactomannan was chosen based on several tested formulations from previous studies.3,15-17 The same was compared to the formulation containing HPMC in the same amount. Matrix rutin tablets (10 mg dose) were prepared by direct compression method. Magnesium stearate and colloidal silicon dioxide were used as lubricant; microcrystalline cellulose as filler-binder; galactomannan and hydroxypropylmethylcellulose (HPMC) as polymer carriers. The total weight was set at 150 mg. Table 1 shows the different studied formulations. All ingredients with the exception of lubricants were blended for 10 min, and then magnesium stearate and colloidal silicon dioxide were added and mixed for 5 min. Tablets were compressed on a single punch tableting machine (type K5, Kilian GmbH, Füllinsdorf, Germany) in order to obtain 60 to 70 N hardness (tablet hardness tester type TB 42 Erweka, Frankfurt, Germany). The average weight of the tablets was 150 mg ± 0.2.
In vitro drug release study The release experiments (six tablets) were performed in a USP 23 dissolution apparatus 2 (Sotax AG, Basel) as a function of time. The matrix tablets were subjected to the paddle dissolution method using 900 mL of distilled water as the dissolution medium, and at sink condition. The dissolution test was performed at 100 rpm and the temperature was set at 37 °C ± 1 °C. 5 mL samples were withdrawn at predetermined time intervals over an 8-hour period and spectrophotometrically assayed at 359 nm. An equal volume (5 mL) of distilled water was replaced after each sampling. Three replicates were performed for each determination and the mean values were employed to obtain the release profiles. The cumulative percentage of dissolved rutin was calculated using a regression equation generated from the standard data. Release rates from controlled release polymeric matrices can be studied using zero-order model (Eqs. (1) and (2) proposed by Higuchi,18 and Eq. (3) proposed by Korsmeyer et al.19 Linear and non-linear least squares fitting methods were used to determine the optimum values for the parameters present in each equation:  where Q is the amount of drug remaining at time t; k is the zero order release constant on Eq. (1) and the Higuchi rate constant on Eq. (2), k1 is the Korsmeyer-Peppas kinetic constant; n is a diffusional exponent that depends on the release mechanism and on the shape of the tested swelling device.20 For cylindrical shaped matrices, values of n = 0.5 indicate Fickian release, values of 0.5 < n < 1.0 indicate an anomalous (non-Fickian or couple diffusion/relaxation) drug release, whereas values of n = 1.0 indicate a case II (purely relaxation controlled) drug release.21
RESULTS AND DISCUSSION Galactomannan isolation and purification There are several methods that employ organic solvents, ethanol or mechanical pressing of all seed.22 However, galactomannan is not completely precipitated from the solution due to the presence of low molecular weight fractions which are soluble at high alcohol concentrations. The isolated endosperm of A. pavonina seeds contain the water soluble galactomannan, while the rest of the seed coat and embryo mainly contain pentoses.14 Both sides of the endosperm were manually separated from the seed coat and embryo and submitted to hot aqueous (lower than 100 °C) extractions. Thus, the main substances present in the seed coat and embryo (proteins, lipids, crude fibers and xylose and pentose polyssacharides) that could contaminate the polysaccharides were separated from the endosperm before galactomannan extraction. Such a procedure avoids contamination of the galactomannan with pentose and xylose, which are the main impurities in the galactomannans. We used temperatures lower than 110 °C to avoid decomposition and hydrolysis of the galactomannan in the aqueous extraction process. Thereafter, the galactomannan were obtained in a very pure form after freeze-drying of the filtrated product. The yield of the pure galactomannan was 13% (in relation to seed mass) from this process. This value is similar to the galactomannan yields reported in the literature obtained from A. pavonina (17.1 wt.%) in work by Cerqueira et al.23 and other seeds such as Caesalpinia pulcherrima (25 wt.%),24 Gleditsia triacanthos (15.4 wt.%),25 Gleditsia ferox (18.9 wt.%)26 and Prosopis juliflora (10-14 wt.% and 16 wt.%).14,27 We are looking for substitutes for the commercial gums mentioned using plants that can produce these polysaccharides locally. Even with lower yields it would still be easier and cheaper to use them than to use commercial gums. Figure 1 shows the chromatogram of A. pavonina galactomannan and the Mw, Mn values and Mw/Mn ratio was 3.9 x 106, 7.6 x 105 and 5.13, respectively. These results are similar to other galactomannans related in the literature.27
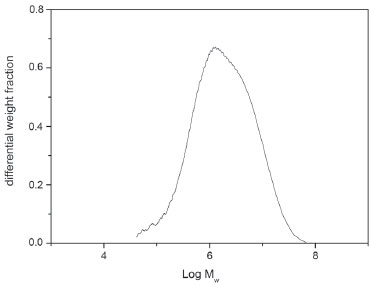 Figure 1. GPC chromatogram for A. pavonina representing the differential weight fraction vs log Mw
Nuclear Magnetic Resonance Spectroscopy (NMR) 1D and 2D NMR analyses were employed to investigate the A. pavonina polysaccharide structure. The 1H-NMR and 13C-BB spectra are shown in Figure 2. The resonances of proton and carbon were assigned based on data reported in the literature for galactomannans.14,25,26,28-31
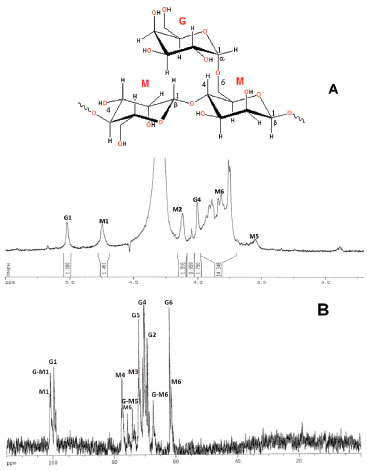 Figure 2. 1H-NMR (A) and 13C-BB NMR (B) spectra of solution (20 mg mL-1) in D2O of galactomannan from Adenanthera pavonina (L.)
We investigated A. pavonina galactomannan using one-dimensional 1H and 13C NMR spectroscopy and two-dimensional (2D) heteronuclear1H/13C HSQC. Two signals at δ H 5.02 and 4.74 ppm were observed in the region for resonance of anomeric protons in the 1H NMR spectrum. These δ H values were assigned to galactose and mannose residues, respectively, and the mannose/galactose (M/G) ratio was directly obtained from the relative areas of the signals.4 The ratio M/G and the statistical distribution of galactosyl residues along with the mannan backbone are of chemotaxonomical value, varying among species. In fact, the three subfamilies of the Fabaceae (Caesalpinioideae, Mimosoideae and Faboideae) can be distinguished by the M/G ratios of their galactomannan seeds.32,33 Moreover, M/G ratio influences galactomannan properties such as viscosity, solubility, gel-forming, and others. This value is frequently used to characterize new gums when they are compared with commercial gums, for example locust bean, guar and tara gums.24 The 1H NMR spectrum (Figure 2A) is somewhat complex and the signals from the anomeric protons at 5.02 and 4.74 ppm were assigned to H-1 of galactose (G1 residue) and mannose (M1 residue), respectively. The M/G ratio found was 1.46. Our results were in accordance with Cerqueira et al.23 which found a value of 1.35 for the M/G ratio. The neighbour signal at 4.74 ppm arises from H-1(M1) which corresponds to the β-D-mannopyranose units. The 125 MHz, 13C-BB spectrum of A. pavonina galactomannan is illustrated in Figure 2B. The 13C-spectrum was well resolved and its assignments were readily identified. The anomeric region (95‑110 ppm) shows three signals (100.78, 100.62 and 99.48 ppm), which were assigned as C-1 of galactose at 99.48 ppm (G1 residue), C-1 of β-D-mannopyranosyl residue branched at HO-6 at 100.62 ppm (G-M1 residue) and C-1 of mannose at 100.78 ppm (M1 residue).34 The signal from C-1(G1) indicates that galactose residues are in the pyranose form and a similar conclusion may be made for mannose. C-6(M6) showed a second signal shifted by +5.86 ppm, indicating substitution at C-6.25,26,28-30 All different carbon lines are resolved, and their chemical shifts are presented in Table 2.
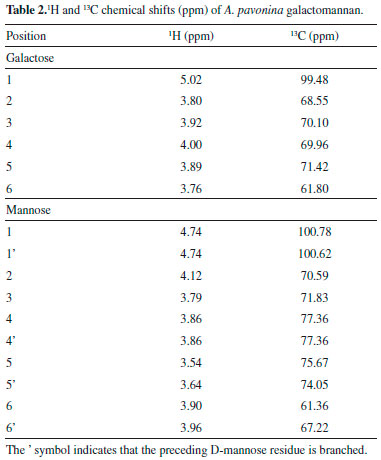
The resonances associated with the galactose and mannose residues were distinguished by making use of the different monomeric compositions of the samples, as determined by 1H-NMR.28 The region of methylene carbons (61.80 and 61.36 ppm) is also well depicted (Table 2). A 13C DEPT 135º experiment was recorded to investigate the presence of 6-O-substitution on mannose residue (Figure 3). The pulse sequence signals of the carbons bearing two protons have opposite amplitude to the CH and CH3 carbons, and the O substitution of carbon leads to a displacement in the range of 6-11 ppm.31 A low field displacement of around 6.12 ppm was observed for the d-value of 6-O substituted mannose in relation to the assignment of the unsubstituted mannose residue for the galactomannan.29
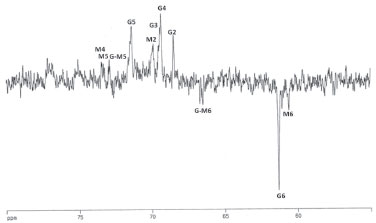 Figure 3. DEPT spectrum (135º) of solution (20 mg/mL) in D2O of galactomannan from Adenanther apavonina (L.). CH carbons are positive and CH2 carbons are negative
Analysis of the positions and fine structure of correlation peaks in the 2D spectrum showed that the polymer consists of manno- and galactopyranose units. The resonance for H-1 of mannopyranose units (4.74 ppm) is consistent with their β-configuration and the chemical shift of the galactopyranose H-1 units (5.02 ppm) with the α-configuration. The 13C NMR spectrum of isolated galactomannan contained resonances for three anomeric C atoms (100.78, 100.62 and 99.48 ppm). Signals in the 13C NMR spectrum (Table 2) were also assigned by analysing the HSQC spectrum and were confirmed by a strong signal of all mannopyranose units on C-4 and C-6. The magnitude of the chemical shifts for C-5 of mannopyranose units suggested their β-configuration. The strong-field chemical shift of C-1 (99.48 ppm) was consistent with the α-configuration of galactopyranose units. The DEPT 135º spectrum of A. pavonina galactomannan has three CH2 signals. The signals at 61.80 and 61.36 ppm were attributed to the C-6 of primary carbons (unsubstituted) of galactose and mannose residues, respectively. The CH2 signal at high frequency (67.22 ppm) was assigned to C-6 of β-D-mannopyranose substituted at this position by the α-D-galactose unit. Thus, we can suggest that the studied A. pavonina polysaccharide is a galactomannan. The 2D spectrum (HSQC) of A. pavonina gum is shown in Figure 4. The HSQC spectrum shows the correlation of the carbons with their respective protons. Their assignments are given in Table 2. The H-1/C-1 correlation for galactose (G residue) is shown at δ 5.02/99.48. The H-1/C-1 correlation for the mannose residue in the HSQC spectrum is well resolved and two signals at 4.74/100.78 ppm and 4.74/100.62 ppm were detected and assigned as unbranched (M) and branched (G-M) units, respectively.29 The three C-6 atoms at opposite amplitudes in the DEPT spectrum exhibited correlations with their protons at 3.96/67.22 ppm, 3.76/61.80 ppm and 3.90/61.36 ppm due to the C-6/H-1 correlations of G-M, G and M residues, respectively. All detected correlations among carbons and their protons are presented in Table 2.
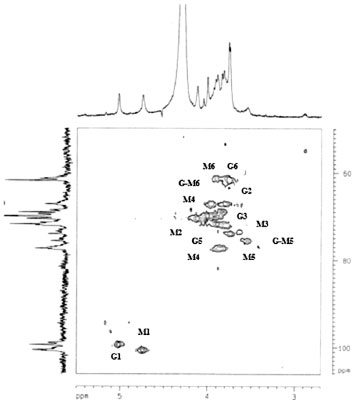 Figure 4. 1H/13C HSQC spectrum of the galactomannan from Adenanthera pavonina
FT-IR spectrum The FT-IR spectrum of A. pavonina galactomannan was analysed (Figure 5). There is an absorption band at 3298 cm-1 indicating the hydroxyl stretching vibration. Another band detected was at 2921 cm-1 indicating -CH stretching.35
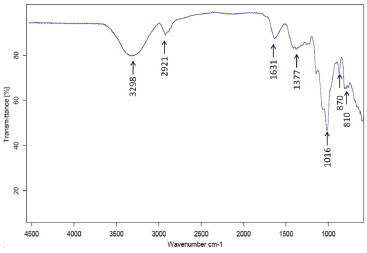 Figure 5. FT-IR spectrum (cm-1) of the Adenantherapavonina (L.) gum
Other common bands for polysaccharides such as bands at 1631, 1377, 1016, 870 and 810 cm-1 were detected in the spectral region between 1600 and 800 cm-1.36 This region exhibits highly coupled C-C-O, C-O-C and C-OH stretching modes of polymer backbone. Figueiró et al.37 detected two adsorption bands at 812 and 871 cm-1 in a film made of A. pavonina galactomannan, suggesting the presence of α-linked D-galactopyranose units and β-linked D-mannopyranose units, respectively. In vitro delivery Figure 6 illustrates drug release profiles from matrices prepared with both polymers (HPMC and galactomannan).
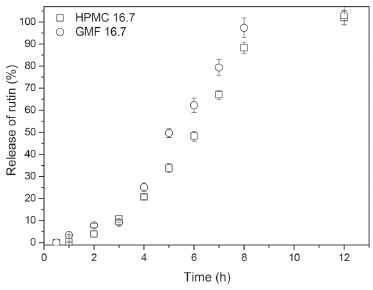 Figure 6. Release profile (duration of 12 h) of matrices containing 16.7 wt.% of galactomannan (GMF 16.7) and 16.7 wt.% HPMC (HPMC 16.7)
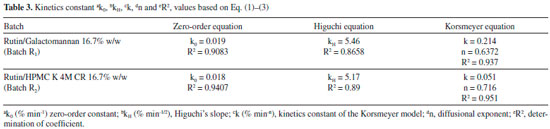
Figure 6 also shows that 16.7 wt.% of galactomannan (Batch R1) can control the drug release. The efficiency of the different polymeric carriers used for rutin was studied by comparing the dissolution profile of rutin/galactomannan (Batch R1) and rutin/HPMC K 4M CR (Batch R2) in distilled water. In comparing the linear regressions shown in Table 3, it can be observed that the release profile of the tablets of the R1 and R2 formulations respond better to zero-order kinetics than that of Higuchi for both polymers. This study showed that the addition of galactomannan instead of HMPC did not affect the release profile of the rutin. However, the results obtained are promising since the substitution of HMPC by galactomannan may possibly be an advantage considering that this type of polysaccharide is non-toxic and is used in the food industry. Furthermore, the applications and effects of galactomannans are intrinsically linked to structural features of the polymers.38 For example, matrix tablets of galactomannan from Senna tora seeds showed better ability for sustained release potential of losartan potassium when compared to the matrix tablets from other galactomannans, such as in those prepared with guar gum.38
Berta et al.39 analysed the applicability of commercial galactomannan from Guar Gum with different molecular weight in low concentrations (5-10%) as a disintegrating agent, and in high concentrations (25%) as a binding agent, under the commercial name of Meyprogat® as an auxiliary agent in tablet-making. Galactomannan provides tablets with a high level of hardness and is suitable for forming hydrophilic matrix tablets of theophylline with different dissolution rates according to the galactomannan used. Several kinetic models describe drug release from immediate and modified release dosage forms. The model that best fits the release data was evaluated by correlation coefficient (r) [Table 3]. The correlation coefficient (r) values were used as the criteria to choose the best model to describe drug release from the R1 and R2 controlled release tablets. For the R1 and R2 formulation tablets, the R2 values (0.9083 and 0.9407) were higher in zero-order models than in the Higuchi equation (0.8658 and 0.89), indicating that the drug release from most of the tablets was according to zero-order kinetics, and thus showing that the drug release rate was independent of the diffusion controlled by the drug. Some systems may be classified as either purely diffusion or erosion controlled, while most systems exhibit a combination of these mechanisms. By using the Korsmeyer-Peppas equation, the n values obtained were 0.6372 and 0.716 [Table 3] for both formulations. These values are characteristic of anomalous kinetics (non-Fickian) and super case-II transport, suggesting that more than one mechanism may be involved in release kinetics, referring to a combination of diffusion and erosion-based drug release mechanisms. Therefore, the drug release mechanism is determined by the structural characteristics of the gel layer (diffusion capability) and by gel layer erosion.40
CONCLUSIONS The galactomannan of Adenanthera pavonina seeds can be easily obtained through the hot aqueous extraction process. This procedure allows a gum yield of 13 wt.%. The M/G ratio (1.46) was determined and the obtained value was similar to that reported in the literature. The NMR study of the Adenanthera pavonina gum suggests that polysaccharide has a structure in agreement with other galactomannans described in the literature. The IR results corroborate with the NMR data obtained in this study. Finally, other chemical or physico-chemical analyses of this galactomannan will help us to better understand their characteristics and can be used in a range of applications within the pharmaceutical industry. Hydrophilic matrices containing galactomannan were able to modulate drug delivery from the oral dosage forms. The release rate of the rutin from the matrix tablets was significantly influenced by the proportion of galactomannan used. Galactomannan matrices show potential as drug encapsulation systems of rutin.
ACKNOWLEDGMENTS The authors are grateful for the support from MCTI/CNPq/Universal 456725/2014-5 (Grant number: 304392/2013-8 N.M.P.S.R), INCT-NanoBioSimes and Central Analítica UFC/SISNANO, CENAUREMN-UFC, and CAPES (C.E.A.S.).
REFERENCES 1. Girish, B.; Pasha, I.; Gowda, D.; Int. J. Pharm. Pharm. Sci. 2012, 4, 120. 2. Krishnaiah Y. S.; Karthikeya, R. S.; Satyanarayana, V.; Int. J. Pharm. 2002, 241, 353. 3. Munday D. L.; Cox, P. J.; Int. J. Pharm. 2000, 203, 179. 4. Siepmann, J.; Peppas, N. A.; Adv. Drug Delivery Rev. 2012, 64,163. 5. Kiil, S.; Dam-Johansen, K.; J. Controlled Release 2003, 90, 1. 6. de Souza, J. R. R.; Feitosa, J. P.; Ricardo, N. M.; Trevisan, M. T. S.; de Paula, H. C. B.; Ulrich, C. M.; Owen, R. W.; Food Hydrocolloids 2013, 33, 10. 7. Sarkar, S., Gupta, S., Variyar, P. S., Sharma, A., Singhal, R. S.; Carbohydr. Polym. 2013, 95, 177. 8. Rashid, F.; Hussain, S.; Ahmed, Z.; Carbohydr. Polym. 2018, 180, 88. 9. Ogunjimi, A. T.; Melo, S. G.G.; Vargas-Rechia, C. G.; Emery, F. S., Lopez, R. F. V.; Carbohydr. Polym. 2017, 157, 1065. 10. de Godoi, A. M.; Faccin-Galhardi, L. C.; Lopes, N.; Nozawa, C.; de Almeida, R. R.; Ricardo, N. M. P. S.; Linhares, R. E.; Curr. Pharm. Biotechnol. 2015, 16, 1024. 11. de Godoi, A. M.; Faccin-Galhardi, L. C.; Lopes, N.; Rechenchoski, D. Z.; de Almeida, R. R.; Ricardo, N. M. P. S.; Nozawa, C.; Linhares, R. E. C.; J. Evidence-Based Complementary Altern. Med. 2014, 2014, 1. 12. Ravishankar, D.; Rajora, A. K.; Greco, F.; Osborn, H. M.; Int. J. Biochem. Cell Biol. 2013, 45, 2821. 13. Abdel Ghaffar, A. M.; Radwan, R. R.; Ali, H. E.; Int J Biol. Macromol. 2016, 92, 957; Cho, N.; Lee, K. Y.; Huh, J.; Choi, J. H.; Yang, H.; Jeong, E. J.; Kim, H. P.; Sung, S. H.; Food Chem. Toxicol. 2013, 58, 355. 14. Vieira, I. G. P.; Mendes, F. N. P.; Galao, M. I.; Brito, E. S.; Food Chem. 2007, 101, 70. 15. Oliveira, E. G.; Campos, R. S.; Machado, A. S.; Pereira, J. F.; Araújo, T. G.; Polímeros 2015, 25, 54 16. Oliveira, E. G.; Cardoso, R. S.; Oliveira, C. L. C. G.; Araújo, T. G.; Lat. Am. J. Pharm. 2017, 36, 19. 17. Aguilar-De-Leyva, A.; Gonçalves-Araujo, T.; Daza, V.; Caraballo, I.; Pharm. Dev. Technol. 2014, 19, 728. 18. Higuchi, T.; J. Pharm. Sci. 1963, 52, 1145. 19. Korsmeyer, R. W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N. A.; Int. J. Pharm. 1983, 15, 25. 20. Ritger, P. L.; Peppas, N. A.; J. Controlled Release. 1987, 5, 23. 21. Gonçalves-Araújo, T.; Rajabi-Siahboomi, A. R.; Caraballo, I.; AAPS PharmSciTech 2010, 11, 558. 22. Jian, H-L.; Lin, X-J.; Zhang, W-A.; Zhang, W-M.; Sun, D-F.; Jiang, J-X. Food Hydrocolloids 2014, 40, 115. 23. Cerqueira, M. A.; Pinheiro, A. C.; Souza, B. W. S.; Lima, A. M. P.; Ribeiro, C.; Miranda, C.; Teixeira, J. A.; Moreira, R. A.; Coimbra, M. A.; Gonçalves, M. P.; Vicente, A. A.; Carbohydr. Polym. 2009, 75, 408; Cerqueira, M. A.; Souza, B. W. S.; Simoes, J.; Teixeira, J. A.; Domingues, M. R. M.; Coimbra, M. A.; Vicente, A. A.; Carbohydr. Polym. 2010, 83, 179. 24. Andrade, C. T.; Azero, E. G.; Luciano, L.; Gonçalves, M. P.; Int. J. Biol. Macromol. 1999, 26, 181. 25. Egorov, A.V.; Mestechkina, N. M. Shcherbukhin, V. D.; Prikladnaia Biokhimiia I Mikrobiologiia 2003, 39, 398. 26. Egorov, A. V.; Mestechkina, N. M.; Shcherbukhin.V. D.; Prikladnaia Biokhimiia I Mikrobiologiia 2004, 40, 314. 27. Azero, E. G.; Andrade, C. T.; Polym. Test. 2002, 21, 551. 28. Grasdalen, H.; Painter, T.; Carbohydr. Res. 1980, 81, 59. 29. Chaubey, M.; Kapoor, V. P.; Carbohydr. Res. 2001, 332, 439. 30. Ramesh, H. P.; Yamaki, K.; Ono, H.; Tsushida, T.; Carbohydr. Polym. 2001, 45, 69. 31. Cunha, P. L. R.; Vieira, I. G.; Arriaga, A. M. C.; de Paula, R. C. M.; Feitosa, J. P. A.; Food Hydrocolloids 2009, 23, 880. 32. Buckeridge, M. S.; Santos, H. P.; Tine, M. A. S.; Plant Physiol. Biochem. 2000, 38, 141. 33. Buckeridge, M. S.; Pavegassi, V. R.; Rocha, D. C.; Dietrich, S. M. C.; Phytochemistry 1995, 38, 871. 34. Garros-Rosa, I.; Reicher, F.; Petkowicz, C. L. O.; Sierakowski, M. R.; Moreira, R. A.; Polímeros 2006, 16, 99. 35. Yuen, S.-N.; Choi, S.-M.; Phillips, D. L.; Ma, C.-Y.; Food Chem. 2009, 114, 1091. 36. Kačuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A.; Carbohydr. Polym. 2000, 43, 195. 37. Figueiró, S. D.; Goes, J. C.; Moreira, R.A.; Sombra, A. S. B.; Carbohydr. Polym. 2004, 56, 313. 38. Gidley, M. J; Reid, J. S. G. Em Food Polysaccharides and Their Applications; Stephen, A. M., Phillips. G. O., Williams, P. A., eds.; CRC Press: Boca Raton, 2006, cap 6. 39. Berta, E.; Hódi, K.; Révész, P.; Miseta, M.; Selmeczi, B.; Acta. Pharm. Hung. 1994, 64, 26. 40. Arora, G.; Malik, K.; Singh, I.; Arora, S.; Rana, V.; J. Adv. Pharm. Technol. Res. 2011, 2, 163. |
On-line version ISSN 1678-7064 Printed version ISSN 0100-4042
Qu�mica Nova
Publica��es da Sociedade Brasileira de Qu�mica
Caixa Postal: 26037
05513-970 S�o Paulo - SP
Tel/Fax: +55.11.3032.2299/+55.11.3814.3602
Free access